Glucobrassicin Metabolites Ameliorate the Development of Portal Hypertension and Cirrhosis in Bile Duct-Ligated Rats
Abstract
:1. Introduction
2. Results
2.1. Body Weight, Hemodynamics, Liver, and Kidney Biochemical Parameters
2.2. Degree of Portosystemic Shunting and Plasma VEGF Concentration
2.3. Mesenteric Angiogenesis
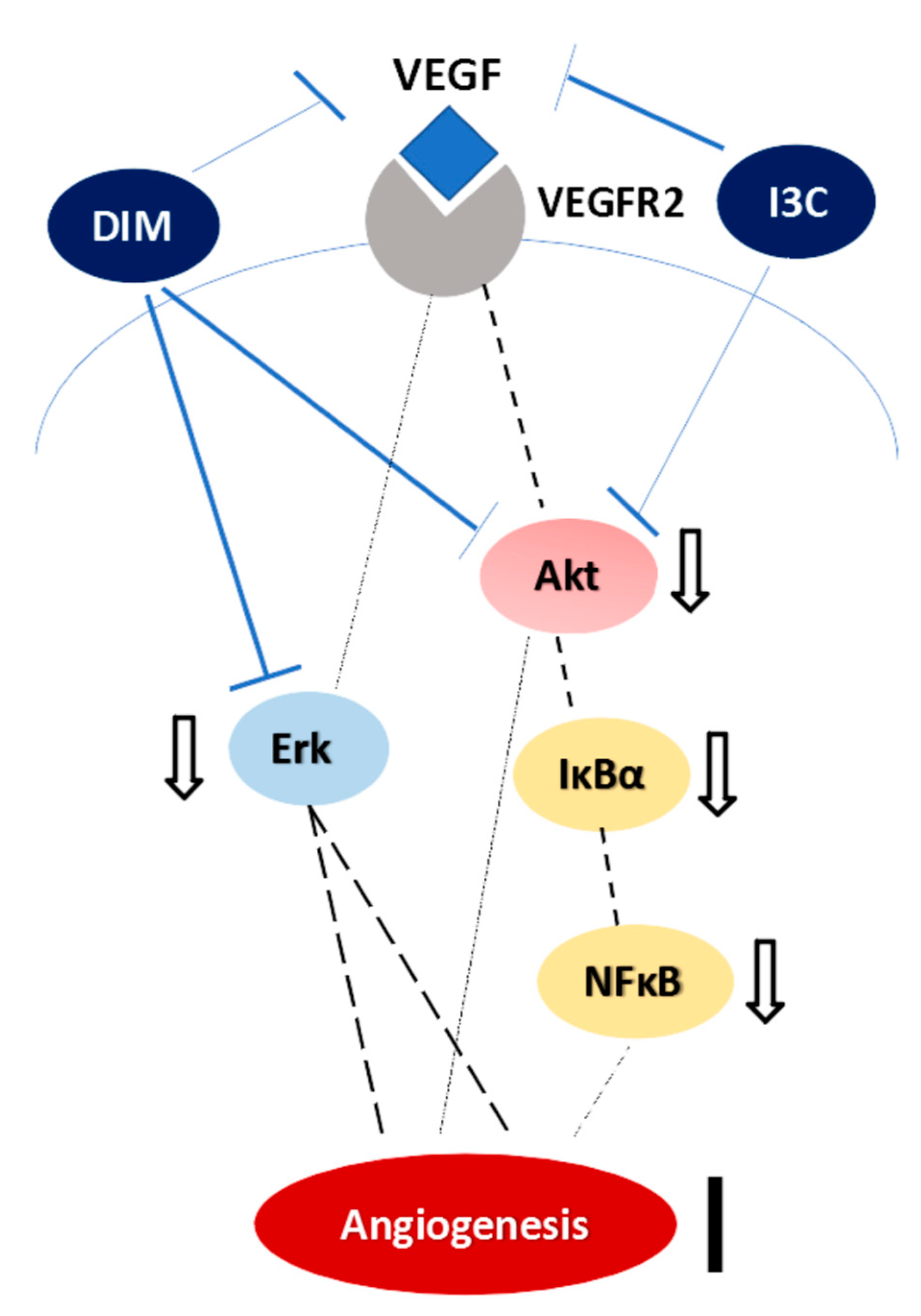
2.4. Angiogenesis-Related Protein Expressions in Mesentery
2.5. Liver Fibrosis
3. Discussion
4. Materials and Methods
4.1. Animal Model: Common Bile Duct Ligation (CBDL) Rats
4.2. Systemic and Splanchnic Hemodynamics and Biochemical Markers Measurement
4.3. Color Microsphere Method for Portosystemic Shunting Degree Analysis
4.4. Immunofluorescent Study for Mesenteric Vascular Density
4.5. Western Blotting for Protein Analysis
4.6. Determination of Plasma VEGF Concentration
4.7. Determination of Hepatic Fibrosis
4.8. Drugs
4.9. Statistical Analysis
4.10. Study Protocol
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miñano, C.; Garcia-Tsao, G. Clinical pharmacology of portal hypertension. Gastroenterol. Clin. North Am. 2010, 39, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.; Vizzutti, F.; Garcia-Pagan, J.C.; Rodes, J.; Bosch, J. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology 2004, 126, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Mejias, M.; Garcia-Pras, E.; Tiani, C.; Miquel, R.; Bosch, J.; Fernandez, M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology 2009, 49, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.H.; Kristal, A.R.; Stanford, J.L. Fruit and vegetable intakes and prostate cancer risk. J. Natl. Cancer Inst. 2000, 92, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Anderton, M.J.; Jukes, R.; Lamb, J.H.; Manson, M.M.; Gescher, A.; Steward, W.P.; Williams, M.L. Liquid chromatographic assay for the simultaneous determination of indole-3-carbinol and its acid condensation products in plasma. J. Chromatogr. B. 2003, 787, 281–291. [Google Scholar] [CrossRef]
- Verhoeven, D.H.T.; Verhagen, H.; Goldbohm, R.A.; van den Brandt, P.A.; van Poppel, G.A. A review of mechanisms underlying anticarcinogenicity by brassica vegetables. Chem. Biol. Interact. 1997, 103, 79–129. [Google Scholar] [CrossRef]
- Chang, Y.C.; Riby, J.; Chang, G.H.F.; Peng, B.C.; Firestone, G.; Bjeldanes, L.F. Cytostatic and antiestrogenic effect of 2-(indole-3-ylmethyl)-3,3′-diindolylmethane, a major in vivo product of dietary indole-3-carbinol. Biochem. Pharm. 1999, 58, 825–834. [Google Scholar] [CrossRef]
- Reed, G.A.; Arneson, D.W.; Putnam, W.C.; Smith, H.J.; Gray, J.C.; Sullivan, D.K.; Mayo, M.S.; Crowell, J.A.; Hurwitz, A. Single-dose and multiple-dose administration of indole-3-carbinol to women: Pharmacokinetics based on 3,39-diindolylmethane. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2477–2481. [Google Scholar] [CrossRef]
- Anderton, M.J.; Manson, M.M.; Verschoyle, R.D.; Gescher, A.; Lamb, J.H.; Farmer, P.B.; Steward, W.P.; Williams, M.L. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin. Cancer Res. 2004, 10, 5233–5241. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Ichikawa, H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle 2005, 4, 1201–1215. [Google Scholar] [CrossRef]
- Grubbs, C.J.; Steele, V.E.; Casebolt, T.; Juliana, M.M.; Eto, I.; Whitaker, L.M.; Dragnev, K.H.; Kelloff, G.J.; Lubet, R.L. Chemoprevention of chemically induced mammary carcinogenesis by indole-3-carbinol. Anticancer Res. 1995, 15, 709–716. [Google Scholar] [PubMed]
- Chen, I.; McDougal, A.; Wang, F.; Safe, S. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis 1998, 19, 1631–1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souli, E.; Machluf, M.; Morgenstern, A.; Sabo, E.; Yannai, S. Indole-3-carbinol (I3C) exhibits inhibitory and preventive effects on prostate tumors in mice. Food Chem. Toxicol. 2008, 46, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Tou, J.C.; Hong, C.; Kim, H.A.; Riby, J.E.; Firestone, G.L.; Bjeldanes, L.F. 3,30-diindolylmethane inhibits angiogenesis and the growth of transplantable human breast carcinoma in athymic mice. Carcinogenesis 2005, 26, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Kroll, J.; Waltenberger, J. The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J. Biol. Chem. 1997, 272, 32521–32527. [Google Scholar] [CrossRef] [PubMed]
- Kanno, S.; Oda, N.; Abe, M.; Terai, Y.; Ito, M.; Shitara, K.; Tabayashi, K.; Shibuya, M.; Sato, Y. Roles of two VEGF receptors, Flt-1 and KDR, in the signal transduction of VEGF effects in human vascular endothelial cells. Oncogene 2000, 19, 2138–2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibuya, M. Vascular endothelial growth factor (VEGF)-Receptor2: Its biological functions, major signaling pathway, and specific ligand VEGF-E. Endothelium 2006, 13, 63–69. [Google Scholar] [CrossRef]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling-in control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef]
- Sumanovski, L.T.; Battegay, E.; Stumm, M.; van der Kooij, M.; Sieber, C.C. Increased angiogenesis in portal hypertensive rats: Role of nitric oxide. Hepatology 1999, 29, 1044–1049. [Google Scholar] [CrossRef]
- Sieber, C.C.; Sumanovski, L.T.; Stumm, M.; van der Kooij, M.; Battegay, E. In vivo angiogenesis in normal and portal hypertensive rats: Role of basic fibroblast growth factor and nitric oxide. J. Hepatol. 2001, 34, 644–650. [Google Scholar] [CrossRef]
- Kroll, J.; Waltenberger, J. A novel function of the vascularendothelial growth factor receptor-2(KDR): Rapid release of nitric oxidein response to VEGF-A stimulation in endothelial cells. Biochem. Biophys. Res. Commun. 1999, 265, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Huang, W.D.; Gao, Q.; Su, M.L.; Yang, Y.F.; Liu, Z.C.; Zhu, B.H. Arnebin-1 promotes angiogenesis by inducing eNOS, VEGF and HIF-1α expression through the PI3K-dependent pathway. Int. J. Mol. Med. 2015, 36, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Vilen, S.T.; Salo, T.; Sorsa, T.; Nyberg, P. Fluctuating Roles of Matrix Metalloproteinase-9 in Oral Squamous Cell Carcinoma. Sci. World J. 2013, 2013, 920595. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Cheng, L.S.; Liu, Y.; Wang, J.Y.; Jiang, W. Indole-3-Carbinol (I3C) and its Major Derivatives: Their Pharmacokinetics and Important Roles in Hepatic Protection. Curr. Drug Metab. 2016, 17, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Ping, J.; Gao, A.M.; Xu, D.; Li, R.W.; Wang, H. Therapeutic effect of indole-3-carbinol on pig serum-induced hepatic fibrosis in rats. Yao Xue Xue Bao 2011, 46, 915–921. [Google Scholar] [PubMed]
- Li, B.; Cong, M.; Zhu, Y.; Xiong, Y.; Jin, W.; Wan, Y.; Zhou, Y.; Ao, Y.; Wang, H. Indole-3-Carbinol Induces Apoptosis of Hepatic Stellate Cells through K63 De-Ubiquitination of RIP1 in Rats. Cell Physiol. Biochem. 2017, 41, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, Z.; Hu, W.; Yin, S.; Wang, C.; Zang, Y.; Chen, J.; Zhang, J.; Dong, L. 3,3’-Diindolylmethane ameliorates experimental hepatic fibrosis via inhibiting miR-21 expression. Br. J. Pharmacol. 2013, 170, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.; Laflamme, L.; Benassou, I.; Cissokho, C.; Guillemette, B.; Gaudreau, L. Low levels of 3,3′-diindolylmethane activate estrogen receptor α and induce proliferation of breast cancer cells in the absence of estradiol. BMC Cancer 2014, 14, 524. [Google Scholar] [CrossRef] [PubMed]
- El-sayed, M.T.; Hamdy, N.A.; Osman, D.A.; Ahmed, K.M. Indoles as anti-cancer agents. Adv. Mod. Oncol. Res. 2015, 1, 20–35. [Google Scholar] [CrossRef]
- Kunimasa, K.; Kobayashi, T.; Sugiyama, S.; Kaji, K.; Ohta, T. Indole-3-carbinol Suppresses Tumor-Induced Angiogenesis by Inhibiting Tube Formation and Inducing Apoptosis. Biosci. Biotechnol. Biochem. 2008, 72, 2243–2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, J.; Huang, H.C. Management of ascites in patients with liver cirrhosis: Recent evidence and controversies. J. Chin. Med. Assoc. 2013, 76, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, J.; Lee, F.Y.; Wang, S.S.; Hsin, I.F.; Lin, T.Y.; Huang, H.C.; Chang, C.C.; Chuang, C.L.; Ho, H.L.; Lin, H.C.; et al. Caffeine ameliorates hemodynamic derangements and portosystemic collaterals in cirrhotic rats. Hepatology 2015, 61, 1672–1684. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, S.S. The Relationship between Serum VEGF Concentration and Prognosis of Lung Cancer. Korean J. Intern. Med. 2003, 18, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; de Boer, W.B.; Adams, L.A.; MacQuillan, G.; Rossi, E.; Rigby, P.; Raftopoulos, S.C.; Bulsara, M.; Jeffrey, G.P. Image analysis of liver collagen using sirius red is more accurate and correlates better with serum fibrosis markers than trichrome. Liver Int. 2013, 33, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Firestone, G.L.; Bjeldanes, L.F. Inhibition of growth factor-induced Ras signaling in vascular endothelial cells and angiogenesis by 3,3’-diindolylmethane. Carcinogenesis 2006, 27, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chinni, S.R.; Sarkar, F.H. Selective growth regulatory and pro-apoptotic effects of DIM is mediated by AKT and NF-κB pathways in prostate cancer cells. Front. Biosci. 2005, 10, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Shih, C.K.; Chang, H.P.; Chen, Y.H. Antiangiogenic activity of indole-3-carbinol in endothelial cells stimulated with activated macrophages. Food Chem. 2012, 134, 811–820. [Google Scholar] [CrossRef]
- Takada, Y.; Andreeff, M.; Aggarwal, B.B. Indole-3-carbinol suppresses NF-kappaB and IkappaBalpha kinase activation, causing inhibition of expression of NF-kappaB-regulated antiapoptotic and metastatic gene products and enhancement of apoptosis in myeloid and leukemia cells. Blood 2005, 106, 641–649. [Google Scholar] [CrossRef]
- Ampofo, E.; Schmitt, B.M.; Menger, M.D.; Laschke, M.W. Targeting the Microcirculation by Indole-3-carbinol and Its Main Derivate 3,3,’-diindolylmethane: Effects on Angiogenesis, Thrombosis and Inflammation. Mini Rev. Med. Chem. 2018, 18, 962–968. [Google Scholar] [CrossRef]
- Choi, Y.; Abdelmegeed, M.A.; Song, B.J. Preventive effects of indole-3-carbinol against alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms: Role of gut-liver-adipose tissue axis. J. Nutr. Biochem. 2018, 55, 12–25. [Google Scholar] [CrossRef]
- Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P.S. Natural indoles, indole-3-carbinol (I3C) and 3,3’-diindolylmethane (DIM), attenuate staphylococcal enterotoxin B-mediated liver injury by downregulating miR-31 expression and promoting caspase-2-mediated apoptosis. PLoS ONE 2015, 10, e0118506. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; She, W.; Wang, F.; Li, J.; Wang, J.; Jiang, W. 3, 3’-Diindolylmethane alleviates steatosis and the progression of NASH partly through shifting the imbalance of Treg/Th17 cells to Treg dominance. Int. Immunopharmacol. 2014, 23, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Gigou, M.; Szekely, A.M.; Bismuth, H. Portal hypertension after bile duct obstruction. Effect of the bile diversion on portal pressure in the rat. Arch Surg. 1979, 114, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Cameron, G.R.; Hasan, S.M. Disturbances of structure and function in the liver as the result of biliary obstruction. J. Pathol. Bacteriol. 1958, 75, 33–49. [Google Scholar] [CrossRef]
- Kountouras, J.; Billing, B.H.; Scheuer, P.J. Prolonged bile duct ligation obstruction: A new experimental model of cirrhosis in the rat. Br. J. Exp. Pathol. 1984, 65, 305–311. [Google Scholar] [PubMed]
- Kushad, M.M.; Brown, A.F.; Kurilich, A.C.; Juvik, J.A.; Klein, B.P.; Wallig, M.A.; Jeffery, E.H. Variation of glucosinolates in vegetable crops of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Designed Nutritional Products. 2003. Available online: http://www.designednutritional.com/Header%20Side%20Door/AnswersBrocoli.html (accessed on 6 August 2019).
- Lee, F.Y.; Wang, S.S.; Tsai, Y.T.; Lin, H.J.; Lin, H.C.; Chu, C.J.; Wu, S.L.; Tai, C.C.; Lee, S.D. Aminoguanidine corrects hyperdynamic circulation without ameliorating portal hypertension and portal hypertensive gastropathy in anesthetized portal hypertensive rats. J. Hepatol. 1997, 26, 687–693. [Google Scholar] [CrossRef]
- Lee, F.Y.; Colombato, L.A.; Albillos, A.; Groszmann, R.J. Administration of Nω-nitro-L- arginine ameliorates portal-systemic shunting in portal-hypertensive rats. Gastroenterology 1993, 105, 1464–1470. [Google Scholar] [CrossRef]
- Albillos, A.; Colombato, L.A.; Groszmann, R.J. Vasodilatation and sodium retention in prehepatic portal hypertension. Gastroenterology 1992, 102, 931–935. [Google Scholar] [CrossRef]
- Chojkier, M.; Groszmann, R.J. Measurement of portal-systemic shunting in the rat by using γ-labeled microspheres. Am. J. Physiol. 1981, 240, G371–G375. [Google Scholar] [CrossRef]
- Hodeige, D.; de Pauw, M.; Eechaute, W.; Weyne, J.; Heyndrickx, G.R. On the validity of blood flow measurement using colored microspheres. Am. J. Physiol. 1999, 276, H1150–H1158. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Ponce, A.M.; Price, R.J. Immunohistochemical identification of an extracellular matrix scaffold that microguides capillary sprouting in vivo. J. Histochem. Cytochem. 2004, 52, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- ImageJ Website. Available online: http://rsb.info.nih.gov/ij/ (accessed on 12 January 2016).
- Huang, H.C.; Wang, S.S.; Hsin, I.F.; Chang, C.C.; Lee, F.Y.; Lin, H.C.; Chuang, C.L.; Lee, J.Y.; Hsieh, H.G.; Lee, S.D. Cannabinoid receptor 2 agonist ameliorates mesenteric angiogenesis and portosystemic collaterals in cirrhotic rats. Hepatology 2012, 56, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Garikapasy, V.P.S.; Ashok, B.T.; Chen, Y.G.; Mittelman, A.; Latropoulos, M.; Tiwari, R.K. Anti-carcinogenic and anti-metastatic properties of indole-3-carbinol in prostate cancer. Oncol. Rep. 2004, 13, 89–93. [Google Scholar]







| CBDL-Vehicle n = 5 | CBDL-DIM n = 7 | CBDL–I3C n = 7 | Sham-Vehicle n = 6 | Sham-DIM n = 6 | Sham-I3C n = 6 | |
|---|---|---|---|---|---|---|
| BW (g) | 388.0 ± 20.2 # | 409.1 ± 4.2 | 408.0 ± 20.9 | 447.8 ± 11.6 | 432.7 ± 7.2 | 451.0 ± 10.8 |
| MAP (mmHg) | 120.7 ± 4.7 # | 108.9 ± 5.6 | 106.6 ± 8.9 | 145.96 ± 4.66 | 145.5 ± 5.0 | 150.0 ± 6.0 |
| HR (beats/min) | 363 ± 10 | 326 ± 11 * | 361 ± 12 | 376 ± 19 | 353 ± 18 | 317 ± 27 |
| PP (mmHg) | 17.8 ± 1.2 ### | 16.2 ± 0.6 * | 16.1 ± 1.1 * | 10.7 ± 0.3 | 9.5 ± 0.5 | 9.5 ± 0.5 |
| CO (mL/min) | 141.5 ± 5.5 | 120.7 ± 8.8 | 123.8 ± 6.0 | 131.3 ± 2.0 | 161.2 ± 11.6 * | 140.1 ± 4.2 |
| CI (mL/min/100 g) | 36.4 ± 2.0 # | 29.4 ± 2.1 | 30.17 ± 3.1 | 29.4 ± 0.9 | 37.2 ± 2.3 ** | 31.1 ± 0.8 |
| SV (mL/beats) | 0.39 ± 0.02 ### | 0.37 ± 0.02 | 0.34 ± 0.01 | 0.06 ± 0.00 | 0.07 ± 0.01 * | 0.08 ± 0.00 * |
| SVR (mmHg/mL/min/100 g) | 3.24 ± 0.56 ## | 3.87 ± 0.27 | 3.70 ± 0.61 | 4.99 ± 0.24 | 3.97 ± 0.23 ** | 4.82 ± 0.13 |
| SMA flow (mL/min/100 g) | 5.9 ± 0.4 # | 6.2 ± 0.4 | 5.8 ± 0.8 | 4.7 ± 0.2 | 5.9 ± 0.6 | 5.6 ± 0.5 |
| SMAR (mmHg/mL/min/100 g) | 18.04 ± 2.15 ## | 15.62 ± 1.64 | 17.54 ± 2.33 | 28.83 ± 1.20 | 24.31 ± 2.84 | 25.81 ± 1.58 |
| ALT (U/L) | 237.8 ± 40.0 | 206.1 ± 32.0 | 189.9 ± 29.3 | 45.8 ± 2.2 | 53.8 ± 2.6 | 48.3 ± 2.5 |
| Total Bilirubin (mg/dL) | 9.35 ± 0.64 | 8.64 ± 0.41 | 8.13 ± 0.82 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00 |
| Creatinine (mg/dL) | 0.52 ± 0.05 | 0.55 ± 0.03 | 0.57 ± 0.06 | 0.43 ± 0.03 | 0.42 ± 0.03 | 0.40 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.; Ho, H.-L.; Hsu, S.-J.; Chang, C.-C.; Tsai, M.-H.; Huo, T.-I.; Huang, H.-C.; Lee, F.-Y.; Hou, M.-C.; Lee, S.-D. Glucobrassicin Metabolites Ameliorate the Development of Portal Hypertension and Cirrhosis in Bile Duct-Ligated Rats. Int. J. Mol. Sci. 2019, 20, 4161. https://doi.org/10.3390/ijms20174161
Chang T, Ho H-L, Hsu S-J, Chang C-C, Tsai M-H, Huo T-I, Huang H-C, Lee F-Y, Hou M-C, Lee S-D. Glucobrassicin Metabolites Ameliorate the Development of Portal Hypertension and Cirrhosis in Bile Duct-Ligated Rats. International Journal of Molecular Sciences. 2019; 20(17):4161. https://doi.org/10.3390/ijms20174161
Chicago/Turabian StyleChang, Ting, Hsin-Ling Ho, Shao-Jung Hsu, Ching-Chih Chang, Ming-Hung Tsai, Teh-Ia Huo, Hui-Chun Huang, Fa-Yauh Lee, Ming-Chih Hou, and Shou-Dong Lee. 2019. "Glucobrassicin Metabolites Ameliorate the Development of Portal Hypertension and Cirrhosis in Bile Duct-Ligated Rats" International Journal of Molecular Sciences 20, no. 17: 4161. https://doi.org/10.3390/ijms20174161
APA StyleChang, T., Ho, H.-L., Hsu, S.-J., Chang, C.-C., Tsai, M.-H., Huo, T.-I., Huang, H.-C., Lee, F.-Y., Hou, M.-C., & Lee, S.-D. (2019). Glucobrassicin Metabolites Ameliorate the Development of Portal Hypertension and Cirrhosis in Bile Duct-Ligated Rats. International Journal of Molecular Sciences, 20(17), 4161. https://doi.org/10.3390/ijms20174161





