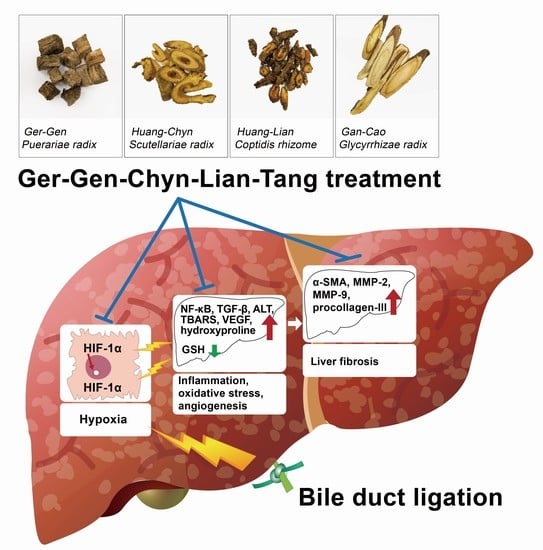
Positive Effects of Ger-Gen-Chyn-Lian-Tang on Cholestatic Liver Fibrosis in Bile Duct Ligation-Challenged Mice
Abstract
:1. Introduction
2. Results
2.1. GGCLT Treatment Effect on Liver Injury and Hepatic Oxidative Stress
2.2. GGCLT Treatment Effect on Fibrogenesis and Angiogenesis-Related Factors in the Liver
3. Discussion
4. Materials and Methods
4.1. Preparation of GGCLT
4.2. Animals and Experimental Protocols
4.3. Analyses of Liver Histology, Cytokines, and Biochemicals
4.4. Quantitative Real-Time PCR
4.5. Western Blot Measurement
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friedman, S.L. Liver fibrosis—from bench to bedside. J. Hepatol. 2003, 38, S38–S53. [Google Scholar] [CrossRef]
- Iredale, J.P. Models of liver fibrosis: Exploring the dynamic nature of inflammation and repair in a solid organ. J. Clin. Invest. 2007, 117, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; Moon, H.E.; Kim, K.W. Hypoxia-induced angiogenesis in human hepatocellular carcinoma. J. Mol. Med. 2002, 80, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Rosmorduc, O.; Housset, C. Hypoxia: A link between fibrogenesis, angiogenesis, and carcinogenesis in liver disease. Semin. Liver Dis. 2010, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Bozova, S.; Elpek, G.O. Hypoxia-inducible factor-1alpha expression in experimental cirrhosis: Correlation with vascular endothelial growth factor expression and angiogenesis. Apmis 2007, 115, 795–801. [Google Scholar] [CrossRef]
- Cannito, S.; Novo, E.; Compagnone, A.; Valfre di Bonzo, L.; Busletta, C.; Zamara, E.; Paternostro, C.; Povero, D.; Bandino, A.; Bozzo, F.; et al. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis 2008, 29, 2267–2278. [Google Scholar] [CrossRef] [PubMed]
- Thabut, D.; Shah, V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: New targets for the treatment of portal hypertension? J. Hepatol. 2010, 53, 976–980. [Google Scholar] [CrossRef] [Green Version]
- Lemoinne, S.; Cadoret, A.; Rautou, P.E.; El Mourabit, H.; Ratziu, V.; Corpechot, C.; Rey, C.; Bosselut, N.; Barbu, V.; Wendum, D. Portal myofibroblasts promote vascular remodeling underlying cirrhosis formation through the release of microparticles. Hepatology 2015, 61, 1041–1055. [Google Scholar] [CrossRef]
- Higgins, D.F.; Kimura, K.; Bernhardt, W.M.; Shrimanker, N.; Akai, Y.; Hohenstein, B.; Saito, Y.; Johnson, R.S.; Kretzler, M.; Cohen, C.D.; et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Invest. 2007, 117, 3810–3820. [Google Scholar] [CrossRef]
- Shi, Y.F.; Fong, C.C.; Zhang, Q.; Cheung, P.Y.; Tzang, C.H.; Wu, R.S.; Yang, M. Hypoxia induces the activation of human hepatic stellate cells LX-2 through TGF-beta signaling pathway. FEBS Lett. 2007, 581, 203–210. [Google Scholar] [CrossRef]
- Meurer, S.K.; Tihaa, L.; Borkham-Kamphorst, E.; Weiskirchen, R. Expression and functional analysis of endoglin in isolated liver cells and its involvement in fibrogenic Smad signalling. Cell Signal. 2011, 23, 683–699. [Google Scholar] [CrossRef]
- Geerts, A.M.; Vanheule, E.; Praet, M.; Van Vlierberghe, H.; De Vos, M.; Colle, I. Comparison of three research models of portal hypertension in mice: macroscopic, histological and portal pressure evaluation. Int. J. Exp. Pathol. 2008, 89, 251–263. [Google Scholar] [CrossRef]
- Kountouras, J.; Billing, B.H.; Scheuer, P.J. Prolonged bile duct obstruction: A new experimental model for cirrhosis in the rat. Br. J. Exp. Pathol. 1984, 65, 305–311. [Google Scholar]
- Popov, Y.; Sverdlov, D.Y.; Bhaskar, K.R.; Sharma, A.K.; Millonig, G.; Patsenker, E.; Krahenbuhl, S.; Krahenbuhl, L.; Schuppan, D. Macrophage-mediated phagocytosis of apoptotic cholangiocytes contributes to reversal of experimental biliary fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G323–G334. [Google Scholar] [CrossRef] [Green Version]
- Scott-Conner, C.E.; Grogan, J.B. The pathophysiology of biliary obstruction and its effect on phagocytic and immune function. J. Surg. Res. 1994, 57, 316–336. [Google Scholar] [CrossRef]
- Minter, R.M.; Fan, M.H.; Sun, J.; Niederbichler, A.; Ipaktchi, K.; Arbabi, S.; Hemmila, M.R.; Remick, D.G.; Wang, S.C.; Su, G.L. Altered Kupffer cell function in biliary obstruction. Surgery 2005, 138, 236–245. [Google Scholar] [CrossRef]
- Saito, J.M.; Maher, J.J. Bile duct ligation in rats induces biliary expression of cytokine-induced neutrophil chemoattractant. Gastroenterology 2000, 118, 1157–1168. [Google Scholar] [CrossRef]
- Prado, I.B.; Santos, M.H.H.; Lopasso, F.P.; Iriya, K.; Laudanna, A.A. Cholestasis in a murine experimental model: lesions include hepatocyte ischemic necrosis. Rev. Hosp. Clín. 2003, 58, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Tag, C.G.; Sauer-Lehnen, S.; Weiskirchen, S.; Borkham-Kamphorst, E.; Tolba, R.H.; Tacke, F.; Weiskirchen, R. Bile duct ligation in mice: induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J. Vis. Exp. 2015, 96, e52438. [Google Scholar] [CrossRef]
- Tuchweber, B.; Desmouliere, A.; Bochaton-Piallat, M.-L.; Rubbia-Brandt, L.; Gabbiani, G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab. Invest. 1996, 74, 265–278. [Google Scholar]
- Desmoulière, A.; Darby, I.; Costa, A.; Raccurt, M.; Tuchweber, B.; Sommer, P.; Gabbiani, G. Extracellular matrix deposition, lysyl oxidase expression, and myofibroblastic differentiation during the initial stages of cholestatic fibrosis in the rat. Lab. Invest. 1997, 76, 765–778. [Google Scholar]
- Moczydlowska, J.; Miltyk, W.; Hermanowicz, A.; Lebensztejn, D.M.; Palka, J.A.; Debek, W. HIF-1 alpha as a Key Factor in Bile Duct Ligation-Induced Liver Fibrosis in Rats. J. Invest. Surg. 2016, 30, 41–46. [Google Scholar] [CrossRef]
- Moon, J.O.; Welch, T.P.; Gonzalez, F.J.; Copple, B.L. Reduced liver fibrosis in hypoxia-inducible factor-1alpha-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G582–G592. [Google Scholar] [CrossRef]
- Corpechot, C.; Barbu, V.; Wendum, D.; Kinnman, N.; Rey, C.; Poupon, R.; Housset, C.; Rosmorduc, O. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology 2002, 35, 1010–1021. [Google Scholar] [CrossRef]
- Copple, B.L.; Kaska, S.; Wentling, C. Hypoxia-inducible factor activation in myeloid cells contributes to the development of liver fibrosis in cholestatic mice. J. Pharmacol. Exp. Ther. 2012, 341, 307–316. [Google Scholar] [CrossRef]
- Ankoma-Sey, V.; Matli, M.; Chang, K.B.; Lalazar, A.; Donner, D.B.; Wong, L.; Warren, R.S.; Friedman, S.L. Coordinated induction of VEGF receptors in mesenchymal cell types during rat hepatic wound healing. Oncogene 1998, 17, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Yoshiji, H.; Kuriyama, S.; Yoshii, J.; Ikenaka, Y.; Noguchi, R.; Hicklin, D.J.; Wu, Y.; Yanase, K.; Namisaki, T.; Yamazaki, M.; et al. Vascular endothelial growth factor and receptor interaction is a prerequisite for murine hepatic fibrogenesis. Gut 2003, 52, 1347–1354. [Google Scholar] [CrossRef] [Green Version]
- Novo, E.; Cannito, S.; Zamara, E.; Valfre di Bonzo, L.; Caligiuri, A.; Cravanzola, C.; Compagnone, A.; Colombatto, S.; Marra, F.; Pinzani, M.; et al. Proangiogenic cytokines as hypoxia-dependent factors stimulating migration of human hepatic stellate cells. Am. J. Pathol. 2007, 170, 1942–1953. [Google Scholar] [CrossRef]
- Margadant, C.; Sonnenberg, A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Ho, F.M.; Liao, Y.H.; Yang, A.J.; Lee Chao, P.D.; Hou, Y.C.; Huang, C.T.; Lin, S.R.; Lee, K.R.; Huang, K.C.; Lin, W.W. Anti-atherosclerotic action of Ger-Gen-Chyn-Lian-Tang and AMPK-dependent lipid lowering effect in hepatocytes. J. Ethnopharmacol. 2012, 142, 175–187. [Google Scholar] [CrossRef]
- Chang, Z.Y.; Lee, T.Y.; Huang, T.H.; Wen, C.K.; Chien, R.N.; Chang, H.H. Hepatoprotective effects of Ger-Gen-Chyn-Lian-Tang in thioacetamide-induced fibrosis in mice. J. Chin. Med. Assoc. 2014, 77, 360–366. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Jin, Q.; Fan, G.; Duan, Y.; Qin, C.; Wen, M. Microwave-assisted extraction of effective constituents from a Chinese herbal medicine Radix puerariae. Analytica. Chimica. Acta. 2001, 436, 41–47. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, H.; Ye, J. Determination of baicalein, baicalin and quercetin in Scutellariae Radix and its preparations by capillary electrophoresis with electrochemical detection. Talanta 2000, 53, 471–479. [Google Scholar] [CrossRef]
- Kong, W.; Li, Z.; Xiao, X.; Zhao, Y. Quality control for Coptidis rhizoma through the determination of five alkaloids by HPLC–ELSD coupled with chemometrics. Nat. Prod. Res. 2010, 24, 1616–1629. [Google Scholar] [CrossRef]
- Lin, Z.J.; Qiu, S.-X.; Wufuer, A.; Shum, L. Simultaneous determination of glycyrrhizin, a marker component in radix Glycyrrhizae, and its major metabolite glycyrrhetic acid in human plasma by LC–MS/MS. J. Chromatogr. B 2005, 814, 201–207. [Google Scholar] [CrossRef]
- Teng, Y.; Cui, H.; Yang, M.; Song, H.; Zhang, Q.; Su, Y.; Zheng, J. Protective effect of puerarin on diabetic retinopathy in rats. Mol. Biol. Rep. 2009, 36, 1129. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, Z.; Wang, J.; Zhang, Z.; Hu, D.; Wang, J. Baicalin inhibits hypoxia-induced pulmonary artery smooth muscle cell proliferation via the AKT/HIF-1α/p27-associated pathway. Int. J. Mol. Sci. 2014, 15, 8153–8168. [Google Scholar] [CrossRef]
- Hamsa, T.; Kuttan, G. Antiangiogenic activity of berberine is mediated through the downregulation of hypoxia-inducible factor-1, VEGF, and proinflammatory mediators. Drug Chem. Toxicol. 2012, 35, 57–70. [Google Scholar] [CrossRef]
- Abe, M.; Koga, H.; Yoshida, T.; Masuda, H.; Iwamoto, H.; Sakata, M.; Hanada, S.; Nakamura, T.; Taniguchi, E.; Kawaguchi, T.; et al. Hepatitis C virus core protein upregulates the expression of vascular endothelial growth factor via the nuclear factor-kappaB/hypoxia-inducible factor-1alpha axis under hypoxic conditions. Hepatol. Res. 2012, 42, 591–600. [Google Scholar] [CrossRef]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef]
- Taura, K.; De Minicis, S.; Seki, E.; Hatano, E.; Iwaisako, K.; Osterreicher, C.H.; Kodama, Y.; Miura, K.; Ikai, I.; Uemoto, S.; et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology 2008, 135, 1729–1738. [Google Scholar] [CrossRef]
- Aleffi, S.; Petrai, I.; Bertolani, C.; Parola, M.; Colombatto, S.; Novo, E.; Vizzutti, F.; Anania, F.A.; Milani, S.; Rombouts, K.; et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology 2005, 42, 1339–1348. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Vagianos, C.E.; Patsoukis, N.; Georgiou, C.; Nikolopoulou, V.; Scopa, C.D. Evidence for intestinal oxidative stress in obstructive jaundice-induced gut barrier dysfunction in rats. Acta. Physiol. Scand. 2004, 180, 177–185. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Scopa, C.D.; Zervoudakis, G.; Mylonas, P.G.; Georgiou, C.; Nikolopoulou, V.; Vagianos, C.E. Bombesin and neurotensin reduce endotoxemia, intestinal oxidative stress, and apoptosis in experimental obstructive jaundice. Ann. Surg. 2005, 241, 159–167. [Google Scholar]
- Guimaraes, E.L.; Franceschi, M.F.; Grivicich, I.; Dal-Pizzol, F.; Moreira, J.C.; Guaragna, R.M.; Borojevic, R.; Margis, R.; Guma, F.C. Relationship between oxidative stress levels and activation state on a hepatic stellate cell line. Liver Int. 2006, 26, 477–485. [Google Scholar] [CrossRef]
- Tong, L. Antipuretic, antimicrobic action of gegen qinlian tang. Zhong Yao Tong Bao 1987, 12, 49–50. [Google Scholar]







| Gene | Forward | Reverse |
|---|---|---|
| HIF-1α | TCAAGTCAGCAACGTGGAAG | TATCGAGGCTGTGTCGACTG |
| VEGF | GAGAGAGGCCGAAGTCCTTT | TTGGAACCGGCATCTTTATC |
| VEGFR1 | GAAGCGGTTCACCTGGACTGAGACC | GGCTTTGCTGGGGGGATTTCTCTAA |
| VEGFR2 | ACAGCAGTGGGATGGTCCTTGCAT | AAACAGGAGGTGAGCTGCAGTGTGG |
| TGF-β | TGCCCTCTACAACCAACACAACCCG | AACTGCTCCACCTTGGGCTTGCGAC |
| MMP-2 | GCTGATACTGACA CTGGTACTG | CAATCTTTTCTGGGAGCTC |
| MMP-9 | CGTCGTGATCCCCACTTACT | AGAGTACTGCTTGCCCAGGA |
| Procollagen-III | CCCCTGGTCCCTGCTGTGG | GAGGCCCGGCTGGAAAGAA |
| GAPDH | CCCTTCATTGACCTCAACTACATGG | CATGGTGGTGAAGACGCCAG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Z.-Y.; Chen, C.-C.; Liu, H.-M.; Yeh, Y.-C.; Lin, T.-Y.; Lee, T.-Y.; Huang, T.-H. Positive Effects of Ger-Gen-Chyn-Lian-Tang on Cholestatic Liver Fibrosis in Bile Duct Ligation-Challenged Mice. Int. J. Mol. Sci. 2019, 20, 4181. https://doi.org/10.3390/ijms20174181
Chang Z-Y, Chen C-C, Liu H-M, Yeh Y-C, Lin T-Y, Lee T-Y, Huang T-H. Positive Effects of Ger-Gen-Chyn-Lian-Tang on Cholestatic Liver Fibrosis in Bile Duct Ligation-Challenged Mice. International Journal of Molecular Sciences. 2019; 20(17):4181. https://doi.org/10.3390/ijms20174181
Chicago/Turabian StyleChang, Zi-Yu, Chin-Chang Chen, Hsuan-Miao Liu, Yuan-Chieh Yeh, Tung-Yi Lin, Tzung-Yan Lee, and Tse-Hung Huang. 2019. "Positive Effects of Ger-Gen-Chyn-Lian-Tang on Cholestatic Liver Fibrosis in Bile Duct Ligation-Challenged Mice" International Journal of Molecular Sciences 20, no. 17: 4181. https://doi.org/10.3390/ijms20174181