The Role of Major Histocompatibility Complex in Organ Transplantation- Donor Specific Anti-Major Histocompatibility Complex Antibodies Analysis Goes to the Next Stage -
Abstract
1. Introduction
2. Major Histocompatibility Complexes (MHC)
2.1. History of Major Histocompatibility Complexes
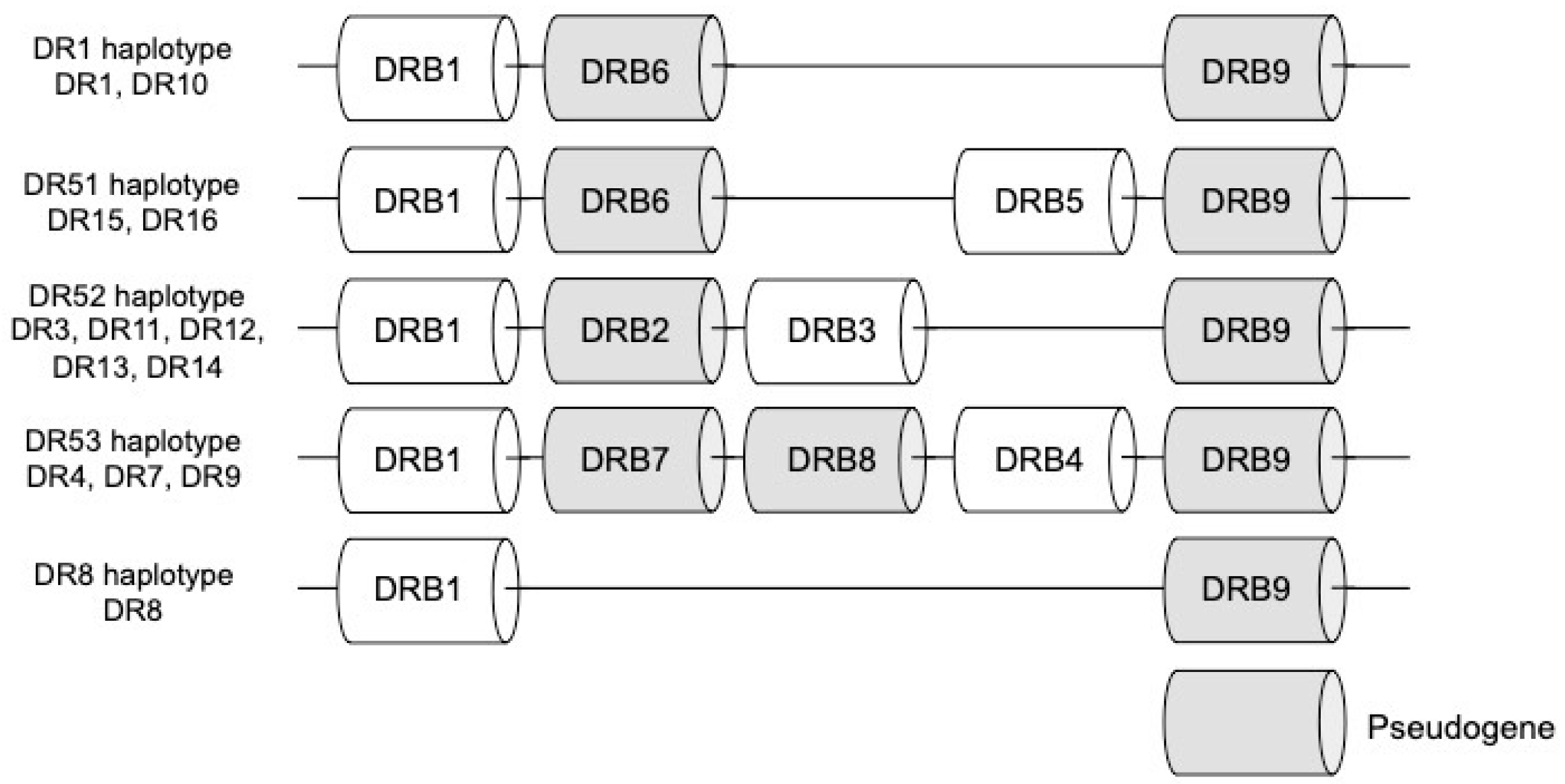
2.2. Types of Major Histocompatibility Complexes
2.2.1. MHC Class I
2.2.2. MHC Class II
2.3. Structure of Major Histocompatibility Complexes
2.3.1. MHC Class I
2.3.2. MHC Class II
2.4. Function of Major Histocompatibility Complexes
2.4.1. MHC Class I
2.4.2. MHC Class II
3. Major Histocompatibility Complex in Organ Transplantation
3.1. Major Histocompatibility Complexes in Cellular Rejection
3.2. Major Histocompatibility Complexes in Antibody-Mediated Rejection
4. Analysis Methods of Anti-Major Histocompatibility Complex Antibodies
4.1. Lymphocyte Cytotoxicity Test (LCT)
4.2. Flow Cytometry Crossmatch (FCXM)
4.3. Immunocomplex Capture Fluorescence Analysis (ICFA)
4.4. Human Leukocyte Antigens (HLA) Antibody Testing
4.4.1. FlowPRA
4.4.2. Single Antigen Beads Assay (SAB)
4.5. Complement Fixation Test
5. Graft ICFA, Intra-Graft Donor Specific Anti-HLA Antibodies
6. Future Perspective
6.1. HLA-Epitope Matching
6.2. HLA-Eplet Matching
6.3. Predicted Indirectly Recognizable HLA Epitopes
6.4. Direct Crossmatch Test by Graft ICFA
7. Concluding Remarks
Funding
Conflicts of Interest
Abbreviations
| AHG | Anti Human Globulin |
| AMR | Antibody-Mediated Rejection |
| CDC | Complement Dependent Cytotoxicity |
| CREGs | Cross-Reacting Groups |
| DSA | Donor-Specific Anti-MHC (HLA) Antibodies |
| DTH | Delayed-Type Hypersensitivity |
| FCXM | Flow Cytometry Crossmatch Test |
| HLA | Human Leukocyte Antigens |
| ICFA | Immunocomplex Capture Fluorescence Analysis |
| LCT | Lymphocyte Cytotoxicity Test |
| MFI | Mean Fluorescence Intensity |
| MHC | Major Histocompatibility Complex |
| MICA | MHC Class I Polypeptide-Related Sequence A |
| MLC | Mixed lymphocytes culture |
| PIRCHE | Predicted Indirectly Recognizable HLA Epitopes |
| SAB | Single Antigen Beads Assay |
| TCR | T Cell Receptor |
References
- Ujvari, B.; Belov, K. Major Histocompatibility Complex (MHC) Markers in Conservation Biology. Int. J. Mol. Sci. 2011, 12, 5168–5186. [Google Scholar] [CrossRef] [PubMed]
- Moreso, F.; Crespo, M.; Ruiz, J.C.; Torres, A.; Gutierrez-Dalmau, A.; Osuna, A.; Perello, M.; Pascual, J.; Torres, I.B.; Redondo-Pachon, D.; et al. Treatment of chronic antibody mediated rejection with intravenous immunoglobulins and rituximab: A multicenter, prospective, randomized, double-blind clinical trial. Am. J. Transplant. 2018, 18, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.M.; Bolton, E.M.; Bradley, J.A.; Pettigrew, G.J. Allorecognition Pathways in Transplant Rejection and Tolerance. Transplantation 2013, 96, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Snell, G.D. T Cells, T Cell Recognition Structures, and the Major Histocompatibility Complex. Immunol. Rev. 1978, 38, 3–69. [Google Scholar] [CrossRef] [PubMed]
- Klein, J. Seeds of time: Fifty years ago Peter A. Gorer discovered the H-2 complex. Immunogenetics 1986, 24, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Marchal, G.; Dausset, J.; Colombani, J. Frequency of anti-platelet iso-antibodies in polytransfused patients. Bibl. Haematol. 1962, 13, 319–323. [Google Scholar] [PubMed]
- Degos, L. Jean Dausset a scientific pioneer: Intuition and creativity for the patients (1916–2009). Haematologica 2009, 94, 1331–1332. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Terasaki, P.I.; McClelland, J.D. microdroplet Assay OF human serum cytotoxins. Nature 1964, 204, 998. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.H.; Amos, D.B.; Whitmore, F.C.; Emery, K.O.; Cooke, H.B.S.; Swift, D.J.P. Hu-1: Major Histocompatibility Locus in Man. Science 1967, 156, 1506–1508. [Google Scholar] [CrossRef]
- Solheim, B.G.; Fuks, A.; Smith, L.; Strominger, J.L.; Thorsby, E. Possible Detection of HLA-DR Alloantigenic Specificities in Man with Unabsorbed Rabbit Antisera. Scand. J. Immunol. 1978, 8, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Bradley, B.A.; Termijtelen, A.; Franks, D.; Van Rood, J.J. Interpretation of data obtained from primed lymphocyte tests (PLTs). Transplant. Proc. 1977, 9, 421–424. [Google Scholar] [PubMed]
- Bodmer, W.; Albert, E.; Bodmer, J.; Dausset, J.; Kissmeyer-Nielsen, F.; Mayr, W.; Payne, R.; Van Rood, J.; Trnka, Z.; Walford, R. Nomenclature for factors of the HLA system 1984. Hum. Immunol. 1984, 11, 117–125. [Google Scholar] [CrossRef]
- Bentley, G.; Higuchi, R.; Hoglund, B.; Goodridge, D.; Sayer, D.; Trachtenberg, E.A.; Erlich, H.A. High-resolution, high-throughput HLA genotyping by next-generation sequencing. Tissue Antigens 2009, 74, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.; Furst, D.; Fae, I.; Wenda, S.; Zollikofer, C.; Mytilineos, J.; Fischer, G.F. HLA typing by next-generation sequencing-getting closer to reality. Tissue Antigens 2014, 83, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Slatkin, M. Linkage disequilibrium—Understanding the evolutionary past and mapping the medical future. Nat. Rev. Genet. 2008, 9, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Hviid, T.V.F.; Christiansen, O.B. Linkage Disequilibrium Between Human Leukocyte Antigen (HLA) Class II and HLA-G—Possible Implications for Human Reproduction and Autoimmune Disease. Hum. Immunol. 2005, 66, 688–699. [Google Scholar] [CrossRef]
- Evseeva, I.; Nicodemus, K.K.; Bonilla, C.; Tonks, S.; Bodmer, W.F. Linkage disequilibrium and age of HLA region SNPs in relation to classic HLA gene alleles within Europe. Eur. J. Hum. Genet. 2010, 18, 924–932. [Google Scholar] [CrossRef]
- Horton, R.; Wilming, L.; Rand, V.; Lovering, R.C.; Bruford, E.A.; Khodiyar, V.K.; Lush, M.J.; Povey, S.; Talbot, C.C.; Wright, M.W.; et al. Gene map of the extended human MHC. Nat. Rev. Genet. 2004, 5, 889–899. [Google Scholar] [CrossRef]
- Miyadera, H.; Ohashi, J.; Lernmark, A.; Kitamura, T.; Tokunaga, K. Cell-surface MHC density profiling reveals instability of autoimmunity-associated HLA. J. Clin. Investig. 2015, 125, 275–291. [Google Scholar] [CrossRef]
- Xu, X.; Yue, M.; Jiang, L.; Deng, X.; Zhang, Y.; Zhang, Y.; Zhu, D.; Xiao, W.; Zhou, Z.; Yao, W.; et al. Genetic Variants in Human Leukocyte Antigen-DP Influence Both Hepatitis C Virus Persistence and Hepatitis C Virus F Protein Generation in the Chinese Han Population. Int. J. Mol. Sci. 2014, 15, 9826–9843. [Google Scholar] [CrossRef]
- Aroviita, P.; Partanen, J.; Sistonen, P.; Teramo, K.; Kekomaki, R. High birth weight is associated with human leukocyte antigen (HLA) DRB1*13 in full-term infants. Eur. J. Immunogenet. 2004, 31, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Gyllensten, U.B.; Erlich, H.A. Ancient roots for polymorphism at the HLA-DQ alpha locus in primates. Proc. Natl. Acad. Sci. USA 1989, 86, 9986–9990. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Ohashi, J.; Nishida, N.; Tokunaga, K. Evolutionary Analysis of Classical HLA Class I and II Genes Suggests That Recent Positive Selection Acted on DPB1* 04: 01 in Japanese Population. PLoS ONE 2012, 7, e46806. [Google Scholar] [CrossRef] [PubMed]
- Nakaoka, H.; Mitsunaga, S.; Hosomichi, K.; Shyh-Yuh, L.; Sawamoto, T.; Fujiwara, T.; Tsutsui, N.; Suematsu, K.; Shinagawa, A.; Inoko, H.; et al. Detection of Ancestry Informative HLA Alleles Confirms the Admixed Origins of Japanese Population. PLoS ONE 2013, 8, e60793. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, D.E.; Koller, B.H.; Orr, H.T. A human major histocompatibility complex class I gene that encodes a protein with a shortened cytoplasmic segment. Proc. Natl. Acad. Sci. USA 1987, 84, 9145–9149. [Google Scholar] [CrossRef] [PubMed]
- Koller, B.H.; E Geraghty, D.; Shimizu, Y.; Demars, R.; Orr, H.T. HLA-E. A novel HLA class I gene expressed in resting T lymphocytes. J. Immunol. 1988, 141, 897–904. [Google Scholar] [PubMed]
- Geraghty, D.E. Human leukocyte antigen F (HLA-F). An expressed HLA gene composed of a class I coding sequence linked to a novel transcribed repetitive element. J. Exp. Med. 1990, 171, 1–18. [Google Scholar] [CrossRef]
- Shiina, T.; Hosomichi, K.; Inoko, H.; Kulski, J.K. The HLA genomic loci map: Expression, interaction, diversity and disease. J. Hum. Genet. 2009, 54, 15–39. [Google Scholar] [CrossRef]
- Megiorni, F.; Pizzuti, A. HLA-DQA1 and HLA-DQB1 in Celiac disease predisposition: Practical implications of the HLA molecular typing. J. Biomed. Sci. 2012, 19, 88. [Google Scholar] [CrossRef]
- Svensson, A.C.; Andersson, G. Presence of retroelements reveal the evolutionary history of the human DR haplotypes. Hereditas 1997, 127, 113–124. [Google Scholar] [CrossRef]
- Berdoz, J.; Gorski, J.; Termijtelen, A.M.; Dayer, J.M.; Irlé, C.; Schendel, D.; Mach, B. Constitutive and induced expression of the individual HLA-DR beta and alpha chain loci in different cell types. J. Immunol. 1987, 139, 1336–1341. [Google Scholar]
- Kotsch, K.; Blasczyk, R. The Noncoding Regions of HLA-DRB Uncover Interlineage Recombinations as a Mechanism of HLA Diversification. J. Immunol. 2000, 165, 5664–5670. [Google Scholar] [CrossRef] [PubMed]
- Horton, R.; Gibson, R.C.; Coggill, P.; Miretti, M.; Allcock, R.J.; Almeida, J.; Forbes, S.; Gilbert, J.G.R.; Halls, K.; Harrow, J.L.; et al. Variation analysis and gene annotation of eight MHC haplotypes: The MHC Haplotype Project. Immunogenetics 2008, 60, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.I.; Borg, N.A.; Dunstone, M.A.; Kjer-Nielsen, L.; Beddoe, T.; McCluskey, J.; Carbone, F.R.; Bottomley, S.P.; Aguilar, M.-I.; Purcell, A.W.; et al. The Structure of H-2Kb and Kbm8 Complexed to a Herpes Simplex Virus Determinant: Evidence for a Conformational Switch That Governs T Cell Repertoire Selection and Viral Resistance. J. Immunol. 2004, 173, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, K.; Li, H.; A Mariuzza, R.; Margulies, D.H. MHC class I molecules, structure and function. Rev. Immunogenet. 1999, 1, 32–46. [Google Scholar]
- Elliott, T.; Cerundolo, V.; Elvin, J.; Townsend, A. Peptide-induced conformational change of the class I heavy chain. Nature 1991, 351, 402. [Google Scholar] [CrossRef] [PubMed]
- Falk, K.; Rötzschke, O.; Stevanovié, S.; Jung, G.; Rammensee, H.-G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature 1991, 351, 290. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, M.; Fremont, D.; Peterson, P.; Wilson, I. Emerging principles for the recognition of peptide antigens by MHC class I molecules. Science 1992, 257, 927–934. [Google Scholar] [CrossRef]
- Hunt, D.; Henderson, R.; Shabanowitz, J.; Sakaguchi, K.; Michel, H.; Sevilir, N.; Cox, A.; Appella, E.; Engelhard, V. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science 1992, 255, 1261–1263. [Google Scholar] [CrossRef]
- Walker, L.E.; Ketler, T.A.; Houghten, R.A.; Schulz, G.; Chersi, A.; Reisfeld, R.A. Human major histocompatibility complex class I antigens: Residues 61-83 of the HLA-B7 heavy chain specify an alloreactive site. Proc. Natl. Acad. Sci. USA 1985, 82, 539–542. [Google Scholar] [CrossRef]
- Bouvier, M.; Wiley, D. Importance of peptide amino and carboxyl termini to the stability of MHC class I molecules. Science 1994, 265, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Ploegh, H.L.; Orr, H.T.; Strominger, J.L. Major histocompatibility antigens: The human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell 1981, 24, 287–299. [Google Scholar] [CrossRef]
- Kaufman, J.; Auffray, C.; Korman, A.; Shackelford, D.; Strominger, J. The class II molecules of the human and murine major histocompatibility complex. Cell 1984, 36, 1–13. [Google Scholar] [CrossRef]
- Brown, J.H.; Jardetzky, T.S.; Gorga, J.C.; Stern, L.J.; Urban, R.G.; Strominger, J.L.; Wiley, D.C. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature 1993, 364, 33. [Google Scholar] [CrossRef] [PubMed]
- Stern, L.J.; Brown, J.H.; Jardetzky, T.S.; Gorga, J.C.; Urban, R.G.; Strominger, J.L.; Wiley, D.C. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature 1994, 368, 215. [Google Scholar] [CrossRef]
- Lundegaard, C.; Lamberth, K.; Harndahl, M.; Buus, S.; Lund, O.; Nielsen, M. NetMHC-3.0: Accurate web accessible predictions of human, mouse and monkey MHC class I affinities for peptides of length 8–11. Nucleic Acids Res. 2008, 36, W509–W512. [Google Scholar] [CrossRef] [PubMed]
- Bessoles, S.; Grandclement, C.; Alari-Pahissa, E.; Gehrig, J.; Jeevan-Raj, B.; Held, W. Adaptations of Natural Killer Cells to Self-MHC Class I. Front. Immunol. 2014, 5, 349. [Google Scholar] [CrossRef]
- Sadegh-Nasseri, S.; Kim, A. Exogenous antigens bind MHC class II first, and are processed by cathepsins later. Mol. Immunol. 2015, 68, 81–84. [Google Scholar] [CrossRef]
- Srinivasan, M.; Domanico, S.Z.; Kaumaya, P.T.P.; Pierce, S.K. Peptides of 23 residues or greater are required to stimulate a high affinity class II-restricted T cell response. Eur. J. Immunol. 1993, 23, 1011–1016. [Google Scholar] [CrossRef]
- O’Garra, A. Cytokines Induce the Development of Functionally Heterogeneous T Helper Cell Subsets. Immunity 1998, 8, 275–283. [Google Scholar] [CrossRef]
- Fehniger, T.A.; Cooper, M.A.; Nuovo, G.J.; Cella, M.; Facchetti, F.; Colonna, M.; Caligiuri, M.A. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: A potential new link between adaptive and innate immunity. Blood 2003, 101, 3052–3057. [Google Scholar] [CrossRef] [PubMed]
- Peters, P.J. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J. Exp. Med. 1991, 173, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.; Moore, T.; Toews, G. Role of T- and B-lymphocytes in pulmonary host defences. Eur. Respir. J. 2001, 18, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Langereis, J.D.; Henriet, S.S.; Kuipers, S.; Weemaes, C.M.; Van Der Burg, M.; De Jonge, M.I.; Van Der Flier, M. IgM Augments Complement Bactericidal Activity with Serum from a Patient with a Novel CD79a Mutation. J. Clin. Immunol. 2018, 38, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Huber-Lang, M.; Younkin, E.M.; Sarma, J.V.; Riedemann, N.; McGuire, S.R.; Lu, K.T.; Kunkel, R.; Younger, J.G.; Zetoune, F.S.; Ward, P.A. Generation of C5a by Phagocytic Cells. Am. J. Pathol. 2002, 161, 1849–1859. [Google Scholar] [CrossRef]
- Dierselhuis, M.; Goulmy, E. The relevance of minor histocompatibility antigens in solid organ transplantation. Curr. Opin. Organ Transplant. 2009, 14, 419–425. [Google Scholar] [CrossRef]
- Toldo, S.; Quader, M.; Salloum, F.N.; Mezzaroma, E.; Abbate, A. Targeting the Innate Immune Response to Improve Cardiac Graft Recovery after Heart Transplantation: Implications for the Donation after Cardiac Death. Int. J. Mol. Sci. 2016, 17, 958. [Google Scholar] [CrossRef]
- Kreisel, D.; Petrowsky, H.; Krasinskas, A.M.; Krupnick, A.S.; Szeto, W.Y.; McLean, A.D.; Popma, S.H.; Gelman, A.E.; Traum, M.K.; Furth, E.E.; et al. The role of passenger leukocyte genotype in rejection and acceptance of rat liver allografts1. Transplantation 2002, 73, 1501–1507. [Google Scholar] [CrossRef]
- Golding, H.; Singer, A. Role of accessory cell processing and presentation of shed H-2 alloantigens in allospecific cytotoxic T lymphocyte responses. J. Immunol. 1984, 133, 597–605. [Google Scholar]
- Herrera, O.B.; Golshayan, D.; Tibbott, R.; Ochoa, F.S.; James, M.J.; Marelli-Berg, F.M.; Lechler, R.I. A novel pathway of alloantigen presentation by dendritic cells. J. Immunol. 2004, 173, 4828–4837. [Google Scholar] [CrossRef]
- Scozzi, D.; Ibrahim, M.; Menna, C.; Krupnick, A.S.; Kreisel, D.; Gelman, A.E. The Role of Neutrophils in Transplanted Organs. Am. J. Transplant. 2017, 17, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Harmon, C.; Sanchez-Fueyo, A.; O’Farrelly, C.; Houlihan, D.D. Natural Killer Cells and Liver Transplantation: Orchestrators of Rejection or Tolerance? Am. J. Transplant. 2016, 16, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ushigome, H. Myeloid-Derived Suppressor Cells as a Regulator of Immunity in Organ Transplantation. Int. J. Mol. Sci. 2018, 19, 2357. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Nakao, T.; Yoshimura, N.; Ashihara, E. Rapamycin Prolongs Cardiac Allograft Survival in a Mouse Model by Inducing Myeloid-Derived Suppressor Cells. Am. J. Transplant. 2015, 15, 2364–2377. [Google Scholar] [CrossRef] [PubMed]
- Grazia, T.J.; Pietra, B.A.; Johnson, Z.A.; Kelly, B.P.; Plenter, R.J.; Gill, R.G. A Two-Step Model of Acute CD4 T-Cell Mediated Cardiac Allograft Rejection. J. Immunol. 2004, 172, 7451–7458. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.L.; Negus, S.L.; Negus, M.; Bolton, E.M.; Bradley, J.A.; Pettigrew, G.J. Pathways of Helper CD4 T Cell Allorecognition in Generating Alloantibody and CD8 T Cell Alloimmunity. Transplantation 2007, 83, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Kreisel, D.; Krupnick, A.S.; Gelman, A.E.; Engels, F.H.; Popma, S.H.; Krasinskas, A.M.; Balsara, K.R.; Szeto, W.Y.; Turka, L.A.; Rosengard, B.R. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: An alternative mechanism of allorecognition. Nat. Med. 2002, 8, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Harper, S.J.; Ali, J.M.; Wlodek, E.; Negus, M.C.; Harper, I.G.; Chhabra, M.; Qureshi, M.S.; Mallik, M.; Bolton, E.; Bradley, J.A.; et al. CD8 T-cell recognition of acquired alloantigen promotes acute allograft rejection. Proc. Natl. Acad. Sci. USA 2015, 112, 12788–12793. [Google Scholar] [CrossRef]
- Sanchez-Fueyo, A.; Domenig, C.M.; Mariat, C.; Alexopoulos, S.; Zheng, X.X.; Strom, T.B. Influence of direct and indirect allorecognition pathways on CD4+CD25+ regulatory T-cell function in transplantation. Transpl. Int. 2007, 20, 534–541. [Google Scholar] [CrossRef]
- Schenk, S.; Kish, D.D.; He, C.; El-Sawy, T.; Chiffoleau, E.; Chen, C.; Wu, Z.; Sandner, S.; Gorbachev, A.V.; Fukamachi, K.; et al. Alloreactive T cell responses and acute rejection of single class II MHC-disparate heart allografts are under strict regulation by CD4+ CD25+ T cells. J. Immunol. 2005, 174, 3741–3748. [Google Scholar] [CrossRef]
- Abrahimi, P.; Qin, L.; Chang, W.G.; Bothwell, A.L.; Tellides, G.; Saltzman, W.M.; Pober, J.S. Blocking MHC class II on human endothelium mitigates acute rejection. JCI Insight 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Qin, L.; Zhang, J.; Abrahimi, P.; Li, H.; Li, G.; Tietjen, G.T.; Tellides, G.; Pober, J.S.; Saltzman, W.M. Ex vivo pretreatment of human vessels with siRNA nanoparticles provides protein silencing in endothelial cells. Nat. Commun. 2017, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Yamada, J.; Hamuro, J.; Terai, K.; Kinoshita, S. Major Histocompatibility Complex Semi-Matching Improves Murine Corneal Allograft Survival Under Oxidative Macrophage Dominancy. Transplantation 2007, 84, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Meehan, S.; Dömer, P.; Josephson, M.; Donoghue, M.; Sadhu, A.; Ho, L.; Aronson, A.; Thistlethwaite, J.; Haas, M. The clinical and pathologic implications of plasmacytic infiltrates in percutaneous renal allograft biopsies. Hum. Pathol. 2001, 32, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Masuzawa, N.; Nakamura, T.; Harada, S.; Nobori, S.; Ushigome, H.; Yoshimura, N.; Konishi, E. Clinicopathological and immunohistochemical analysis of plasma cell-rich rejection in renal transplantation: Involvement of intratubular Th1/Th2 balance in plasma cell enrichment. Nephrology 2018, 23, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Abbas, K.; Mubarak, M.; Zafar, M.N.; Aziz, T.; Abbas, H.; Muzaffar, R.; Rizvi, S.A.H. Plasma cell-rich acute rejections in living-related kidney transplantation: A clinicopathological study of 50 cases. Clin. Transplant. 2015, 29, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Conlon, T.M.; Saeb-Parsy, K.; Cole, J.L.; Motallebzadeh, R.; Qureshi, M.S.; Rehakova, S.; Negus, M.C.; Callaghan, C.J.; Bolton, E.M.; Bradley, J.A.; et al. Germinal centre alloantibody responses are mediated exclusively by indirect-pathway CD4 T follicular helper cells. J. Immunol. 2012, 188, 2643–2652. [Google Scholar] [CrossRef] [PubMed]
- Ballet, C.; Renaudin, K.; Degauque, N.; Mai, H.L.; Boeffard, F.; Lair, D.; Berthelot, L.; Feng, C.; Smit, H.; Usal, C.; et al. Indirect CD4+ TH1 response, antidonor antibodies and diffuse C4d graft deposits in long-term recipients conditioned by donor antigens priming. Am. J. Transplant. 2009, 9, 697–708. [Google Scholar] [CrossRef]
- Loupy, A.; Haas, M.; Solez, K.; Racusen, L.; Glotz, D.; Seron, D.; Nankivell, B.J.; Colvin, R.B.; Afrouzian, M.; Akalin, E.; et al. The Banff 2015 Kidney Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting Molecular Pathology. Am. J. Transplant. 2017, 17, 28–41. [Google Scholar] [CrossRef]
- Demetris, A.J.; Bellamy, C.; Hübscher, S.G.; O’Leary, J.; Randhawa, P.S.; Feng, S.; Neil, D.; Colvin, R.B.; McCaughan, G.; Fung, J.J.; et al. Comprehensive Update of the Banff Working Group on Liver Allograft Pathology: Introduction of Antibody-Mediated Rejection. Am. J. Transplant. 2016, 16, 2816–2835. [Google Scholar] [CrossRef]
- Berry, G.J.; Burke, M.M.; Andersen, C.; Bruneval, P.; Fedrigo, M.; Fishbein, M.C.; Goddard, M.; Hammond, E.H.; Leone, O.; Marboe, C.; et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J. Heart Lung Transplant. 2013, 32, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.J.; Glanville, A.R.; Aboyoun, C.; Belperio, J.; Benden, C.; Berry, G.J.; Hachem, R.; Hayes, D.; Neil, D.; Reinsmoen, N.L.; et al. Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2016, 35, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Clerkin, K.J.; Farr, M.A.; Restaino, S.W.; Zorn, E.; Latif, F.; Vasilescu, E.R.; Marboe, C.C.; Colombo, P.C.; Mancini, D.M. Donor-specific anti-HLA antibodies with antibody-mediated rejection and long-term outcomes following heart transplantation. J. Heart Lung Transplant. 2017, 36, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Mine, K.L.; Tedesco-Silva, H.; Mourão, T.B.; Campos, É.F.; Salzedas, L.A.; Aguiar, B.; Felipe, C.R.; Medina-Pestana, J.O.; Gerbase-DeLima, M. Heightened expression of HLA-DQB1 and HLA-DQB2 in pre-implantation biopsies predicts poor late kidney graft function. Hum. Immunol. 2018, 79, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, S.; van Bergen, C.A.; van Luxemburg-Heijs, S.A.; van der Zouwen, B.; Jordanova, E.S.; Kruisselbrink, A.B.; van de Meent, M.; Harskamp, J.C.; Claas, F.H.; Marijt, E.W.; et al. HLA class II upregulation during viral infection leads to HLA-DP-directed graft-versus-host disease after CD4+ donor lymphocyte infusion. Blood 2013, 122, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.L.; Coles, M.I.; Griffin, R.J.; Pomerance, A.; Yacoub, M.H. expression of class i and class II major histocompatability antigens in normal and transplanted human heart. Transplantation 1986, 41, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Fuggle, S.V.; McWhinnie, D.L.; Chapman, J.R.; Taylor, H.M.; Morris, P.J. sequential analysis of hla-class II antigen expression in human renal allografts Induction of Tubular class II Antigens and Correlation with Clinical Parameters. Transplantation 1986, 42, 144–149. [Google Scholar] [CrossRef]
- Steinhoff, G.; Wonigeit, K.; Pichlmayr, R. Analysis OF sequential changes in major histocompatibility complex expression IN Human liver grafts after transplantation. Transplantation 1988, 45, 394–401. [Google Scholar] [CrossRef]
- Sablik, K.A.; Groningen, M.C.C.-V.; Looman, C.W.; Damman, J.; Roelen, D.L.; Van Agteren, M.; Betjes, M.G. Chronic-active antibody-mediated rejection with or without donor-specific antibodies has similar histomorphology and clinical outcome—A retrospective study. Transpl. Int. 2018, 31, 900–908. [Google Scholar] [CrossRef]
- Wozniak, L.J.; Hickey, M.J.; Venick, R.S.; Vargas, J.H.; Farmer, D.G.; Busuttil, R.W.; McDiarmid, S.V.; Reed, E.F. Donor-specific HLA antibodies are associated with late allograft dysfunction after pediatric liver transplantation. Transplantation 2015, 99, 1416–1422. [Google Scholar] [CrossRef]
- Roux, A.; Le Lan, I.B.; Holifanjaniaina, S.; Thomas, K.A.; Picard, C.; Grenet, D.; De Miranda, S.; Douvry, B.; Beaumont-Azuar, L.; Sage, E.; et al. Characteristics of Donor-Specific Antibodies Associated with Antibody-Mediated Rejection in Lung Transplantation. Front. Med. 2017, 4, 155. [Google Scholar] [CrossRef] [PubMed]
- Snanoudj, R.; Tinel, C.; Legendre, C. Immunological risks of minimization strategies. Transpl. Int. 2015, 28, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ushigome, H.; Shirouzu, T.; Yoshimura, N. Donor Specific anti-HLA Antibodies in Organ Transplantation-Transition from SERUM DSA to intra-Graft DSA-; 5 November 2018 ed.; Mahdi, B.M., Ed.; Intech: Rijeka, Croatia, 2019; pp. 19–41. [Google Scholar]
- E Feucht, H. Complement C4d in graft capillaries—The missing link in the recognition of humoral alloreactivity. Am. J. Transplant. 2003, 3, 646–652. [Google Scholar] [CrossRef]
- Feucht, H.E.; Felber, E.; Gokel, M.J.; Hillebrand, G.; Nattermann, U.; Brockmeyer, C.; Held, E.; Riethmüller, G.; Land, W.; Albert, E. Vascular deposition of complement-split products in kidney allografts with cell-mediated rejection. Clin. Exp. Immunol. 1991, 86, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Van Huyen, J.-P.D.; Mooney, N.; Delahousse, M.; Legendre, C.; Hill, G.S.; Zeevi, A.; Loupy, A.; Lefaucheur, C.; Vernerey, D.; Prugger, C.; et al. Complement-Binding Anti-HLA Antibodies and Kidney-Allograft Survival. N. Engl. J. Med. 2013, 369, 1215–1226. [Google Scholar]
- O’Leary, J.G.; Kaneku, H.; Banuelos, N.; Jennings, L.W.; Klintmalm, G.B.; Terasaki, P.I. Impact of IgG3 Subclass and C1q-Fixing Donor-Specific HLA Alloantibodies on Rejection and Survival in Liver Transplantation. Am. J. Transplant. 2015, 15, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Sicard, A.; Ducreux, S.; Rabeyrin, M.; Couzi, L.; McGregor, B.; Badet, L.; Scoazec, J.Y.; Bachelet, T.; Lepreux, S.; Visentin, J.; et al. Detection of C3d-binding donor-specific anti-HLA antibodies at diagnosis of humoral rejection predicts renal graft loss. J. Am. Soc. Nephrol. JASN 2015, 26, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Metzgar, R.S.; E Seigler, H.; E Ward, F.; Rowlands, D.T. immunological studies on eluates from human renal allografts. Transplantation 1972, 13, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Guignier, F.; Mousson, C.; Rageot, D.; Justrabo, E.; Rifle, G. Detection of donor-specific anti-HLA antibodies with flow cytometry in eluates and sera from renal transplant recipients with chronic allograft nephropathy1. Transplantation 2003, 76, 395–400. [Google Scholar] [CrossRef]
- Bocrie, O.; Aly, A.A.H.; Guignier, F.; De La Vega, M.F.; Rifle, G.; Mousson, C.; Martin, L. Distribution of donor-specific antibodies in the cortex and the medulla of renal transplants with chronic allograft nephropathy. Transpl. Immunol. 2007, 17, 227–229. [Google Scholar] [CrossRef]
- Bachelet, T.; Couzi, L.; Lepreux, S.; Legeret, M.; Pariscoat, G.; Guidicelli, G.; Merville, P.; Taupin, J.-L. Kidney Intragraft Donor-Specific Antibodies as Determinant of Antibody-Mediated Lesions and Poor Graft Outcome. Am. J. Transplant. 2013, 13, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Neau-Cransac, M.; Le Bail, B.; Guidicelli, G.; Visentin, J.; Moreau, K.; Quinart, A.; Boueilh, A.; Laurent, C.; Taupin, J.-L. Evolution of serum and intra-graft donor-specific anti-HLA antibodies in a patient with two consecutive liver transplantations. Transpl. Immunol. 2015, 33, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Visentin, J.; Chartier, A.; Massara, L.; Linares, G.; Guidicelli, G.; Blanchard, E.; Parrens, M.; Begueret, H.; Dromer, C.; Taupin, J.-L. Lung intragraft donor-specific antibodies as a risk factor for graft loss. J. Heart Lung Transplant. 2016, 35, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Milongo, D.; Kamar, N.; Del Bello, A.; Guilbeau-Frugier, C.; Sallusto, F.; Esposito, L.; Dörr, G.; Blancher, A.; Congy-Jolivet, N. Allelic and epitopic characterization of intra-kidney-allograft anti-HLA antibodies at allograft nephrectomy. Am. J. Transplant. 2016, 17, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ushigome, H.; Watabe, K.; Imanishi, Y.; Masuda, K.; Matsuyama, T.; Harada, S.; Koshino, K.; Iida, T.; Nobori, S.; et al. Graft Immunocomplex Capture Fluorescence Analysis to Detect Donor-Specific Antibodies and HLA Antigen Complexes in the Allograft. Immunol. Investig. 2017, 46, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ushigome, H.; Watabe, K.; Imanishi, Y.; Masuda, K.; Matsuyama, T.; Harada, S.; Koshino, K.; Iida, T.; Nobori, S.; et al. Influences of Pre-formed Donor-Specific Anti–Human Leukocyte Antigen Antibodies in Living-Donor Renal Transplantation: Results with Graft Immunocomplex Capture Fluorescence Analysis. Transplant. Proc. 2017, 49, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Yoshimura, N.; Akioka, K.; Shirouzu, T.; Kawai, S.; Imanishi, Y.; Matsuyama, T.; Harada, S.; Nobori, S.; Ushigome, H. Clearance of Intra-graft Donor Specific Anti-HLA Antibodies in the Early Stage of Antibody-Mediated Rejection Following Rituximab and Apheresis Therapy in Renal Transplantation. Transplant. Proc. 2019, 51, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Shirouzu, T.; Kawai, S.; Matsuyama, T.; Harada, S.; Nobori, S.; Yoshimura, N.; Ushigome, H. Graft Immunocomplex Capture Fluorescence Analysis Can Detect Intragraft Anti–Major Histocompatibility Complex Antibodies in Mice Cardiac Transplant. Transplant. Proc. 2019, 51, 1531–1535. [Google Scholar] [CrossRef]
- Nakamura, T.; Shirouzu, T.; Kawai, S.; Imanishi, Y.; Matsuyama, T.; Harada, S.; Nobori, S.; Yoshimura, N.; Ushigome, H. Detection of Intragraft Anti-Blood Group A and B Antibodies Following Renal Transplantation. Transplant. Proc. 2019, 51, 1371–1377. [Google Scholar] [CrossRef]
- Pedersen, N.C. The role of humoral antibody in the rejection of primary renal allografts in sheep. J. Exp. Med. 1974, 140, 619–630. [Google Scholar] [CrossRef]
- Jeannet, M.; Lambert, P.H. Immunological studies on eluates from human kidney grafts. Clin. Immunol. Immunopathol. 1975, 4, 478–488. [Google Scholar] [CrossRef]
- Möschl, P.; Lubec, G.; Keiler, A.; Salem, G.; Glöckler, M. Donor- and Organ-Specific Evaluation of Antibodies Eluted from Canine Lung Allografts Rejected by Immunosuppressively Treated and Untreated Recipients. Respiration 1979, 38, 12–17. [Google Scholar] [CrossRef] [PubMed]
- McPhaul, J.J.; Stastny, P.; Freeman, R.B. Specificities of Antibodies Eluted from Human Cadaveric Renal Allografts. J. Clin. Investig. 1981, 67, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Mohanakumar, T.; Waldrep, J.C.; Phibbs, M.; Mendez-Picon, G.; Kaplan, A.M.; Lee, H.M. serological characterization of antibodies eluted from chronically rejected human renal allografts. Transplantation 1981, 32, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.; Flye, M.W.; Mohanakumar, T. characterization of kidney cell-specific, non-major histocompatibility complex alloantigen using antibodies eluted from rejected human renal ALLOGRAFTS. Transplantation 1988, 46, 362–369. [Google Scholar] [CrossRef]
- Lucchiari, N.; Panajotopoulos, N.; Xu, C.; Rodrigues, H.; Ianhez, L.E.; Kalil, J.; Glotz, D. Antibodies eluted from acutely rejected renal allografts bind to and activate human endothelial cells. Hum. Immunol. 2000, 61, 518–527. [Google Scholar] [CrossRef]
- Zou, Y.; Heinemann, F.M.; Grosse-Wilde, H.; Sireci, G.; Wang, Z.; Lavingia, B.; Stastny, P. Detection of Anti-MICA Antibodies in Patients Awaiting Kidney Transplantation, during the Post-transplant Course, and in Eluates from Rejected Kidney Allografts by Luminex Flow Cytometry. Hum. Immunol. 2006, 67, 230–237. [Google Scholar] [CrossRef]
- Heinemann, F.M.; Roth, I.; Rebmann, V.; Arnold, M.-L.; Spriewald, B.M.; Grosse-Wilde, H. Characterization of anti-HLA antibodies eluted from explanted renal allografts. Clin. Transpl. 2006, 371–378. [Google Scholar]
- Heinemann, F.M.; Roth, I.; Rebmann, V.; Arnold, M.-L.; Witzke, O.; Wilde, B.; Spriewald, B.M.; Grosse-Wilde, H. Immunoglobulin isotype-specific characterization of anti-human leukocyte antigen antibodies eluted from explanted renal allografts. Hum. Immunol. 2007, 68, 500–506. [Google Scholar] [CrossRef]
- Martin, L.; Charon-Barra, C.; Bocrie, O.; Guignier, F.; D’Athis, P.; Dautin, G.; De La Vega, M.F.; Justrabo, E.; Rifle, G.; Mousson, C. Detection of plasma cells, C4d deposits and donor-specific antibodies on sequential graft biopsies of renal transplant recipients with chronic dysfunction. Transpl. Immunol. 2010, 22, 110–114. [Google Scholar] [CrossRef]
- Nocera, A.; Tagliamacco, A.; Cioni, M.; Innocente, A.; Fontana, I.; Barbano, G.; Carrea, A.; Ramondetta, M.; Sementa, A.; Basso, S.; et al. Kidney Intragraft Homing of De Novo Donor-Specific HLA Antibodies Is an Essential Step of Antibody-Mediated Damage but Not Per Se Predictive of Graft Loss. Am. J. Transplant. 2017, 17, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Courant, M.; Visentin, J.; Linares, G.; Dubois, V.; Lepreux, S.; Guidicelli, G.; Thaunat, O.; Merville, P.; Couzi, L.; Taupin, J.-L. The disappointing contribution of anti-human leukocyte antigen donor-specific antibodies characteristics for predicting allograft loss. Nephrol. Dial. Transplant. 2018, 33, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Duquesnoy, R.J. HLA epitope based matching for transplantation. Transpl. Immunol. 2014, 31, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Duquesnoy, R.J. Antibody-reactive epitope determination with HLAMatchmaker and its clinical applications. Tissue Antigens 2011, 77, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Duquesnoy, R.J. Update of the HLA class I eplet database in the website based registry of antibody-defined HLA epitopes. Tissue Antigens 2014, 83, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Claas, F.H. Predictive parameters for in vivo alloreactivity. Transpl. Immunol. 2002, 10, 137–142. [Google Scholar] [CrossRef]
- Papassavas, A.C.; Barnardo, M.C.; Bunce, M.; Welsh, K.I. Is there MHC Class II restriction of the response to MHC Class I in transplant patients? Transplantation 2002, 73, 642–651. [Google Scholar] [CrossRef]
- Otten, H.G.; Calis, J.J.; Kesmir, C.; Van Zuilen, A.D.; Spierings, E. Predicted indirectly recognizable HLA epitopes presented by HLA-DR correlate with the de novo development of donor-specific HLA IgG antibodies after kidney transplantation. Hum. Immunol. 2013, 74, 290–296. [Google Scholar] [CrossRef]
- Lachmann, N.; Niemann, M.; Reinke, P.; Budde, K.; Schmidt, D.; Halleck, F.; Pruß, A.; Schönemann, C.; Spierings, E.; Staeck, O. Donor-Recipient Matching Based on Predicted Indirectly Recognizable HLA Epitopes Independently Predicts the Incidence of De Novo Donor-Specific HLA Antibodies Following Renal Transplantation. Am. J. Transplant. 2017, 17, 3076–3086. [Google Scholar] [CrossRef]


| Author | Refs. | Year | Species | Organ | Sample | Methods | Detector | Remarkable Findings |
|---|---|---|---|---|---|---|---|---|
| Metzger | [99] | 1972 | Human | Kidney | removed grafts | Acid Elution | LCT/MLC | Well antibody activity could be recovered from allografts with hyper acute antibody-mediated rejection. |
| Pedersen | [111] | 1974 | Sheep | Kidney | removed grafts | Acid Elution | LCT/MLC | DSA were bound to graft antigens during the rejection process. |
| Jeannet | [112] | 1975 | Human | Kidney | removed grafts | Acid Elution | LCT/MLC | The balance between intra-graft cytotoxic and blocking factors might determine the outcome of allografts. |
| Moschi | [113] | 1979 | Dog | Lung | removed grafts | Acid Elution | LCT/MLC | A considerable amount of DSA was confirmed in recipients without immunosuppression. |
| McPhaul | [114] | 1981 | Human | Kidney | removed grafts | Acid Elution | LCT/MLC, IH | g-DSA contained two types: 1. cytotoxic Abs to mononuclear cells; 2. Abs with specificity for kidneys. |
| Mohanakumar | [115] | 1981 | Human | Kidney | removed grafts | Acid Elution | LCT/MLC, IH | Rejected allograft contained multispecific alloantibodies, not only reactive to MHC class I and II. |
| Joyce | [116] | 1988 | Human | Kidney | removed grafts | Acid Elution | LCT/MLC, IH | Eluted DSA recognized organ-specific antigens expressed on the kidney cells. |
| Lucchiari | [117] | 2000 | Human | Kidney | removed grafts | Acid Elution | LCT/MLC, FCM | Eluted antibodies activated human endothelial cells, resulting in upregulation of adhesion molecules. |
| Martin | [100] | 2003 | Human | Kidney | removed grafts | Acid Elution | FCM | The detection rate of intra-graft DSA is greater than in serum before the removal of chronic rejected allografts. |
| Zou* | [118] | 2006 | Human | Kidney | removed grafts | Acid Elution | Luminex | MICA-DSA were detected in allografts of patients on transplantation waiting list. |
| Heinemann | [119] | 2006 | Human | Kidney | removed grafts | Acid Elution | ELISA/Luminex | Allografts harbor DSA, including non-complement binding DSA. |
| Bocrie | [101] | 2007 | Human | Kidney | Biopsy | Acid Elution | Luminex | The distribution of intra-graft DSA between the cortex and medulla is roughly concordant. |
| Heinemann | [120] | 2007 | Human | Kidney | removed grafts | Acid Elution | ELISA/Luminex | Allografts harbor DSA, including non-complement binding DSA. |
| Martin | [121] | 2010 | Human | Kidney | Biopsy | Acid Elution | FCM | Graft eluates contained non-DSA. The rate of detecting s and g-DSA is almost the same in patients with graft dysfunction. |
| Bachelet | [102] | 2013 | Human | Kidney | Biopsy | Acid Elution | Luminex | g-DSA, not s-DSA, are a severity and prognostic marker of AMR. |
| Neau-Cransac | [103] | 2015 | Human | Liver | Biopsy | Acid Elution | Luminex | AMR detected as g-DSA deposition in liver allograft might explain graft dysfunction. |
| Milongo | [105] | 2016 | Human | Kidney | removed grafts | Acid Elution | Luminex | g-DSA are generally directed against the donor at an epitopic level. |
| Visentin | [104] | 2016 | Human | Lung | Biopsy | Acid Elution | Luminex | The presence of g-DSA means a higher risk for graft loss. |
| Nakamura | [106] | 2017 | Human | Kidney | Biopsy | ICFA | Luminex | Graft ICFA is a useful technique to make an early and accurate diagnosis of AMR. |
| Nakamura | [107] | 2017 | Human | Kidney | Biopsy | ICFA | Luminex | g-DSA measured by graft ICFA are a marker of effective de-sensitization in crossmatch positive renal transplantation. |
| Norcera | [122] | 2017 | Human | Kidney | Biopsy | Acid Elution | Luminex | The presence of g-DSA indicates clinically relevant antibodies which should be monitored. |
| Courant | [123] | 2018 | Human | Kidney | Biopsy | Acid Elution | Luminex | Results of this study did not associate g-DSA with graft loss. |
| Nakamura | [93] | 2019 | Human | Multiple | Biopsy, removed organs | ICFA | Luminex | g-DSA in heart, lung, liver, pancreas and intestine as well as kidney grafts are also detected by graft ICFA technique. |
| Nakamura | [108] | 2019 | Human | Kidney, Liver | Biopsy | ICFA | Luminex | g-DSA measured by graft ICFA are a marker to predict therapeutic responses in chronic active AMR recipients. |
| Nakamura | [109] | 2019 | Mice | Heart | Biopsy | ICFA | Luminex | Graft ICFA can be applied in mice transplantation models. In the acute phase, class I DSA play important roles. |
| Nakamura** | [110] | 2019 | Human | Kidney | Biopsy | ICFA | Luminex | ABO-DSA can also be detected by graft ICFA technique. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, T.; Shirouzu, T.; Nakata, K.; Yoshimura, N.; Ushigome, H. The Role of Major Histocompatibility Complex in Organ Transplantation- Donor Specific Anti-Major Histocompatibility Complex Antibodies Analysis Goes to the Next Stage -. Int. J. Mol. Sci. 2019, 20, 4544. https://doi.org/10.3390/ijms20184544
Nakamura T, Shirouzu T, Nakata K, Yoshimura N, Ushigome H. The Role of Major Histocompatibility Complex in Organ Transplantation- Donor Specific Anti-Major Histocompatibility Complex Antibodies Analysis Goes to the Next Stage -. International Journal of Molecular Sciences. 2019; 20(18):4544. https://doi.org/10.3390/ijms20184544
Chicago/Turabian StyleNakamura, Tsukasa, Takayuki Shirouzu, Katsuya Nakata, Norio Yoshimura, and Hidetaka Ushigome. 2019. "The Role of Major Histocompatibility Complex in Organ Transplantation- Donor Specific Anti-Major Histocompatibility Complex Antibodies Analysis Goes to the Next Stage -" International Journal of Molecular Sciences 20, no. 18: 4544. https://doi.org/10.3390/ijms20184544
APA StyleNakamura, T., Shirouzu, T., Nakata, K., Yoshimura, N., & Ushigome, H. (2019). The Role of Major Histocompatibility Complex in Organ Transplantation- Donor Specific Anti-Major Histocompatibility Complex Antibodies Analysis Goes to the Next Stage -. International Journal of Molecular Sciences, 20(18), 4544. https://doi.org/10.3390/ijms20184544





