TRP Channels as Sensors of Chemically-Induced Changes in Cell Membrane Mechanical Properties
VIB Center for Brain & Disease Research, Herestraat 49, Campus Gasthuisberg O&N1 bus 802, 3000 Leuven, Belgium
Abstract
:1. TRP Channels and Mechanosensitivity
2. TRP Channel Modulation by the Local Lipid Environment
3. TRP Channel Modulation by the Conically-Shaped Lipid Diacylglycerol
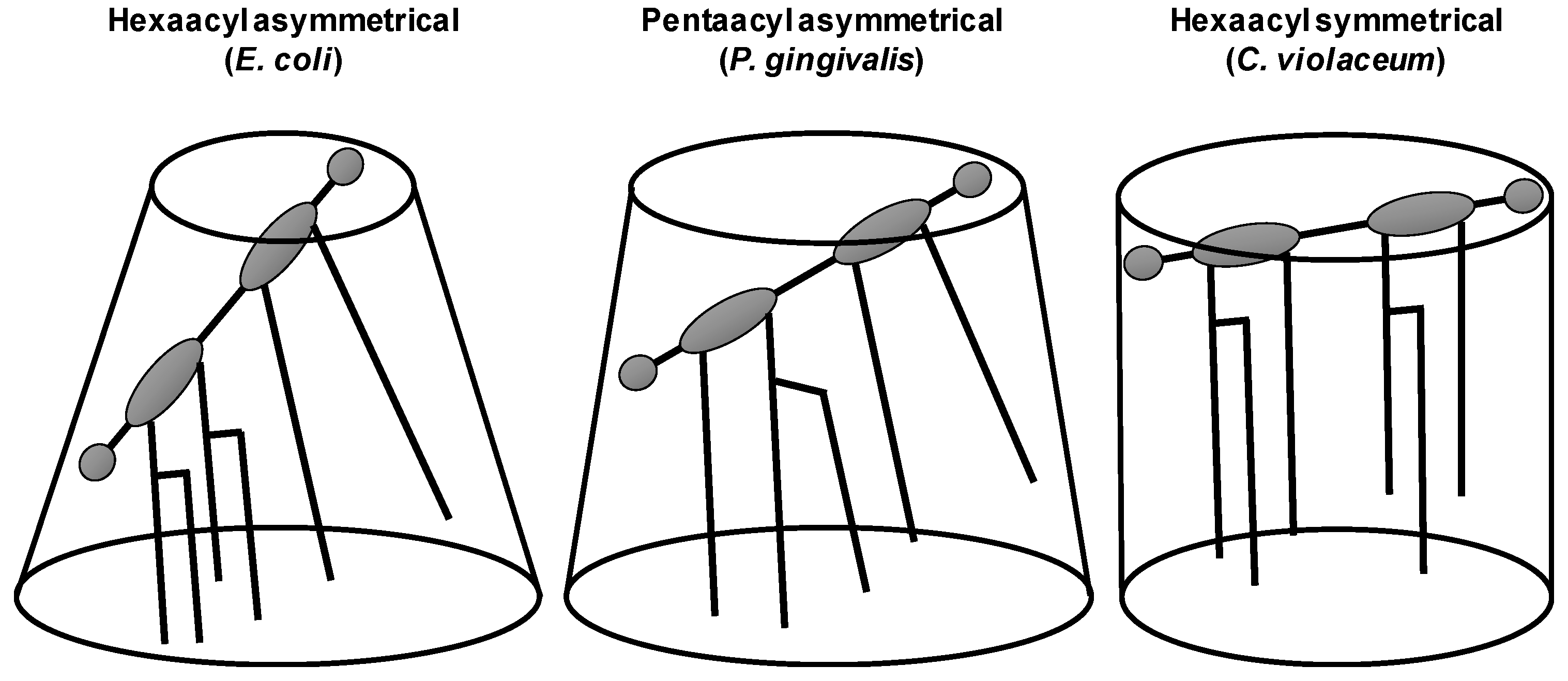
4. TRP Channel Modulation by the Natural Membrane Modulator Lipopolysaccharide
5. TRP Channel Modulation by Lipophilic Compounds
Author Contributions
Funding
Conflicts of Interest
References
- Pedersen, S.F.; Nilius, B. Chapter ten—Transient receptor potential channels in mechanosensing and cell volume regulation. In Methods in Enzymology; Häussinger, D., Sies, H., Eds.; Academic Press: New York, NY, USA, 2007; Volume 428, pp. 183–207. [Google Scholar]
- White, C.R.; Frangos, J.A. The shear stress of it all: The cell membrane and mechanochemical transduction. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Huiskes, R.; Ruimerman, R.; van Lenthe, G.H.; Janssen, J.D. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature 2000, 405, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, P.G.; Müller, U. Mechanotransduction by hair cells: Models, molecules, and mechanisms. Cell 2009, 139, 33–44. [Google Scholar] [CrossRef] [PubMed]
- García-Añoveros, J.; Corey, D.P. The molecules of mechanosensation. Ann. Rev. Neurosci. 1997, 20, 567–594. [Google Scholar] [CrossRef]
- Stukel, J.M.; Willits, R.K. Mechanotransduction of neural cells through cell-substrate interactions. Tissue Eng. Part B Rev. 2016, 22, 173–182. [Google Scholar] [CrossRef]
- Sukharev, S.; Sachs, F. Molecular force transduction by ion channels—Diversity and unifying principles. J. Cell Sci. 2012, 125, 3075–3083. [Google Scholar] [CrossRef] [PubMed]
- Bagriantsev, S.N.; Peyronnet, R.; Clark, K.A.; Honoré, E.; Minor, D.L. Multiple modalities converge on a common gate to control k(2p) channel function. EMBO J. 2011, 30, 3594–3606. [Google Scholar] [CrossRef]
- Hao, J.; Padilla, F.; Dandonneau, M.; Lavebratt, C.; Lesage, F.; Noël, J.; Delmas, P. Kv1.1 channels act as mechanical brake in the senses of touch and pain. Neuron 2013, 77, 899–914. [Google Scholar] [CrossRef]
- Hamill, O.P.; Martinac, B. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 2001, 81, 685–740. [Google Scholar] [CrossRef]
- Boonen, B.; Startek, J.B.; Talavera, K. Chemical activation of sensory trp channels. In Taste and Smell; Krautwurst, D., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 73–113. [Google Scholar]
- Voets, T.; Droogmans, G.; Wissenbach, U.; Janssens, A.; Flockerzi, V.; Nilius, B. The principle of temperature-dependent gating in cold- and heat-sensitive trp channels. Nature 2004, 430, 748–754. [Google Scholar] [CrossRef]
- Talavera, K.; Nilius, B.; Voets, T. Neuronal trp channels: Thermometers, pathfinders and life-savers. Trends Neurosci. 2008, 31, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Vriens, J.; Nilius, B.; Voets, T. Peripheral thermosensation in mammals. Nat. Rev. Neurosci. 2014, 15, 573–589. [Google Scholar] [CrossRef]
- Liu, C.; Montell, C. Forcing open trp channels: Mechanical gating as a unifying activation mechanism. Biochem. Biophys. Res. Commun. 2015, 460, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Montell, C.; Rubin, G.M. Molecular characterization of the drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron 1989, 2, 1313–1323. [Google Scholar] [CrossRef]
- Hardie, R.C.; Minke, B. The trp gene is essential for a light-activated ca2+ channel in drosophila photoreceptors. Neuron 1992, 8, 643–651. [Google Scholar] [CrossRef]
- Clapham, D.E.; Miller, C. A thermodynamic framework for understanding temperature sensing by transient receptor potential (trp) channels. Proc. Natl. Acad. Sci. USA 2011, 108, 19492–19497. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for trp channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef]
- Startek, J.B.; Voets, T.; Talavera, K. To flourish or perish: Evolutionary trips into the sensory biology of plant-herbivore interactions. Pflugers Arch. 2018. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Montell, C. Trp channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef]
- Moran, M.M.; McAlexander, M.A.; Biro, T.; Szallasi, A. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 601–620. [Google Scholar] [CrossRef]
- Alonso-Carbajo, L.; Kecskes, M.; Jacobs, G.; Pironet, A.; Syam, N.; Talavera, K.; Vennekens, R. Muscling in on trp channels in vascular smooth muscle cells and cardiomyocytes. Cell Calcium 2017, 66, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Laing, R.J.; Dhaka, A. Thermotrps and pain. Neuroscientist 2016, 22, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y. Trps and pain. Semin. Immunopathol. 2016, 38, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Flockerzi, V. Mammalian transient receptor potential (trp) cation channels. Preface. Handb. Exp. Pharmacol. 2014, 223, v–vi. [Google Scholar] [PubMed]
- Geffeney, S. Frontiers in neuroscience sensory mechanotransduction and thermotransduction in invertebrates. In Neurobiology of Trp Channels; Emir, T.L.R., Ed.; Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2017; pp. 65–83. [Google Scholar]
- Colbert, H.A.; Smith, T.L.; Bargmann, C.I. Osm-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in caenorhabditis elegans. J. Neurosci. 1997, 17, 8259–8269. [Google Scholar] [CrossRef]
- Ciura, S.; Bourque, C.W. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J. Neurosci. 2006, 26, 9069–9075. [Google Scholar] [CrossRef]
- Sharif Naeini, R.; Witty, M.F.; Séguéla, P.; Bourque, C.W. An n-terminal variant of trpv1 channel is required for osmosensory transduction. Nat. Neurosci. 2006, 9, 93–98. [Google Scholar] [CrossRef]
- Lu, G.; Henderson, D.; Liu, L.; Reinhart, P.H.; Simon, S.A. Trpv1b, a functional human vanilloid receptor splice variant. Mol. Pharmacol. 2005, 67, 1119–1127. [Google Scholar] [CrossRef]
- Beech, D.J.; Muraki, K.; Flemming, R. Non-selective cationic channels of smooth muscle and the mammalian homologues of drosophila trp. J. Physiol. 2004, 559, 685–706. [Google Scholar] [CrossRef]
- Muraki, K.; Iwata, Y.; Katanosaka, Y.; Ito, T.; Ohya, S.; Shigekawa, M.; Imaizumi, Y. Trpv2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ. Res. 2003, 93, 829–838. [Google Scholar] [CrossRef]
- Penna, A.; Juvin, V.; Chemin, J.; Compan, V.; Monet, M.; Rassendren, F.-A. Pi3-kinase promotes trpv2 activity independently of channel translocation to the plasma membrane. Cell Calcium 2006, 39, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Chen, M.Z.; Wang, Y.J.; Sun, H.Q.; Wei, Y.; Martinez, M.; Yin, H.L. Hypertonic stress increases phosphatidylinositol 4,5-bisphosphate levels by activating pip5kibeta. J. Biol. Chem. 2006, 281, 32630–32638. [Google Scholar] [CrossRef] [PubMed]
- Stokes, A.J.; Shimoda, L.M.N.; Koblan-Huberson, M.; Adra, C.N.; Turner, H. A trpv2–pka signaling module for transduction of physical stimuli in mast cells. J. Exp. Med. 2004, 200, 137. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W.; Choe, Y.; Martí-Renom, M.A.; Bell, A.M.; Denis, C.S.; AndrejŠali; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid receptor–related osmotically activated channel (vr-oac), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef]
- Vriens, J.; Watanabe, H.; Janssens, A.; Droogmans, G.; Voets, T.; Nilius, B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel trpv4. Proc. Natl. Acad. Sci. USA 2004, 101, 396. [Google Scholar] [CrossRef]
- Vriens, J.; Owsianik, G.; Fisslthaler, B.; Suzuki, M.; Janssens, A.; Voets, T.; Morisseau, C.; Hammock, B.D.; Fleming, I.; Busse, R.; et al. Modulation of the ca2 permeable cation channel trpv4 by cytochrome p450 epoxygenases in vascular endothelium. Circ. Res. 2005, 97, 908–915. [Google Scholar] [CrossRef]
- Watanabe, H.; Vriens, J.; Prenen, J.; Droogmans, G.; Voets, T.; Nilius, B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate trpv4 channels. Nature 2003, 424, 434–438. [Google Scholar] [CrossRef]
- Kinnunen, P.K.J. Lipid bilayers as osmotic response elements. Cell. Physiol. Biochem. 2000, 10, 243–250. [Google Scholar] [CrossRef]
- Pedersen, S.F.; Poulsen, K.A.; Lambert, I.H. Roles of phospholipase a2 isoforms in swelling- and melittin-induced arachidonic acid release and taurine efflux in nih3t3 fibroblasts. Am. J. Physiol.-Cell Physiol. 2006, 291, C1286–C1296. [Google Scholar] [CrossRef]
- Spassova, M.A.; Hewavitharana, T.; Xu, W.; Soboloff, J.; Gill, D.L. A common mechanism underlies stretch activation and receptor activation of trpc6 channels. Proc. Natl. Acad. Sci. USA 2006, 103, 16586–16591. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Jensen, L.J.; Shi, J.; Morita, H.; Nishida, M.; Honda, A.; Ito, Y. Transient receptor potential channels in cardiovascular function and disease. Circ. Res. 2006, 99, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Welsh, D.G.; Morielli, A.D.; Nelson, M.T.; Brayden, J.E. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ. Res. 2002, 90, 248–250. [Google Scholar] [CrossRef]
- Grimm, C.; Kraft, R.; Sauerbruch, S.; Schultz, G.; Harteneck, C. Molecular and functional characterization of the melastatin-related cation channel trpm3. J. Biol. Chem. 2003, 278, 21493–21501. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Baylie, R.L.; Tavares, M.J.; Brayden, J.E. Trpm4 channels couple purinergic receptor mechanoactivation and myogenic tone development in cerebral parenchymal arterioles. J. Cereb. Blood Flow Metab. 2014, 34, 1706–1714. [Google Scholar] [CrossRef]
- Earley, S.; Waldron, B.J.; Brayden, J.E. Critical role for transient receptor potential channel trpm4 in myogenic constriction of cerebral arteries. Circ. Res. 2004, 95, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A.; Chubanov, V.; Kalwa, H.; Rost, B.R.; Gudermann, T. Cation channels of the transient receptor potential superfamily: Their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol. Ther. 2006, 112, 744–760. [Google Scholar] [CrossRef]
- Numata, T.; Shimizu, T.; Okada, Y. Trpm7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am. J. Physiol. Cell Physiol. 2007, 292, C460–C467. [Google Scholar] [CrossRef]
- Oancea, E.; Wolfe, J.T.; Clapham, D.E. Functional trpm7 channels accumulate at the plasma membrane in response to fluid flow. Circ. Res. 2006, 98, 245–253. [Google Scholar] [CrossRef]
- Corey, D.P. New trp channels in hearing and mechanosensation. Neuron 2003, 39, 585–588. [Google Scholar] [CrossRef]
- Corey, D.P.; García-Añoveros, J.; Holt, J.R.; Kwan, K.Y.; Lin, S.Y.; Vollrath, M.A.; Amalfitano, A.; Cheung, E.L.; Derfler, B.H.; Duggan, A.; et al. Trpa1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 2004, 432, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Sotomayor, M.; Corey, D.P.; Schulten, K. In search of the hair-cell gating spring elastic properties of ankyrin and cadherin repeats. Structure 2005, 13, 669–682. [Google Scholar] [CrossRef]
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. Trpa1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Kwan, K.Y.; Allchorne, A.J.; Vollrath, M.A.; Christensen, A.P.; Zhang, D.S.; Woolf, C.J.; Corey, D.P. Trpa1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 2006, 50, 277–289. [Google Scholar] [CrossRef]
- Bhattacharya, M.R.C.; Bautista, D.M.; Wu, K.; Haeberle, H.; Lumpkin, E.A.; Julius, D. Radial stretch reveals distinct populations of mechanosensitive mammalian somatosensory neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 20015. [Google Scholar] [CrossRef] [PubMed]
- Vilceanu, D.; Stucky, C.L. Trpa1 mediates mechanical currents in the plasma membrane of mouse sensory neurons. PLoS ONE 2010, 5, e12177. [Google Scholar] [CrossRef] [PubMed]
- Rugiero, F.; Wood, J.N. The mechanosensitive cell line nd-c does not express functional thermotrp channels. Neuropharmacology 2009, 56, 1138–1146. [Google Scholar] [CrossRef]
- Brierley, S.M.; Hughes, P.A.; Page, A.J.; Kwan, K.Y.; Martin, C.M.; O’Donnell, T.A.; Cooper, N.J.; Harrington, A.M.; Adam, B.; Liebregts, T.; et al. The ion channel trpa1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology 2009, 137, 2084–2095.e2083. [Google Scholar] [CrossRef]
- Kwan, K.Y.; Glazer, J.M.; Corey, D.P.; Rice, F.L.; Stucky, C.L. Trpa1 modulates mechanotransduction in cutaneous sensory neurons. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 4808–4819. [Google Scholar] [CrossRef]
- Brierley, S.M.; Castro, J.; Harrington, A.M.; Hughes, P.A.; Page, A.J.; Rychkov, G.Y.; Blackshaw, L.A. Trpa1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity. J. Physiol. 2011, 589, 3575–3593. [Google Scholar] [CrossRef]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meents, J.E.; Fischer, M.J.; McNaughton, P.A. Sensitization of trpa1 by protein kinase a. PLoS ONE 2017, 12, e0170097. [Google Scholar] [CrossRef]
- Singer, S.J.; Nicolson, G.L. The fluid mosaic model of the structure of cell membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Sampaio, J.L. Membrane organization and lipid rafts. Cold Spring Harb. Perspect. Biol. 2011, 3, a004697. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Vaz, W.L. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 269–295. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Magaz, S.; Calandra, P. Amphiphiles self-assembly: Basic concepts and future perspectives of supramolecular approaches. Adv. Condens. Matter Phys. 2015, 2015, 22. [Google Scholar] [CrossRef]
- Garidel, P.; Kaconis, Y.; Heinbockel, L.; Wulf, M.; Gerber, S.; Munk, A.; Vill, V.; Brandenburg, K. Self-organisation, thermotropic and lyotropic properties of glycolipids related to their biological implications. Open Biochem. J. 2015, 9, 49–72. [Google Scholar] [PubMed]
- Janiak, M.J.; Small, D.M.; Shipley, G.G. Nature of the thermal pretransition of synthetic phospholipids: Dimyristolyl- and dipalmitoyllecithin. Biochemistry 1976, 15, 4575–4580. [Google Scholar] [CrossRef]
- Janiak, M.J.; Small, D.M.; Shipley, G.G. Temperature and compositional dependence of the structure of hydrated dimyristoyl lecithin. J. Biol. Chem. 1979, 254, 6068–6078. [Google Scholar]
- Tardieu, A.; Luzzati, V.; Reman, F.C. Structure and polymorphism of the hydrocarbon chains of lipids: A study of lecithin-water phases. J. Mol. Biol. 1973, 75, 711–733. [Google Scholar] [CrossRef]
- Eeman, M.; Deleu, M. From biological membranes to biomimetic model membranes. BASE 2010, 14, 719–736. [Google Scholar]
- Bloom, M.; Evans, E.; Mouritsen, O.G. Physical properties of the fluid lipid-bilayer component of cell membranes: A perspective. Q. Rev. Biophys. 1991, 24, 293–397. [Google Scholar] [CrossRef]
- Corvera, E.; Mouritsen, O.G.; Singer, M.A.; Zuckermann, M.J. The permeability and the effect of acyl-chain length for phospholipid bilayers containing cholesterol: Theory and experiment. Biochim. Biophys. Acta (BBA) Biomembr. 1992, 1107, 261–270. [Google Scholar] [CrossRef]
- Raffy, S.; Teissié, J. Control of lipid membrane stability by cholesterol content. Biophys. J. 1999, 76, 2072–2080. [Google Scholar] [CrossRef]
- Ciardo, M.G.; Ferrer-Montiel, A. Lipids as central modulators of sensory trp channels. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1615–1628. [Google Scholar] [CrossRef] [PubMed]
- Vaz, W.L.C.; Begley, T.P. Lipid bilayers: Properties. In Wiley Encyclopedia of Chemical Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Heimburg, T. Membrane structure. In Thermal Biophysics of Membranes; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Ambudkar, I.S.; Brazer, S.C.; Liu, X.; Lockwich, T.; Singh, B. Plasma membrane localization of trpc channels: Role of caveolar lipid rafts. Nov. Found. Symp. 2004, 258, 63–70, discussion 70–64, 98–102, 263–106. [Google Scholar]
- Lockwich, T.P.; Liu, X.; Singh, B.B.; Jadlowiec, J.; Weiland, S.; Ambudkar, I.S. Assembly of trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J. Biol. Chem. 2000, 275, 11934–11942. [Google Scholar] [CrossRef]
- Brownlow, S.L.; Sage, S.O. Transient receptor potential protein subunit assembly and membrane distribution in human platelets. Thromb. Haemost. 2005, 94, 839–845. [Google Scholar]
- Graziani, A.; Rosker, C.; Kohlwein, S.D.; Zhu, M.X.; Romanin, C.; Sattler, W.; Groschner, K.; Poteser, M. Cellular cholesterol controls trpc3 function: Evidence from a novel dominant-negative knockdown strategy. Biochem. J. 2006, 396, 147–155. [Google Scholar] [CrossRef]
- Szoke, E.; Börzsei, R.; Tóth, D.M.; Lengl, O.; Helyes, Z.; Sándor, Z.; Szolcsányi, J. Effect of lipid raft disruption on trpv1 receptor activation of trigeminal sensory neurons and transfected cell line. Eur. J. Pharmacol. 2010, 628, 67–74. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, A.; Sardar, P.; Yadav, M.; Majhi, R.K.; Goswami, C. Influence of membrane cholesterol in the molecular evolution and functional regulation of trpv4. Biochem. Biophys. Res. Commun. 2015, 456, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Morenilla-Palao, C.; Pertusa, M.; Meseguer, V.; Cabedo, H.; Viana, F. Lipid raft segregation modulates trpm8 channel activity. J. Biol. Chem. 2009, 284, 9215–9224. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, W.; Wu, D.; Priestley, J.V. Trpv1, but not p2x, requires cholesterol for its function and membrane expression in rat nociceptors. Eur. J. Neurosci. 2006, 24, 1–6. [Google Scholar] [CrossRef]
- Sághy, É.; Szőke, É.; Payrits, M.; Helyes, Z.; Börzsei, R.; Erostyák, J.; Jánosi, T.Z.; Sétáló, G.; Szolcsányi, J. Evidence for the role of lipid rafts and sphingomyelin in ca2+-gating of transient receptor potential channels in trigeminal sensory neurons and peripheral nerve terminals. Pharmacol. Res. 2015, 100, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Saghy, E.; Payrits, M.; Biro-Suto, T.; Skoda-Foldes, R.; Szanti-Pinter, E.; Erostyak, J.; Makkai, G.; Setalo, G., Jr.; Kollar, L.; Koszegi, T.; et al. Carboxamido steroids inhibit the opening properties of transient receptor potential ion channels by lipid raft modulation. J. Lipid Res. 2018, 59, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Lakk, M.; Yarishkin, O.; Baumann, J.M.; Iuso, A.; Krizaj, D. Cholesterol regulates polymodal sensory transduction in müller glia. Glia 2017, 65, 2038–2050. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Katz, B.; Lev, S.; Zaguri, R.; Gutorov, R.; Minke, B. Depletion of membrane cholesterol suppresses drosophila transient receptor potential-like (trpl) channel activity. Curr. Top. Membr. 2017, 80, 233–254. [Google Scholar]
- Naylor, J.; Li, J.; Milligan, C.J.; Zeng, F.; Sukumar, P.; Hou, B.; Sedo, A.; Yuldasheva, N.; Majeed, Y.; Beri, D.; et al. Pregnenolone sulphate- and cholesterol-regulated trpm3 channels coupled to vascular smooth muscle secretion and contraction. Circ. Res. 2010, 106, 1507–1515. [Google Scholar] [CrossRef]
- Hill, K.; Schaefer, M. Trpa1 is differentially modulated by the amphipathic molecules trinitrophenol and chlorpromazine. J. Biol. Chem. 2007, 282, 7145–7153. [Google Scholar] [CrossRef]
- Meseguer, V.; Alpizar, Y.A.; Luis, E.; Tajada, S.; Denlinger, B.; Fajardo, O.; Manenschijn, J.A.; Fernández-Peña, C.; Talavera, A.; Kichko, T.; et al. Trpa1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 2014, 5, 3125. [Google Scholar] [CrossRef]
- Startek, J.B.; Talavera, K.; Voets, T.; Alpizar, Y.A. Differential interactions of bacterial lipopolysaccharides with lipid membranes: Implications for trpa1-mediated chemosensation. Sci. Rep. 2018, 8, 12010. [Google Scholar] [CrossRef] [PubMed]
- McMahon, H.T.; Gallop, J.L. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 2005, 438, 590. [Google Scholar] [CrossRef] [PubMed]
- Callan-Jones, A.; Sorre, B.; Bassereau, P. Curvature-Driven Lipid Sorting in Biomembranes. Cold Spring Harbor Perspectives in Biology 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Frolov, V.A.; Shnyrova, A.V.; Zimmerberg, J. Lipid Polymorphisms and Membrane Shape. Cold Spring Harbor Perspectives in Biology 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.; Rietschel, E.T. Invited review: Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 2001, 7, 167–202. [Google Scholar] [CrossRef]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide endotoxins. Ann. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef]
- Steimle, A.; Autenrieth, I.B.; Frick, J.-S. Structure and function: Lipid a modifications in commensals and pathogens. Int. J. Med. Microbiol. 2016, 306, 290–301. [Google Scholar] [CrossRef]
- Molinaro, A.; Holst, O.; Di Lorenzo, F.; Callaghan, M.; Nurisso, A.; D’Errico, G.; Zamyatina, A.; Peri, F.; Berisio, R.; Jerala, R.; et al. Chemistry of lipid a: At the heart of innate immunity. Chemistry 2015, 21, 500–519. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Reeves, P.R. The variation of o antigens in gram-negative bacteria. Subcell Biochem. 2010, 53, 123–152. [Google Scholar]
- Erridge, C.; Bennett-Guerrero, E.; Poxton, I.R. Structure and function of lipopolysaccharides. Microbes Infect. 2002, 4, 837–851. [Google Scholar] [CrossRef]
- Schromm, A.B.; Brandenburg, K.; Loppnow, H.; Moran, A.P.; Koch, M.H.; Rietschel, E.T.; Seydel, U. Biological activities of lipopolysaccharides are determined by the shape of their lipid a portion. Eur. J. Biochem. 2000, 267, 2008–2013. [Google Scholar] [CrossRef] [PubMed]
- Galanos, C.; Luderitz, O.; Rietschel, E.T.; Westphal, O.; Brade, H.; Brade, L.; Freudenberg, M.; Schade, U.; Imoto, M.; Yoshimura, H.; et al. Synthetic and natural escherichia coli free lipid a express identical endotoxic activities. Eur. J. Biochem. 1985, 148, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Owen, K.A.; Ly, K.T.; Park, D.; Black, S.G.; Wilson, J.M.; Sifri, C.D.; Ravichandran, K.S.; Ernst, P.B.; Casanova, J.E. Brain angiogenesis inhibitor 1 (bai1) is a pattern recognition receptor that mediates macrophage binding and engulfment of gram-negative bacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Rosadini, C.V.; Kagan, J.C. Early innate immune responses to bacterial lps. Curr. Opin. Immunol. 2017, 44, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Seydel, U.; Oikawa, M.; Fukase, K.; Kusumoto, S.; Brandenburg, K. Intrinsic conformation of lipid a is responsible for agonistic and antagonistic activity. Eur. J. Biochem. 2000, 267, 3032–3039. [Google Scholar] [CrossRef]
- Netea, M.G.; van Deuren, M.; Kullberg, B.J.; Cavaillon, J.M.; Van der Meer, J.W. Does the shape of lipid A determine the interaction of LPS with Toll-like receptors? Trends Immunol. 2002, 23, 135–139. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Boonen, B.; Alpizar, Y.A.; Meseguer, V.M.; Talavera, K. Trp channels as sensors of bacterial endotoxins. Toxins 2018, 10, 326. [Google Scholar] [CrossRef]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Vande Walle, L.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef]
- Hagar, J.A.; Powell, D.A.; Aachoui, Y.; Ernst, R.K.; Miao, E.A. Cytoplasmic lps activates caspase-11: Implications in tlr4-independent endotoxic shock. Science 2013, 341, 1250–1253. [Google Scholar] [CrossRef]
- Alpizar, Y.A.; Boonen, B.; Sanchez, A.; Jung, C.; Lopez-Requena, A.; Naert, R.; Steelant, B.; Luyts, K.; Plata, C.; De Vooght, V.; et al. Trpv4 activation triggers protective responses to bacterial lipopolysaccharides in airway epithelial cells. Nat. Commun. 2017, 8, 1059. [Google Scholar] [CrossRef] [PubMed]
- Soldano, A.; Alpizar, Y.A.; Boonen, B.; Franco, L.; Lopez-Requena, A.; Liu, G.; Mora, N.; Yaksi, E.; Voets, T.; Vennekens, R.; et al. Gustatory-mediated avoidance of bacterial lipopolysaccharides via trpa1 activation in drosophila. eLife 2016, 5. [Google Scholar] [CrossRef]
- Zappia, K.J.; O’Hara, C.L.; Moehring, F.; Kwan, K.Y.; Stucky, C.L. Sensory neuron-specific deletion of trpa1 results in mechanical cutaneous sensory deficits. eNeuro 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, F.; Davis, B.; Rittig, M.; Bonev, B.B.; O’Shea, P. Receptor-independent interaction of bacterial lipopolysaccharide with lipid and lymphocyte membranes; the role of cholesterol. PLoS ONE 2012, 7, e38677. [Google Scholar] [CrossRef] [PubMed]
- Brauckmann, S.; Effenberger-Neidnicht, K.; de Groot, H.; Nagel, M.; Mayer, C.; Peters, J.; Hartmann, M. Lipopolysaccharide-induced hemolysis: Evidence for direct membrane interactions. Sci. Rep. 2016, 6, 35508. [Google Scholar] [CrossRef] [PubMed]
- Pöschl, J.M.; Leray, C.; Ruef, P.; Cazenave, J.P.; Linderkamp, O. Endotoxin binding to erythrocyte membrane and erythrocyte deformability in human sepsis and in vitro. Crit. Care Med. 2003, 31, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.C.; Mollitt, D.L. Sepsis-induced alterations in the erythrocyte membrane. Am. Surg. 1994, 60, 954–957. [Google Scholar] [CrossRef]
- Portolés, M.T.; Pagani, R.; Díaz-Laviada, I.; Municio, A.M. Effect of escherichia coli lipopolysaccharide on the microviscosity of liver plasma membranes and hepatocyte suspensions and monolayers. Cell Biochem. Funct. 1987, 5, 55–61. [Google Scholar] [CrossRef]
- Nagel, M.; Brauckmann, S.; Moegle-Hofacker, F.; Effenberger-Neidnicht, K.; Hartmann, M.; de Groot, H.; Mayer, C. Impact of bacterial endotoxin on the structure of dmpc membranes. Biochim. Biophys. Acta 2015, 1848, 2271–2276. [Google Scholar] [CrossRef]
- Tanamoto, K.-I.; Azumi, S. Salmonella-type heptaacylated lipid a is inactive and acts as an antagonist of lipopolysaccharide action on human line cells. J. Immunol. 2000, 164, 3149–3156. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Armache, J.P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the trpa1 ion channel suggests regulatory mechanisms. Nature 2015, 525, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.G. Lipid-protein interactions in biological membranes: A structural perspective. Biochim. Biophys. Acta 2003, 1612, 1–40. [Google Scholar] [CrossRef]
- Hurley, J.C. Endotoxemia: Methods of detection and clinical correlates. Clin. Microbiol. Rev. 1995, 8, 268–292. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, R.C.; Gomes, B.P.; Shah, H.N.; Ferraz, C.C.; Zaia, A.A.; Souza-Filho, F.J. Quantification of endotoxins in necrotic root canals from symptomatic and asymptomatic teeth. J. Med. Microbiol. 2005, 54, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Kanaan, S.A.; Saadé, N.E.; Haddad, J.J.; Abdelnoor, A.M.; Atweh, S.F.; Jabbur, S.J.; Safieh-Garabedian, B. Endotoxin-induced local inflammation and hyperalgesia in rats and mice: A new model for inflammatory pain. Pain 1996, 66, 373–379. [Google Scholar] [CrossRef]
- Okamoto, T.; Gohil, K.; Finkelstein, E.I.; Bove, P.; Akaike, T.; van der Vliet, A. Multiple contributing roles for nos2 in lps-induced acute airway inflammation in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L198–L209. [Google Scholar] [CrossRef]
- Speyer, C.L.; Neff, T.A.; Warner, R.L.; Guo, R.F.; Sarma, J.V.; Riedemann, N.C.; Murphy, M.E.; Murphy, H.S.; Ward, P.A. Regulatory effects of inos on acute lung inflammatory responses in mice. Am. J. Pathol. 2003, 163, 2319–2328. [Google Scholar] [CrossRef]
- Schuijs, M.J.; Willart, M.A.; Vergote, K.; Gras, D.; Deswarte, K.; Ege, M.J.; Madeira, F.B.; Beyaert, R.; van Loo, G.; Bracher, F.; et al. Farm dust and endotoxin protect against allergy through a20 induction in lung epithelial cells. Science 2015, 349, 1106–1110. [Google Scholar] [CrossRef]
- Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Lopez-Requena, A.; Voets, T.; Talavera, K. Differential effects of lipopolysaccharide on mouse sensory trp channels. Cell Calcium 2018, 73, 72–81. [Google Scholar] [CrossRef]
- Sachs, F. Stretch-activated ion channels: What are they? Physiology (Bethesda) 2010, 25, 50–56. [Google Scholar] [CrossRef]
- Parasassi, T.; Krasnowska, E.K.; Bagatolli, L.; Gratton, E. Laurdan and prodan as polarity-sensitive fluorescent membrane probes. J. Fluoresc. 1998, 8, 365–373. [Google Scholar] [CrossRef]
- Parasassi, T.; Gratton, E. Membrane lipid domains and dynamics as detected by laurdan fluorescence. J Fluoresc. 1995, 5, 59–69. [Google Scholar] [CrossRef]
- Parasassi, T.; Ravagnan, G.; Rusch, R.M.; Gratton, E. Modulation and dynamics of phase properties in phospholipid mixtures detected by laurdan fluorescence. Photochem. Photobiol. 1993, 57, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, G.; Le Grimellec, C.; Giocondi, M.C.; Amiel, C. Benzyl alcohol increases membrane fluidity and modulates cyclic AMP synthesis in intact renal epithelial cells. Biochim. Biophys. Acta 1987, 903, 341–348. [Google Scholar] [CrossRef]
- Scholz, A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br. J. Anaesth. 2002, 89, 52–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragsdale, D.S.; McPhee, J.C.; Scheuer, T.; Catterall, W.A. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated na+ channels. Proc. Natl. Acad. Sci. USA 1996, 93, 9270–9275. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Mizogami, M. Interaction of local anesthetics with biomembranes consisting of phospholipids and cholesterol: Mechanistic and clinical implications for anesthetic and cardiotoxic effects. Anesthesiol. Res. Pract. 2013, 2013, 297141. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Shimokawa, N.; Takagi, M. Thermal stability of phase-separated domains in multicomponent lipid membranes with local anesthetics. Membranes 2017, 7, 33. [Google Scholar] [CrossRef]
- Yun, I.; Cho, E.-S.; Jang, H.-O.; Kim, U.-K.; Choi, C.-H.; Chung, I.-K.; Kim, I.-S.; Wood, W.G. Amphiphilic effects of local anesthetics on rotational mobility in neuronal and model membranes. Biochim. Biophys. Acta (BBA) Biomembr. 2002, 1564, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Pristerà, A.; Baker, M.D.; Okuse, K. Association between tetrodotoxin resistant channels and lipid rafts regulates sensory neuron excitability. PLoS ONE 2012, 7, e40079. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Structure and function of voltage-gated sodium channels at atomic resolution. Exp. Physiol. 2014, 99. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Shimokawa, N.; Takagi, M. Destabilization of phase-separated structures in local anesthetic-containing model biomembranes. Chem. Lett. 2015, 44, 1604–1606. [Google Scholar] [CrossRef]
- Strichartz, G.R.; Sanchez, V.; Arthur, G.R.; Chafetz, R.; Martin, D. Fundamental properties of local anesthetics. Ii. Measured octanol:Buffer partition coefficients and pka values of clinically used drugs. Anesth. Analg. 1990, 71, 158–170. [Google Scholar] [CrossRef]
- Gray, E.; Karslake, J.; Machta, B.B.; Veatch, S.L. Liquid general anesthetics lower critical temperatures in plasma membrane vesicles. Biophys. J. 2013, 105, 2751–2759. [Google Scholar] [CrossRef] [PubMed]
- Leffler, A.; Lattrell, A.; Kronewald, S.; Niedermirtl, F.; Nau, C. Activation of trpa1 by membrane permeable local anesthetics. Mol. Pain 2011, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Leffler, A.; Fischer, M.J.; Rehner, D.; Kienel, S.; Kistner, K.; Sauer, S.K.; Gavva, N.R.; Reeh, P.W.; Nau, C. The vanilloid receptor trpv1 is activated and sensitized by local anesthetics in rodent sensory neurons. J. Clin. Investig. 2008, 118, 763–776. [Google Scholar] [CrossRef]
- Eberhardt, M.; Stueber, T.; de la Roche, J.; Herzog, C.; Leffler, A.; Reeh, P.W.; Kistner, K. Trpa1 and trpv1 are required for lidocaine-evoked calcium influx and neuropeptide release but not cytotoxicity in mouse sensory neurons. PLoS ONE 2017, 12, e0188008. [Google Scholar] [CrossRef]
- Butterworth, J.F.T. Models and mechanisms of local anesthetic cardiac toxicity: A review. Reg. Anesth. Pain Med. 2010, 35, 167–176. [Google Scholar] [CrossRef]
- Xiao, B.; Dubin, A.E.; Bursulaya, B.; Viswanath, V.; Jegla, T.J.; Patapoutian, A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian trpa1 channels. J. Neurosci. 2008, 28, 9640–9651. [Google Scholar] [CrossRef]
- Matta, J.A.; Cornett, P.M.; Miyares, R.L.; Abe, K.; Sahibzada, N.; Ahern, G.P. General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc. Natl. Acad. Sci. USA 2008, 105, 8784–8789. [Google Scholar] [CrossRef] [Green Version]
- Cometto-Muniz, J.E.; Cain, W.S. Relative sensitivity of the ocular trigeminal, nasal trigeminal and olfactory systems to airborne chemicals. Chem. Senses 1995, 20, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Obata, K.; Ikeda, Y.; Ohkoshi, K.; Okumura, H.; Ozawa, N.; Ogawa, T.; Katsumura, Y.; Kawai, J.; Tatsumi, H.; et al. Evaluation of human skin irritation by carboxylic acids, alcohols, esters and aldehydes, with nitrocellulose-replica method and closed patch testing. Contact Dermat. 1996, 34, 12–16. [Google Scholar] [CrossRef]
- Komatsu, T.; Uchida, K.; Fujita, F.; Zhou, Y.; Tominaga, M. Primary alcohols activate human trpa1 channel in a carbon chain length-dependent manner. Pflügers Arch. Eur. J. Physiol. 2012, 463, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Morita, A.; Iwasaki, Y.; Kobata, K.; Iida, T.; Higashi, T.; Oda, K.; Suzuki, A.; Narukawa, M.; Sasakuma, S.; Yokogoshi, H.; et al. Lipophilicity of capsaicinoids and capsinoids influences the multiple activation process of rat trpv1. Life Sci. 2006, 79, 2303–2310. [Google Scholar] [CrossRef]
- Ursu, D.; Knopp, K.; Beattie, R.E.; Liu, B.; Sher, E. Pungency of trpv1 agonists is directly correlated with kinetics of receptor activation and lipophilicity. Eur. J. Pharmacol. 2010, 641, 114–122. [Google Scholar] [CrossRef]
- Lee, S.P.; Buber, M.T.; Yang, Q.; Cerne, R.; Cortés, R.Y.; Sprous, D.G.; Bryant, R.W. Thymol and related alkyl phenols activate the htrpa1 channel. Br. J. Pharmacol. 2008, 153, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Fujita, F.; Moriyama, T.; Higashi, T.; Shima, A.; Tominaga, M. Methyl p-hydroxybenzoate causes pain sensation through activation of trpa1 channels. Br. J. Pharmacol. 2007, 151, 153–160. [Google Scholar] [CrossRef]
- Maher, M.; Ao, H.; Banke, T.; Nasser, N.; Wu, N.T.; Breitenbucher, J.G.; Chaplan, S.R.; Wickenden, A.D. Activation of trpa1 by farnesyl thiosalicylic acid. Mol. Pharmacol. 2008, 73, 1225–1234. [Google Scholar] [CrossRef]
- Morera, E.; De Petrocellis, L.; Morera, L.; Moriello, A.S.; Nalli, M.; Di Marzo, V.; Ortar, G. Synthesis and biological evaluation of [6]-gingerol analogues as transient receptor potential channel trpv1 and trpa1 modulators. Bioorg. Med. Chem. Lett. 2012, 22, 1674–1677. [Google Scholar] [CrossRef]
- Riera, C.E.; Menozzi-Smarrito, C.; Affolter, M.; Michlig, S.; Munari, C.; Robert, F.; Vogel, H.; Simon, S.A.; le Coutre, J. Compounds from sichuan and melegueta peppers activate, covalently and non-covalently, trpa1 and trpv1 channels. Br. J. Pharmacol. 2009, 157, 1398–1409. [Google Scholar] [CrossRef]
- Chianese, G.; Fattorusso, E.; Putra, M.Y.; Calcinai, B.; Bavestrello, G.; Moriello, A.S.; De Petrocellis, L.; Di Marzo, V.; Taglialatela-Scafati, O. Leucettamols, bifunctionalized marine sphingoids, act as modulators of trpa1 and trpm8 channels. Mar. Drugs 2012, 10, 2435–2447. [Google Scholar] [CrossRef] [PubMed]
- Mazzella, N.; Syakti, A.D.; Molinet, J.; Gilewicz, M.; Doumenq, P.; Artaud, J.; Bertrand, J.C. Effects of crude oil on phospholipid fatty acid compositions of marine hydrocarbon degraders: Estimation of the bacterial membrane fluidity. Environ. Res. 2005, 97, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Odjélé, A.; Weber, J.-M. Pcb-153 and temperature cause restructuring of goldfish membranes: Homeoviscous response to a chemical fluidiser. Aquat. Toxicol. 2013, 144–145, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Wieslander, A.; Rilfors, L.; Lindblom, G. Metabolic changes of membrane lipid composition in acholeplasma laidlawii by hydrocarbons, alcohols, and detergents: Arguments for effects on lipid packing. Biochemistry 1986, 25, 7511–7517. [Google Scholar] [CrossRef] [PubMed]
- Davy De Virville, J.; Cantrel, C.; Bousquet, A.L.; Hoffelt, M.; Tenreiro, A.M.; Vaz Pinto, V.; Arrabaça, J.D.; Caiveau, O.; Moreau, F.; Zachowski, A. Homeoviscous and functional adaptations of mitochondrial membranes to growth temperature in soybean seedlings. Plant Cell Environ. 2002, 25, 1289–1297. [Google Scholar] [CrossRef] [Green Version]
- Guschina, I.A.; Harwood, J.L. Mechanisms of temperature adaptation in poikilotherms. FEBS Lett. 2006, 580, 5477–5483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oger, P.M.; Cario, A. Adaptation of the membrane in archaea. Biophys. Chem. 2013, 183, 42–56. [Google Scholar]
- Gladyshev, M.I.; Semenchenko, V.P.; Dubovskaya, O.P.; Fefilova, E.B.; Makhutova, O.N.; Buseva, Z.F.; Sushchik, N.N.; Razlutskij, V.I.; Lepskaya, E.V.; Baturina, M.A.; et al. Effect of temperature on contents of essential highly unsaturated fatty acids in freshwater zooplankton. Limnologica 2011, 41, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Behan, M.K.; Macdonald, A.G.; Jones, G.R.; Cossins, A.R. Homeoviscous adaptation under pressure: The pressure dependence of membrane order in brain myelin membranes of deep-sea fish. Biochim. Biophys. Acta (BBA) Biomembr. 1992, 1103, 317–323. [Google Scholar] [CrossRef]
- Behan-Martin, M.K.; Jones, G.R.; Bowler, K.; Cossins, A.R. A near perfect temperature adaptation of bilayer order in vertebrate brain membranes. Biochim. Biophys. Acta (BBA) Biomembr. 1993, 1151, 216–222. [Google Scholar] [CrossRef]
- Cossins, A.R.; Prosser, C.L. Evolutionary adaptation of membranes to temperature. Proc. Natl. Acad. Sci. USA 1978, 75, 2040–2043. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.L.; Minton, K.W.; Li, G.C.; Hahn, G.M. Temperature-induced homeoviscous adaptation of chinese hamster ovary cells. Biochim. Biophys. Acta (BBA) Biomembr. 1981, 641, 334–348. [Google Scholar] [CrossRef]
- Dymond Marcus, K. Mammalian phospholipid homeostasis: Evidence that membrane curvature elastic stress drives homeoviscous adaptation in vivo. J. R. Soc. Interface 2016, 13, 20160228. [Google Scholar] [CrossRef]
- Laroche, C.; Beney, L.; Marechal, P.; Gervais, P. The effect of osmotic pressure on the membrane fluidity of saccharomyces cerevisiae at different physiological temperatures. Appl. Microbiol. Biotechnol. 2001, 56, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Lisi, A.; Pozzi, D.; Pasquali, E.; Serafino, A.; Grimaldi, S. Effect of extremely low frequency (elf) magnetic field exposure on morphological and biophysical properties of human lymphoid cell line (raji). Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1997, 1357, 281–290. [Google Scholar] [CrossRef]
- Balogh, G.; Horváth, I.; Nagy, E.; Hoyk, Z.; Benkõ, S.; Bensaude, O.; Vígh, L. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. FEBS J. 2005, 272, 6077–6086. [Google Scholar] [CrossRef] [Green Version]
- Balogh, G.; Maulucci, G.; Gombos, I.; Horváth, I.; Török, Z.; Péter, M.; Fodor, E.; Páli, T.; Benko, S.; Parasassi, T.; et al. Heat stress causes spatially-distinct membrane re-modelling in k562 leukemia cells. PLoS ONE 2011, 6, e21182. [Google Scholar] [CrossRef]
- Balogh, G.; Péter, M.; Glatz, A.; Gombos, I.; Török, Z.; Horváth, I.; Harwood, J.L.; Vígh, L. Key role of lipids in heat stress management. FEBS Lett. 2013, 587, 1970–1980. [Google Scholar] [CrossRef] [Green Version]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Models Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef]
- Di Paolo, G.; Kim, T.-W. Linking lipids to alzheimer’s disease: Cholesterol and beyond. Nat. Rev. Neurosci. 2011, 12, 284–296. [Google Scholar] [CrossRef]
- Li, Z.; Vance, D.E. Phosphatidylcholine and choline homeostasis. J. Lipid Res. 2008, 49, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Agellon, L.B.; Vance, D.E. Phosphatidylcholine homeostasis and liver failure. J. Biol. Chem. 2005, 280, 37798–37802. [Google Scholar] [CrossRef] [PubMed]
- Rob, N.M.W. Lipid composition of cell membranes and its relevance in type 2 diabetes mellitus. Curr. Diabetes Rev. 2012, 8, 390–400. [Google Scholar]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Startek, J.B.; Boonen, B.; Talavera, K.; Meseguer, V. TRP Channels as Sensors of Chemically-Induced Changes in Cell Membrane Mechanical Properties. Int. J. Mol. Sci. 2019, 20, 371. https://doi.org/10.3390/ijms20020371
Startek JB, Boonen B, Talavera K, Meseguer V. TRP Channels as Sensors of Chemically-Induced Changes in Cell Membrane Mechanical Properties. International Journal of Molecular Sciences. 2019; 20(2):371. https://doi.org/10.3390/ijms20020371
Chicago/Turabian StyleStartek, Justyna B., Brett Boonen, Karel Talavera, and Victor Meseguer. 2019. "TRP Channels as Sensors of Chemically-Induced Changes in Cell Membrane Mechanical Properties" International Journal of Molecular Sciences 20, no. 2: 371. https://doi.org/10.3390/ijms20020371
APA StyleStartek, J. B., Boonen, B., Talavera, K., & Meseguer, V. (2019). TRP Channels as Sensors of Chemically-Induced Changes in Cell Membrane Mechanical Properties. International Journal of Molecular Sciences, 20(2), 371. https://doi.org/10.3390/ijms20020371





