Maternal Obesity during Pregnancy Alters Daily Activity and Feeding Cycles, and Hypothalamic Clock Gene Expression in Adult Male Mouse Offspring
Abstract
:1. Introduction
2. Results
2.1. Maternal Obesity and High-Fat Diet Alters Metabolic Phenotype and Behaviour in the Offspring
2.2. Maternal Obesity and High-Fat Diet Disrupts the Daily Expression Patterns of Clock Gene in the Suprachiasmatic Nucleus (SCN) of the Offspring Brain
2.3. Maternal Obesity and High-Fat Diet Alters the Daily Expression Patterns of Appetite Genes in the Arcuate Nucleus (ARC) of the Offspring Brain
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Behavioural Analysis
4.3. Collection of SCN and ARC Samples
4.4. RNA Extraction and Quantitative Real-Time PCR
4.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Dietary Composition | Control Diet | High-Fat Diet |
|---|---|---|
| Percentage weight: | ||
| Carbohydrate | 66.7 | 39.8 |
| Protein | 14.4 | 23.0 |
| Lipid | 2.7 | 22.6 |
| Percentage energy (kcal): | ||
| Carbohydrate | 75.1 | 35.0 |
| Protein | 17.5 | 20.0 |
| Lipid | 7.4 | 45.0 |
| Energy (AFE MJ/kg) | 13.8 | 19.1 |
| Fat breakdown (% AFE): | ||
| Saturated Fatty Acids (SFA) | 0.5 | 6.5 |
| Monounsaturated Fatty Acids (MUFA) | 0.9 | 6.5 |
| Polyunsaturated Fatty Acids (PUFA) | 0.9 | 4.2 |
References
- Shaw, J.; Sicree, R.; Zimmet, P. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, A.; Bass, J. A sense of time: How molecular clocks organize metabolism. Trends Endocrinol. Metab. 2007, 18, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Cagampang, F.R.; Bruce, K.D. The role of the circadian clock system in nutrition and metabolism. Br. J. Nutr. 2012, 108, 381–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowrey, P.L.; Takahashi, J.S. Genetics of circadian rhythms in Mammalian model organisms. Genet. Genom. Fish Phenomics 2011, 74, 175–230. [Google Scholar]
- Dibner, C.; Schibler, U.; Albrecht, U. The Mammalian Circadian Timing System: Organization and Coordination of Central and Peripheral Clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [Green Version]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef]
- Oishi, K.; Atsumi, G.; Sugiyama, S.; Kodomari, I.; Kasamatsu, M.; Machida, K.; Ishida, N. Disrupted fat absorption attenuates obesity induced by a high-fat diet in Clock mutant mice. FEBS Lett. 2006, 580, 127–130. [Google Scholar] [CrossRef]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-Fat Diet Disrupts Behavioral and Molecular Circadian Rhythms in Mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Challet, E. Circadian Clocks, Food Intake, and Metabolism. Prog. Mol. Biol. Transl. Sci. 2013, 119, 105–135. [Google Scholar]
- Schwartz, M.W. Central Nervous System Regulation of Food Intake. Obesity 2006, 14, 1S–8S. [Google Scholar] [CrossRef] [PubMed]
- Gehlert, D.R.; Chronwall, B.M.; Schafer, M.P.; O’Donohue, T.L. Localization of neuropeptide Y messenger ribonucleic acid in rat and mouse brain by in situ hybridization. Synapse 1987, 1, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, H.; Takenoya, F.; Hirako, S.; Wada, N.; Kintaka, Y.; Inoue, S.; Ota, E.; Ogawa, T.; Shioda, S. Neuronal circuits involving neuropeptide Y in hypothalamic arcuate nucleus-mediated feeding regulation. Neuropeptides 2012, 46, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, D.; Betley, J.N.; Su, H.H.; Sternson, S.M. Deconstruction of a neural circuit for hunger. Nature 2012, 488, 172–177. [Google Scholar] [CrossRef]
- Clark, J.T.; Kalra, P.S.; Kalra, S.P. Neuropeptide Y Stimulates Feeding but Inhibits Sexual Behavior in Rats. Endocrinology 1985, 117, 2435–2442. [Google Scholar] [CrossRef]
- Sousa-Ferreira, L.; Garrido, M.; Nascimento-Ferreira, I.; Nobrega, C.; Santos-Carvalho, A.; Álvaro, A.R.; Rosmaninho-Salgado, J.; Kaster, M.; Kügler, S.; De Almeida, L.P.; et al. Moderate Long-Term Modulation of Neuropeptide Y in Hypothalamic Arcuate Nucleus Induces Energy Balance Alterations in Adult Rats. PLoS ONE 2011, 6, e22333. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Gilbey, S.G.; Mannan, M.; Aslam, N.; Ghatei, M.A.; Bloom, S.R. Increased neuropeptide Y content in individual hypothalamic nuclei, but not neuropeptide Y mRNA, in diet-induced obesity in rats. J. Endocrinol. 1992, 132, 299–304. [Google Scholar] [CrossRef]
- Dryden, S.; Pickavance, L.; Frankish, H.M.; Williams, G. Increased neuropeptide Y secretion in the hypothalamic paraventricular nucleus of obese (fa/fa) Zucker rats. Brain Res. 1995, 690, 185–188. [Google Scholar] [CrossRef]
- Huang, X.-F.; Han, M.; Storlien, L.H. The level of NPY receptor mRNA expression in diet-induced obese and resistant mice. Mol. Brain Res. 2003, 115, 21–28. [Google Scholar] [CrossRef]
- Souza, G.F.P.; Solon, C.; Nascimento, L.F.; De-Lima-Junior, J.C.; Nogueira, G.; Moura, R.; Rocha, G.Z.; Fioravante, M.; Bobbo, V.; Morari, J.; et al. Defective regulation of POMC precedes hypothalamic inflammation in diet-induced obesity. Sci. Rep. 2016, 6, 29290. [Google Scholar] [CrossRef] [PubMed]
- Diane, A.; Pierce, W.D.; Russell, J.C.; Heth, C.D.; Vine, D.F.; Richard, D.; Proctor, S.D. Down-regulation of hypothalamic pro-opiomelanocortin (POMC) expression after weaning is associated with hyperphagia-induced obesity in JCR rats overexpressing neuropeptide Y. Br. J. Nutr. 2014, 111, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Kalra, P.S.; Farmerie, W.G.; Kalra, S.P. Daily Changes in Hypothalamic Gene Expression of Neuropeptide Y, Galanin, Proopiomelanocortin, and Adipocyte Leptin Gene Expression and Secretion: Effects of Food Restriction 1. Endocrinology 1999, 140, 2868–2875. [Google Scholar] [CrossRef] [PubMed]
- Stütz, A.M.; Staszkiewicz, J.; Ptitsyn, A.; Argyropoulos, G. Circadian Expression of Genes Regulating Food Intake*. Obesity 2007, 15, 607–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buijs, R.M.; Hou, Y.-X.; Shinn, S.; Renaud, L.P. Ultrastructural evidence for intra- and extranuclear projections of GABAergic neurons of the suprachiasmatic nucleus. J. Comp. Neurol. 1994, 340, 381–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, M.; Herzog, E.D.; Yamazaki, S.; Straume, M.; Tei, H.; Sakaki, Y.; Menaker, M.; Block, G.D. Circadian Rhythms in Isolated Brain Regions. J. Neurosci. 2002, 22, 350–356. [Google Scholar] [CrossRef]
- Buijs, F.N.; Guzman-Ruiz, M.; Leon-Mercado, L.; Basualdo, M.C.; Escobar, C.; Kalsbeek, A.; Buijs, R.M. Suprachiasmatic Nucleus Interaction with the Arcuate Nucleus; Essential for Organizing Physiological Rhythms. Eneuro 2017, 4, 2. [Google Scholar] [CrossRef]
- Drake, A.J.; Reynolds, R.M. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction 2010, 140, 387–398. [Google Scholar] [CrossRef]
- Suter, M.; Bocock, P.; Showalter, L.; Hu, M.; Shope, C.; McKnight, R.; Grove, K.; Lane, R.; Aagaard-Tillery, K. Epigenomics: Maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates. FASEB J. 2011, 25, 714–726. [Google Scholar] [CrossRef]
- Borengasser, S.J.; Kang, P.; Faske, J.; Gomez-Acevedo, H.; Blackburn, M.L.; Badger, T.M.; Shankar, K. High fat diet and in utero exposure to maternal obesity disrupts circadian rhythm and leads to metabolic programming of liver in rat offspring. PLoS ONE 2014, 9, e84209. [Google Scholar] [CrossRef]
- Bruce, K.D.; Szczepankiewicz, D.; Sihota, K.K.; Ravindraanandan, M.; Thomas, H.; Lillycrop, K.A.; Burdge, G.C.; Hanson, M.A.; Byrne, C.D.; Cagampang, F.R. Altered cellular redox status, sirtuin abundance and clock gene expression in a mouse model of developmentally primed NASH. Biochim. Biophys. Acta 2016, 1861, 584–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruce, K.D.; Cagampang, F.R.; Argenton, M.; Zhang, J.; Ethirajan, P.L.; Burdge, G.C.; Bateman, A.C.; Clough, G.F.; Poston, L.; Hanson, M.A.; et al. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology 2009, 50, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.M.; Cagampang, F.R.; Mukhtar, D.; Anthony, F.W.; Ohri, S.K.; Hanson, M.A. Long-term maternal high-fat feeding from weaning through pregnancy and lactation predisposes offspring to hypertension, raised plasma lipids and fatty liver in mice. Br. J. Nutr. 2009, 102, 514. [Google Scholar] [CrossRef] [PubMed]
- Sellayah, D.; Thomas, H.; Lanham, S.A.; Cagampang, F.R. Maternal Obesity During Pregnancy and Lactation Influences Offspring Obesogenic Adipogenesis but Not Developmental Adipogenesis in Mice. Nutrients 2019, 11, 495. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Jellyman, J.K.; Han, G.; Beall, M.; Lane, R.H.; Ross, M.G.; Han, G. Maternal obesity and high-fat diet program offspring metabolic syndrome. Am. J. Obstet. Gynecol. 2014, 211, 237.e1–237.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuelsson, A.-M.; Matthews, P.A.; Argenton, M.; Christie, M.R.; McConnell, J.M.; Jansen, E.H.M.; Piersma, A.H.; Ozanne, S.E.; Twinn, D.F.; Remacle, C.; et al. Diet-Induced Obesity in Female Mice Leads to Offspring Hyperphagia, Adiposity, Hypertension, and Insulin Resistance: A Novel Murine Model of Developmental Programming. Hypertension 2008, 51, 383–392. [Google Scholar] [CrossRef]
- Ellacott, K.L.; Morton, G.J.; Woods, S.C.; Tso, P.; Schwartz, M.W. Assessment of feeding behavior in laboratory mice. Cell Metab. 2010, 12, 10–17. [Google Scholar] [CrossRef]
- Rajia, S.; Morris, M.J.; Chen, H. Maternal overnutrition impacts offspring adiposity and brain appetite markers-modulation by postweaning diet. J. Neuroendocr. 2010, 22, 905–914. [Google Scholar] [CrossRef] [Green Version]
- Sclafani, A. Animal models of obesity: Classification and characterization. Int. J. Obes. 1984, 8, 491–508. [Google Scholar]
- Fenton, P.F.; Carr, C.J. The nutrition of the mouse. XI. Response of four strains to diets differing in fat content. J. Nutr. 1951, 45, 225–233. [Google Scholar] [CrossRef]
- Bunger, M.K.; Wilsbacher, L.D.; Moran, S.M.; Clendenin, C.; Radcliffe, L.A.; HogenEsch, J.B.; Simon, M.C.; Takahashi, J.S.; Bradfield, C.A. Mop3 Is an Essential Component of the Master Circadian Pacemaker in Mammals. Cell 2000, 103, 1009–1017. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Larkin, D.W.; Albrecht, U.; Sun, Z.S.; Sage, M.; Eichele, G.; Lee, C.C.; Bradley, A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 1999, 400, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.; Jin, X.; Maywood, E.S.; Hastings, M.H.; Reppert, S.M.; Weaver, D.R. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 2001, 30, 525–536. [Google Scholar] [CrossRef]
- Van Der Horst, G.T.J.; Muijtjens, M.; Kobayashi, K.; Takano, R.; Kanno, S.-I.; Takao, M.; De Wit, J.; Verkerk, A.; Eker, A.P.M.; Van Leenen, D.; et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 1999, 398, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Bonaconsa, M.; Malpeli, G.; Montaruli, A.; Carandente, F.; Grassi-Zucconi, G.; Bentivoglio, M. Differential modulation of clock gene expression in the suprachiasmatic nucleus, liver and heart of aged mice. Exp. Gerontol. 2014, 55, 70–79. [Google Scholar] [CrossRef]
- Akabayashi, A.; Levin, N.; Paez, X.; Alexander, J.T.; Leibowitz, S.F. Hypothalamic Neuropeptide Y and Its Gene Expression: Relation to Light/Dark Cycle and Circulating Corticosterone. Mol. Cell. Neurosci. 1994, 5, 210–218. [Google Scholar] [CrossRef]
- Steiner, R.A.; Kabigting, E.; Lent, K.; Clifton, D.K. Diurnal Rhythm in Proopiomelanocortin mRNA in the Arcuate Nucleus of the Male Rat. J. Neuroendocr. 1994, 6, 603–608. [Google Scholar] [CrossRef]
- Guerrero-Vargas, N.N.; Espitia-Bautista, E.; Buijs, R.M.; Escobar, C. Shift-work: Is time of eating determining metabolic health? Evidence from animal models. Proc. Nutr. Soc. 2018, 77, 199–215. [Google Scholar] [CrossRef]
- Espitia-Bautista, E.; Velasco-Ramos, M.; Osnaya-Ramírez, I.; Ángeles-Castellanos, M.; Buijs, R.M.; Escobar, C. Social jet-lag potentiates obesity and metabolic syndrome when combined with cafeteria diet in rats. Metabolism 2017, 72, 83–93. [Google Scholar] [CrossRef]
- Brum, M.C.B.; Filho, F.F.D.; Schnorr, C.C.; Bottega, G.B.; Rodrigues, T.C. Shift work and its association with metabolic disorders. Diabetol. Metab. Syndr. 2015, 7, 45. [Google Scholar] [CrossRef]
- Fonken, L.K.; Workman, J.L.; Walton, J.C.; Weil, Z.M.; Morris, J.S.; Haim, A.; Nelson, R.J. Light at night increases body mass by shifting the time of food intake. Proc. Natl. Acad. Sci. USA 2010, 107, 18664–18669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietroiusti, A.; Neri, A.; Somma, G.; Coppeta, L.; Iavicoli, I.; Bergamaschi, A.; Magrini, A. Incidence of metabolic syndrome among night-shift healthcare workers. Occup. Environ. Med. 2010, 67, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Oosterman, J.E.; Kalsbeek, A.; la Fleur, S.E.; Belsham, D.D. Impact of nutrients on circadian rhythmicity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R337–R350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pendergast, J.S.; Branecky, K.L.; Yang, W.; Ellacott, K.L.; Niswender, K.D.; Yamazaki, S. High-fat diet acutely affects circadian organisation and eating behavior. Eur. J. Neurosci. 2013, 37, 1350–1356. [Google Scholar] [CrossRef] [Green Version]
- Krämer, A.; Yang, F.-C.; Snodgrass, P.; Li, X.; Scammell, T.E.; Davis, F.C.; Weitz, C.J. Regulation of Daily Locomotor Activity and Sleep by Hypothalamic EGF Receptor Signaling. Science 2001, 294, 2511–2515. [Google Scholar] [CrossRef]
- Jordan, L.M. Initiation of locomotion in mammals. Ann. N. Y. Acad. Sci. 1998, 860, 83–93. [Google Scholar] [CrossRef]
- Ibrahim, N.; Bosch, M.A.; Smart, J.L.; Qiu, J.; Rubinstein, M.; Rønnekleiv, O.K.; Low, M.J.; Kelly, M.J. Hypothalamic Proopiomelanocortin Neurons Are Glucose Responsive and Express K ATP Channels. Endocrinology 2003, 144, 1331–1340. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 778. [Google Scholar] [CrossRef]
- Gropp, E.; Shanabrough, M.; Borok, E.; Xu, A.W.; Janoschek, R.; Buch, T.; Plum, L.; Balthasar, N.; Hampel, B.; Waisman, A.; et al. Agouti-related peptide–expressing neurons are mandatory for feeding. Nat. Neurosci. 2005, 8, 1289–1291. [Google Scholar] [CrossRef]
- Dalvi, P.S.; Chalmers, J.A.; Luo, V.; Han, D.Y.; Wellhauser, L.; Liu, Y.; Tran, D.Q.; Castel, J.; Luquet, S.; Wheeler, M.B.; et al. High fat induces acute and chronic inflammation in the hypothalamus: Effect of high-fat diet, palmitate and TNF-alpha on appetite-regulating NPY neurons. Int. J. Obes. 2017, 41, 149–158. [Google Scholar] [CrossRef]
- Roseberry, A.G.; Liu, H.; Jackson, A.C.; Cai, X.; Friedman, J.M. Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice. Neuron 2004, 41, 711–722. [Google Scholar] [CrossRef]
- Klein, M.O.; Mackay, H.; Edwards, A.; Park, S.-B.; Kiss, A.C.I.; Felicio, L.F.; Abizaid, A. POMC and NPY mRNA expression during development is increased in rat offspring brain from mothers fed with a high fat diet. Int. J. Dev. Neurosci. 2018, 64, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Catavero, C.; Rinaman, L. Maternal high-fat diet increases independent feeding in pre-weanling rat pups. Physiol. Behav. 2016, 157, 237–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrold, J.A.; Dovey, T.M.; Blundell, J.E.; Halford, J.C. CNS regulation of appetite. Neuropharmacology 2012, 63, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Amin, T.; Mercer, J.G. Hunger and Satiety Mechanisms and Their Potential Exploitation in the Regulation of Food Intake. Curr. Obes. Rep. 2016, 5, 106–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremblay, A.; Bellisle, F. Nutrients, satiety, and control of energy intake. Appl. Physiol. Nutr. Metab. 2015, 40, 971–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, Z.Y.; Muhlhausler, B.S. Maternal “junk-food” feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB J. 2011, 25, 2167–2179. [Google Scholar] [CrossRef]
- Nascimento, L.F.; Souza, G.F.; Morari, J.; Barbosa, G.O.; Solon, C.; Moura, R.F.; Victorio, S.C.; Ignacio-Souza, L.M.; Razolli, D.S.; Carvalho, H.F.; et al. n-3 Fatty Acids Induce Neurogenesis of Predominantly POMC-Expressing Cells in the Hypothalamus. Diabetes 2016, 65, 673–686. [Google Scholar] [CrossRef]
- Benzler, M.; Benzler, J.; Stoehr, S.; Hempp, C.; Rizwan, M.Z.; Heyward, P.; Tups, A. “Insulin-like” effects of palmitate compromise insulin signalling in hypothalamic neurons. J. Comp. Physiol. B 2019, 189, 413–424. [Google Scholar] [CrossRef]
- Kuljis, D.A.; Loh, D.H.; Truong, D.; Vosko, A.M.; Ong, M.L.; McClusky, R.; Arnold, A.P.; Colwell, C.S. Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology 2013, 154, 1501–1512. [Google Scholar] [CrossRef]
- Aagaard-Tillery, K.M.; Grove, K.; Bishop, J.; Ke, X.; Fu, Q.; McKnight, R.; Lane, R.H. Developmental origins of disease and determinants of chromatin structure: Maternal diet modifies the primate fetal epigenome. J. Mol. Endocrinol. 2008, 41, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Keleher, M.R.; Zaidi, R.; Shah, S.; Oakley, M.E.; Pavlatos, C.; El Idrissi, S.; Xing, X.; Li, D.; Wang, T.; Cheverud, J.M. Maternal high-fat diet associated with altered gene expression, DNA methylation, and obesity risk in mouse offspring. PLoS ONE 2018, 13, e0192606. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, T.G.; Allen, T.-J.; Davies, A.; Harno, E.; Sefton, C.; Murgatroyd, C.; White, A. Maternal overnutrition programs epigenetic changes in the regulatory regions of hypothalamic Pomc in the offspring of rats. Int. J. Obes. 2018, 42, 1431–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Lopez, O.; Samblas, M.; Milagro, F.I.; Riezu-Boj, J.I.; Crujeiras, A.; Martinez, J.A.; Project, M. Circadian gene methylation profiles are associated with obesity, metabolic disturbances and carbohydrate intake. Chronobiol. Int. 2018, 35, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Qin, Y.; Shu, H.; Li, X. Methylation analyses on promoters of mPer1, mPer2, and mCry1 during perinatal development. Biochem. Biophys. Res. Commun. 2010, 391, 1742–1747. [Google Scholar] [CrossRef]
- Doi, M.; Hirayama, J.; Sassone-Corsi, P. Circadian Regulator CLOCK Is a Histone Acetyltransferase. Cell 2006, 125, 497–508. [Google Scholar] [CrossRef]
- Sridhar, S.B.; Darbinian, J.; Ehrlich, S.F.; Markman, M.A.; Gunderson, E.P.; Ferrara, A.; Hedderson, M.M. Maternal gestational weight gain and offspring risk for childhood overweight or obesity. Am. J. Obstet. Gynecol. 2014, 211, 259.e1–259.e8. [Google Scholar] [CrossRef] [Green Version]
- Parlee, S.D.; MacDougald, O.A. Maternal nutrition and risk of obesity in offspring: The Trojan horse of developmental plasticity. Biochim. Biophys. Acta 2014, 1842, 495–506. [Google Scholar] [CrossRef]
- Bruce, K.D.; Cagampang, F.R.; Argenton, M.; Zhang, J.L.; Ethirajan, P.; Clough, G.F.; Poston, L.; Hanson, M.A.; McConnell, J.; Byrne, C.D. Maternal Exposure to a High Fat Diet Increases Susceptibility to Adult Onset of Non-Alcoholic Fatty Liver Disease (NAFLD). Reprod. Sci. 2009, 16, 240A–241A. [Google Scholar]
- Zhang, J.; Zhang, F.; Didelot, X.; Bruce, K.D.; Cagampang, F.R.; Vatish, M.; Hanson, M.; Lehnert, H.; Ceriello, A.; Byrne, C.D. Maternal high fat diet during pregnancy and lactation alters hepatic expression of insulin like growth factor-2 and key microRNAs in the adult offspring. BMC Genom. 2009, 10, 478. [Google Scholar] [CrossRef]
- Chong, N.W.S.; Cagampang, F.R.A.; Coen, C.W.; Campbell, I.C.; Powell, J.F. Rapid identification of novel genes expressed in a circadian manner in rat suprachiasmatic nuclei. NeuroReport 1996, 7, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Cleal, J.K.; Shepherd, J.N.; Shearer, J.L.; Bruce, K.D.; Cagampang, F.R. Sensitivity of housekeeping genes in the suprachiasmatic nucleus of the mouse brain to diet and the daily light–dark cycle. Brain Res. 2014, 1575, 72–77. [Google Scholar] [CrossRef] [PubMed]
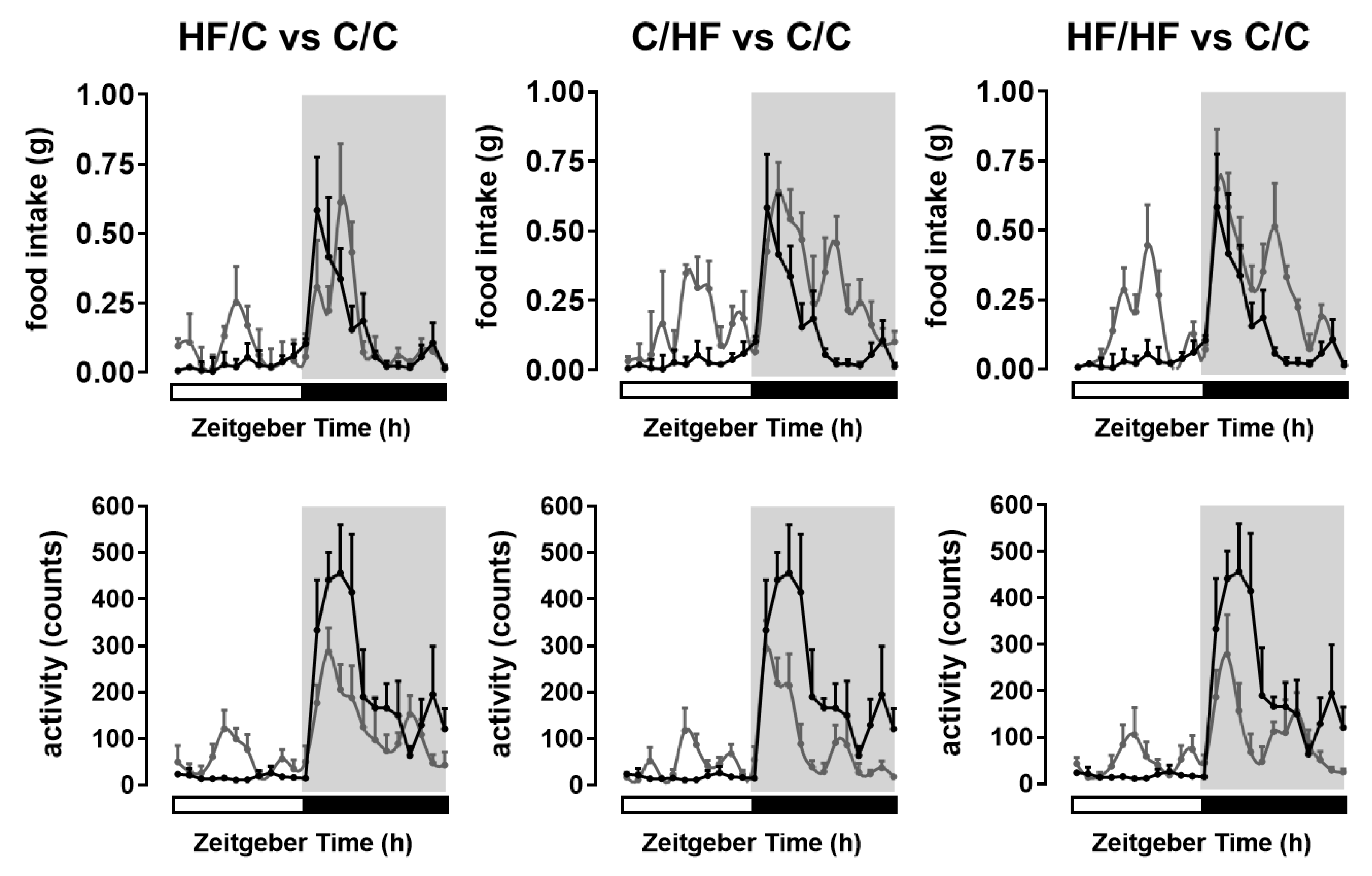


| Offspring of Lean C-fed Dams | Offspring of Obese HF-fed Dams | p-Values | |||||
|---|---|---|---|---|---|---|---|
| Maternal Obesity × Postweaning Diet | Effect of Maternal Obesity | Effect of Postweaning HF Diet | |||||
| Control (C/C) | HF Diet (C/HF) | Control (HF/C) | HF Diet (HF/HF) | ||||
| Body weight (g) | 24.2 ± 0.7 a | 33.4 ± 0.4 b | 30.6 ± 0.7 c | 41.5 ± 0.8 d | ns | <0.0001 | <0.0001 |
| Total body fat (%BW) | 5.2 ± 0.5 a | 12.8 ± 0.9 b | 9.2 ± 1.2 c | 17.5 ± 1.5 d | ns | <0.0001 | <0.0001 |
| Daily food intake (g) | |||||||
| Daytime | 0.40 ± 0.01 a | 1.82 ± 0.03 b | 1.03 ± 0.02 c | 1.67 ± 0.04 d | <0.0001 | <0.0001 | <0.0001 |
| Nighttime | 1.98 ± 0.05 a | 2.96 ± 0.05 b | 2.02 ± 0.05 a | 3.77 ± 0.06 d | <0.0001 | <0.0001 | <0.0001 |
| Daily activity (counts) | |||||||
| Daytime | 206 ± 6 a | 558 ± 19 b | 653 ± 24 c | 592 ± 23 b | <0.0001 | <0.0001 | <0.0001 |
| Nighttime | 2829 ± 72 a | 1168 ± 34 b | 1598 ± 46 c | 1313 ± 39 b | <0.0001 | <0.0001 | <0.0001 |
| Offspring of Lean C-fed Dams | Offspring of Obese HF-fed Dams | |||||
|---|---|---|---|---|---|---|
| Control (C/C) | HF Diet (C/HF) | Control (HF/C) | HF Diet (HF/HF) | |||
| CLOCK genes | ||||||
| Clock | Mensor | 0.062 | 0.071 | 0.064 | 0.051 | |
| Amplitude | 0.016 | NSR | NSR | 0.017 | ||
| Acrophase (ZT:min) | 23.41 | NSR | NSR | 1.93 * | ||
| Bmal1 | Mensor | 0.012 | 0.006 * | 0.008 * | 0.014 | |
| Amplitude | 0.012 | NSR | NSR | NSR | ||
| Acrophase (ZT:min) | 0.39 | NSR | NSR | NSR | ||
| Per2 | Mensor | 0.221 | 0.084 * | 0.161 | 0.138 | |
| Amplitude | 0.089 | NSR | 0.077 | NSR | ||
| Acrophase (ZT:min) | 7.01 | NSR | 10.00 | NSR | ||
| Cry2 | Mensor | 0.401 | 0.122 * | 0.641 * | 0.373 | |
| Amplitude | 0.150 | NSR | 0.117 | NSR | ||
| Acrophase (ZT:min) | 8.34 | NSR | 2.01 * | NSR | ||
| APPETITE genes | ||||||
| Npy | Mensor | 2.654 | 3.900 | 2.859 | 3.348 | |
| Amplitude | 1.721 | NSR | NSR | NSR | ||
| Acrophase (ZT:min) | 17.97 | NSR | NSR | NSR | ||
| Pomc | Mensor | 1.321 | 2.084 * | 0.936 | 1.924 | |
| Amplitude | 0.897 | NSR | NSR | NSR | ||
| Acrophase (ZT:min) | 9.27 | NSR | NSR | NSR | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cleal, J.K.; Bruce, K.D.; Shearer, J.L.; Thomas, H.; Plume, J.; Gregory, L.; Shepard, J.N.; Spiers-Fitzgerald, K.L.; Mani, R.; Lewis, R.M.; et al. Maternal Obesity during Pregnancy Alters Daily Activity and Feeding Cycles, and Hypothalamic Clock Gene Expression in Adult Male Mouse Offspring. Int. J. Mol. Sci. 2019, 20, 5408. https://doi.org/10.3390/ijms20215408
Cleal JK, Bruce KD, Shearer JL, Thomas H, Plume J, Gregory L, Shepard JN, Spiers-Fitzgerald KL, Mani R, Lewis RM, et al. Maternal Obesity during Pregnancy Alters Daily Activity and Feeding Cycles, and Hypothalamic Clock Gene Expression in Adult Male Mouse Offspring. International Journal of Molecular Sciences. 2019; 20(21):5408. https://doi.org/10.3390/ijms20215408
Chicago/Turabian StyleCleal, Jane K., Kimberley D. Bruce, Jasmin L. Shearer, Hugh Thomas, Jack Plume, Louise Gregory, James N. Shepard, Kerry L. Spiers-Fitzgerald, Ravi Mani, Rohan M. Lewis, and et al. 2019. "Maternal Obesity during Pregnancy Alters Daily Activity and Feeding Cycles, and Hypothalamic Clock Gene Expression in Adult Male Mouse Offspring" International Journal of Molecular Sciences 20, no. 21: 5408. https://doi.org/10.3390/ijms20215408





