High-Energy Diet and Shorter Light Exposure Drives Markers of Adipocyte Dysfunction in Visceral and Subcutaneous Adipose Depots of Psammomys obesus
Abstract
:1. Introduction
2. Results
2.1. Daily Rhythms of Adipogenesis Markers are Differentially Expressed in Visceral Adipose Tissue with Changes in Photoperiod and Diet
2.2. Daily Rhythms of Adipogenesis Markers Are Differentially Expressed in Subcutaneous Adipose Tissue with Changes in Photoperiod and Diet
2.3. High-Energy Diet and Short Photoperiod Influences Daily Rhythm Expression of Browning Markers
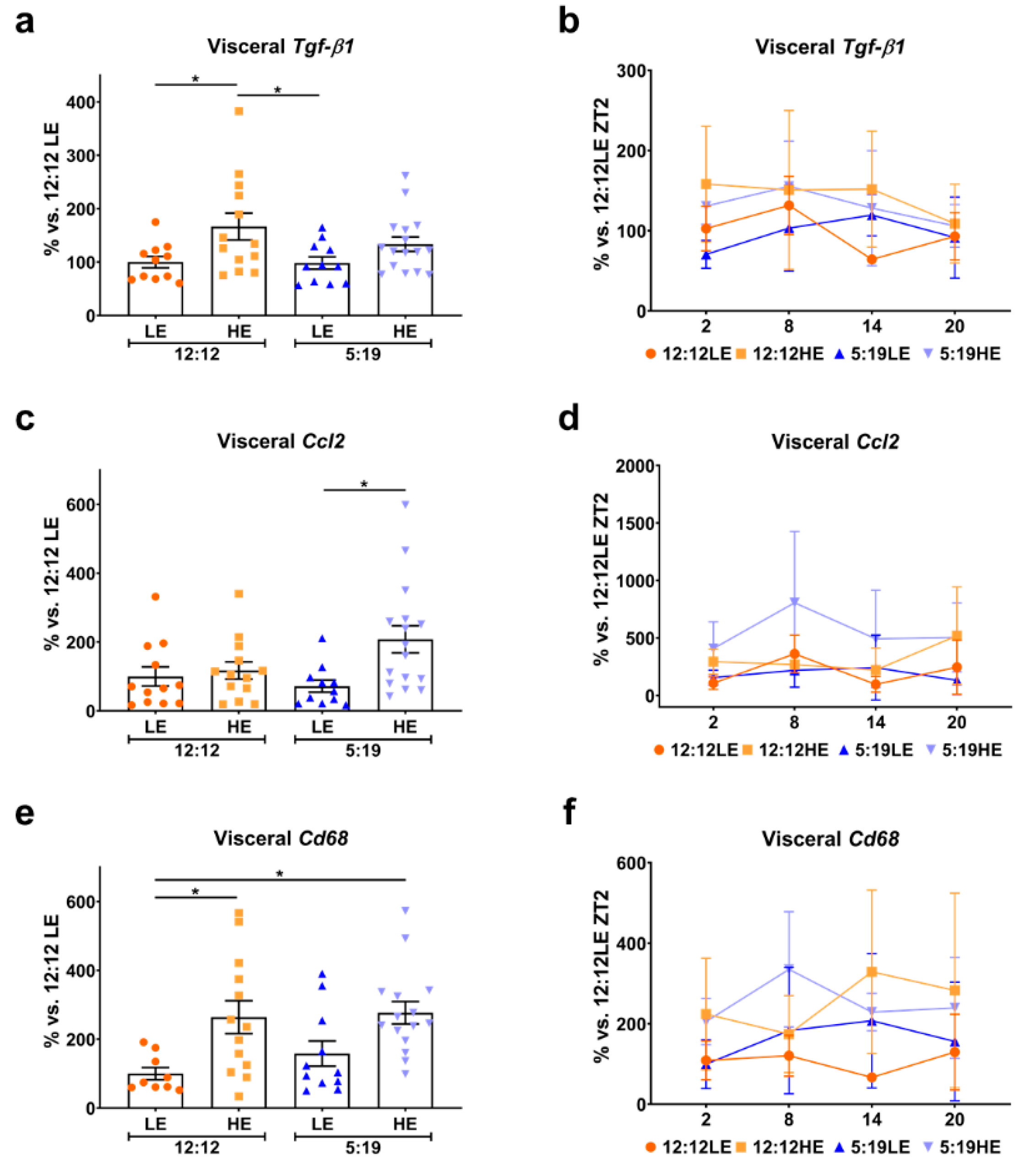
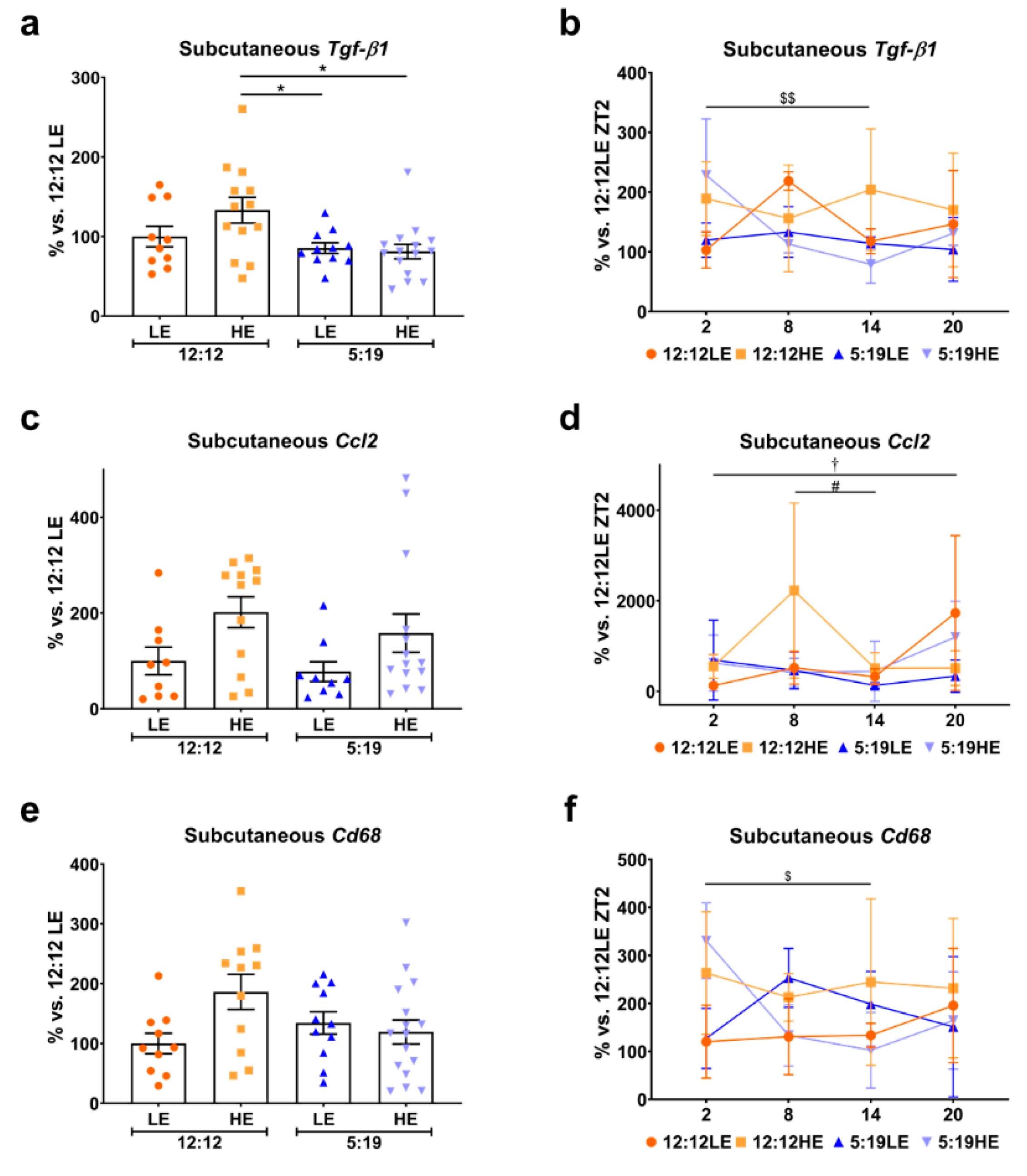
2.4. High-Energy Diet Drives Inflammation in Visceral Adipose Tissue
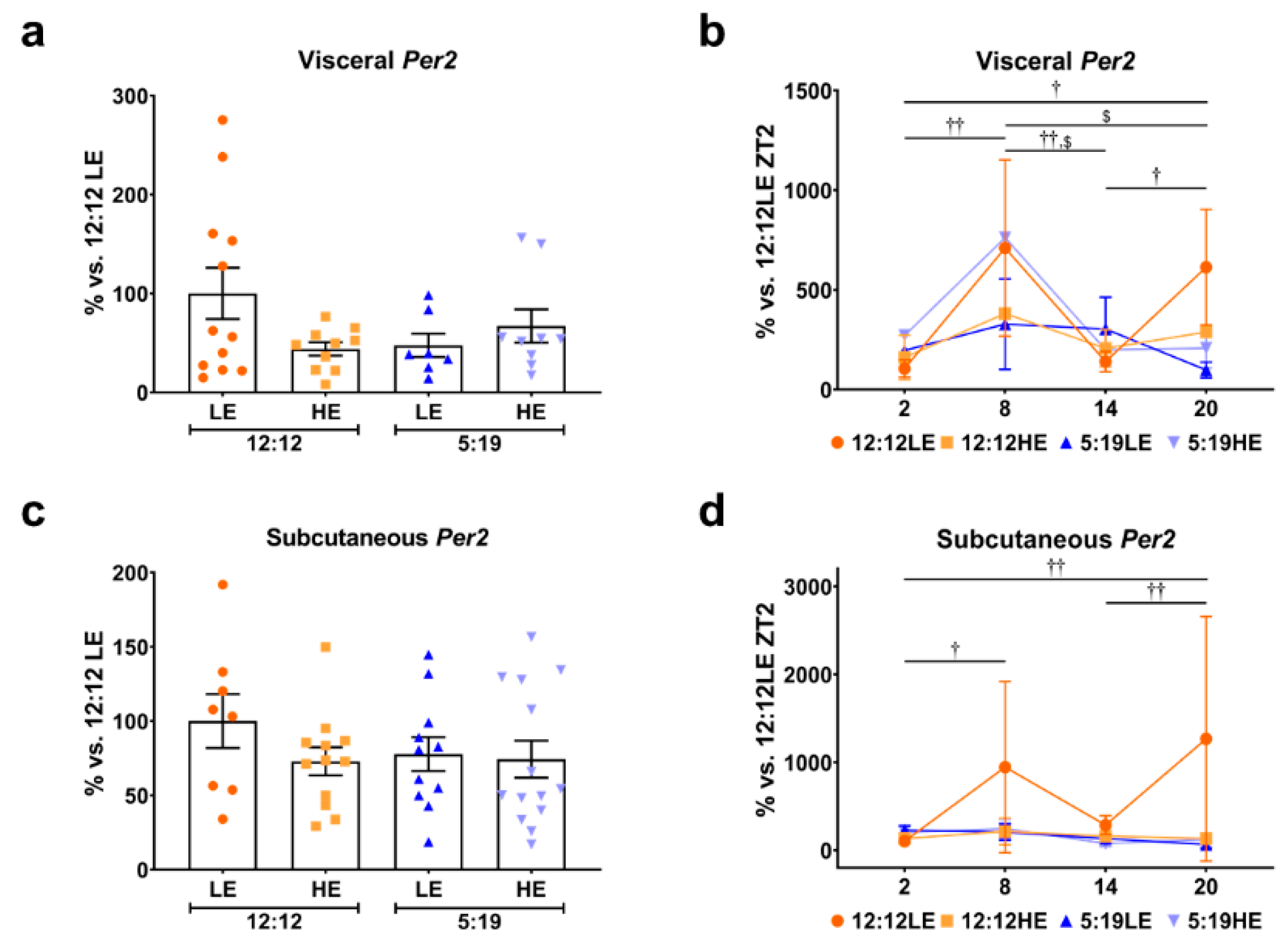
2.5. Daily Rhythms of Per2 in Visceral and Subcutaneous Adipose Depots
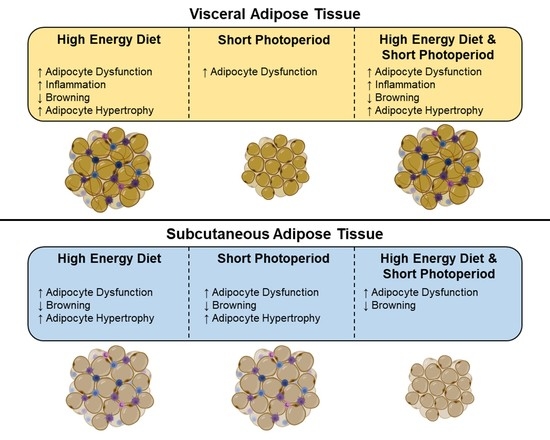
2.6. High-Energy Diet Induces Morphological Changes in Adipose Depots
3. Discussion
4. Materials and Methods
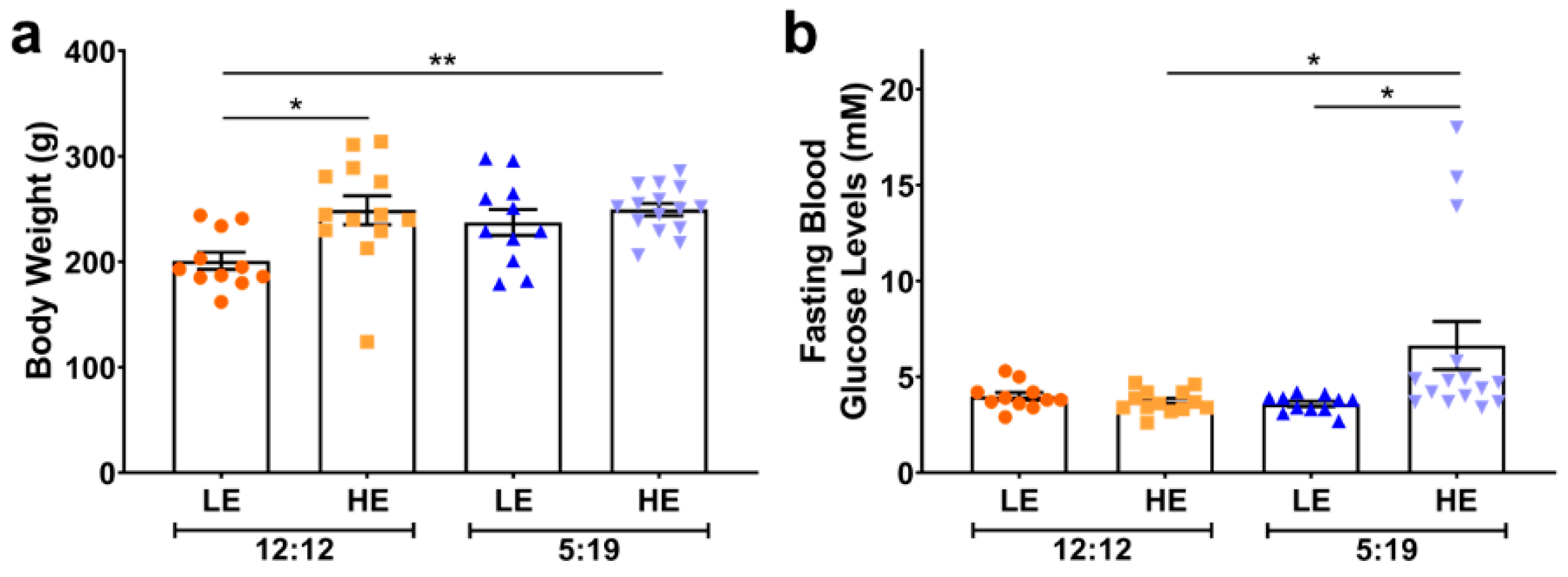
4.1. Animal Studies
4.2. Gene Expression Analysis
4.3. Determination of Adipocyte Size
4.4. Statistical Analysis
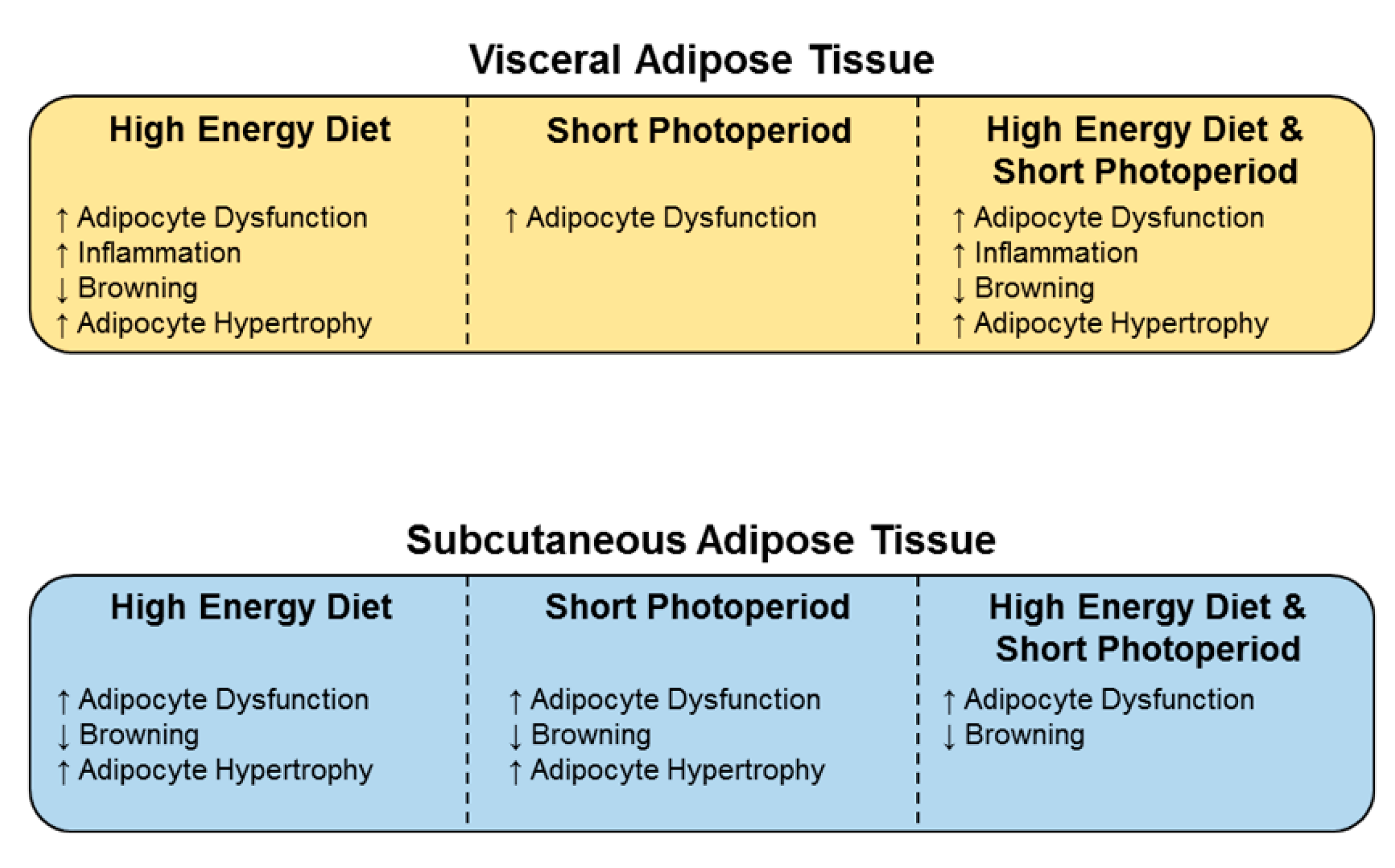
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Adipoq | Adiponectin |
| C/ebpα | CCAAT/enhancer binding protein α |
| Ccl2 | C-C motif chemokine ligand 2 |
| HE | High energy |
| LE | Low energy |
| Per2 | Period 2 |
| PGC-1α | PPAR gamma coactivator-1α |
| PPAR | Peroxisome proliferator-activated receptor |
| TGF-β1 | Transforming growth factor-β1 |
| Ucp1 | Uncoupling protein 1 |
References
- Bjorndal, B.; Burri, L.; Staalesen, V.; Skorve, J.; Berge, R.K. Different adipose depots: Their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J. Obes. 2011, 2011, 490650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, N.; Moreno-Villegas, Z.; Gonzalez-Bris, A.; Egido, J.; Lorenzo, O. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Wu, Y.; Fried, S.K. Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol. Asp. Med. 2013, 34, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras, C.; Nogueiras, R.; Dieguez, C.; Medina-Gomez, G.; Lopez, M. Hypothalamus and thermogenesis: Heating the BAT, browning the WAT. Mol. Cell. Endocrinol. 2016, 438, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Astafev, A.A.; Patel, S.A.; Kondratov, R.V. Calorie restriction effects on circadian rhythms in gene expression are sex dependent. Sci. Rep. 2017, 7, 9716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiehn, J.T.; Tsang, A.H.; Heyde, I.; Leinweber, B.; Kolbe, I.; Leliavski, A.; Oster, H. Circadian Rhythms in Adipose Tissue Physiology. Compr. Physiol. 2017, 7, 383–427. [Google Scholar] [CrossRef]
- Froy, O.; Garaulet, M. The Circadian Clock in White and Brown Adipose Tissue: Mechanistic, Endocrine, and Clinical Aspects. Endocr. Rev. 2018, 39, 261–273. [Google Scholar] [CrossRef]
- De Bacquer, D.; Van Risseghem, M.; Clays, E.; Kittel, F.; De Backer, G.; Braeckman, L. Rotating shift work and the metabolic syndrome: A prospective study. Int. J. Epidemiol. 2009, 38, 848–854. [Google Scholar] [CrossRef] [Green Version]
- Di Lorenzo, L.; De Pergola, G.; Zocchetti, C.; L’Abbate, N.; Basso, A.; Pannacciulli, N.; Cignarelli, M.; Giorgino, R.; Soleo, L. Effect of shift work on body mass index: Results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1353–1358. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, B.; Knutsson, A.; Lindahl, B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup. Environ. Med. 2001, 58, 747–752. [Google Scholar] [CrossRef] [Green Version]
- Mokdad, A.H.; Ford, E.S.; Bowman, B.A.; Dietz, W.H.; Vinicor, F.; Bales, V.S.; Marks, J.S. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003, 289, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Forrestel, A.C.; Miedlich, S.U.; Yurcheshen, M.; Wittlin, S.D.; Sellix, M.T. Chronomedicine and type 2 diabetes: Shining some light on melatonin. Diabetologia 2017, 60, 808–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, S. The arrival of circadian medicine. Nat. Rev. Endocrinol. 2019, 15, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Stenvers, D.J.; Jongejan, A.; Atiqi, S.; Vreijling, J.P.; Limonard, E.J.; Endert, E.; Baas, F.; Moerland, P.D.; Fliers, E.; Kalsbeek, A.; et al. Diurnal rhythms in the white adipose tissue transcriptome are disturbed in obese individuals with type 2 diabetes compared with lean control individuals. Diabetologia 2019, 62, 704–716. [Google Scholar] [CrossRef] [Green Version]
- Grimaldi, B.; Bellet, M.M.; Katada, S.; Astarita, G.; Hirayama, J.; Amin, R.H.; Granneman, J.G.; Piomelli, D.; Leff, T.; Sassone-Corsi, P. PER2 controls lipid metabolism by direct regulation of PPARgamma. Cell Metab. 2010, 12, 509–520. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, N.; Nesher, R.; Donath, M.Y.; Fraenkel, M.; Behar, V.; Magnan, C.; Ktorza, A.; Cerasi, E.; Leibowitz, G. Psammomys obesus, a model for environment-gene interactions in type 2 diabetes. Diabetes 2005, 54 (Suppl. 2), S137–S144. [Google Scholar] [CrossRef] [Green Version]
- Shafrir, E.; Gutman, A. Psammomys obesus of the Jerusalem colony: A model for nutritionally induced, non-insulin-dependent diabetes. J. Basic Clin. Physiol. Pharmacol. 1993, 4, 83–99. [Google Scholar] [CrossRef]
- Walder, K.R.; Fahey, R.P.; Morton, G.J.; Zimmet, P.Z.; Collier, G.R. Characterization of obesity phenotypes in Psammomys obesus (Israeli sand rats). Int. J. Exp. Diabetes Res. 2000, 1, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Bilu, C.; Zimmet, P.; Vishnevskia-Dai, V.; Einat, H.; Agam, G.; Grossman, E.; Kronfeld-Schor, N. Diurnality, Type 2 Diabetes, and Depressive-Like Behavior. J. Biol. Rhythms 2019, 34, 69–83. [Google Scholar] [CrossRef]
- Lekkas, D.; Paschos, G.K. The circadian clock control of adipose tissue physiology and metabolism. Auton. Neurosci. 2019, 219, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Rajala, M.W.; Scherer, P.E. Minireview: The adipocyte—At the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology 2003, 144, 3765–3773. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, J.P.; Richards, A.A.; Hickman, I.J.; Macdonald, G.A.; Prins, J.B. Adiponectin—A key adipokine in the metabolic syndrome. Diabetes Obes. Metab. 2006, 8, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, F. The Beige Adipocyte as a Therapy for Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 5058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohlgruber, A.; Lynch, L. Adipose tissue inflammation in the pathogenesis of type 2 diabetes. Curr. Diabetes Rep. 2015, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P. Endocrine and signalling role of adipose tissue: New perspectives on fat. Acta Physiol. Scand. 2005, 184, 285–293. [Google Scholar] [CrossRef]
- Scherer, P.E. The many secret lives of adipocytes: Implications for diabetes. Diabetologia 2019, 62, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Drucker, D.J. Never Waste a Good Crisis: Confronting Reproducibility in Translational Research. Cell Metab. 2016, 24, 348–360. [Google Scholar] [CrossRef] [Green Version]
- Tchkonia, T.; Giorgadze, N.; Pirtskhalava, T.; Tchoukalova, Y.; Karagiannides, I.; Forse, R.A.; DePonte, M.; Stevenson, M.; Guo, W.; Han, J.; et al. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R1286–R1296. [Google Scholar] [CrossRef] [Green Version]
- Fruhbeck, G.; Gomez-Ambrosi, J. Control of body weight: A physiologic and transgenic perspective. Diabetologia 2003, 46, 143–172. [Google Scholar] [CrossRef] [Green Version]
- Corrales, P.; Vidal-Puig, A.; Medina-Gomez, G. PPARs and Metabolic Disorders Associated with Challenged Adipose Tissue Plasticity. Int. J. Mol. Sci. 2018, 19, 2124. [Google Scholar] [CrossRef] [Green Version]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Zhang, Y.; Steger, D.J.; Schupp, M.; Schug, J.; Cristancho, A.; Feng, D.; Zhuo, D.; Stoeckert, C.J., Jr.; Liu, X.S.; et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008, 22, 2941–2952. [Google Scholar] [CrossRef] [Green Version]
- Kawai, M.; Rosen, C.J. PPARgamma: A circadian transcription factor in adipogenesis and osteogenesis. Nat. Rev. Endocrinol. 2010, 6, 629–636. [Google Scholar] [CrossRef] [Green Version]
- Dollet, L.; Zierath, J.R. Interplay between diet, exercise and the molecular circadian clock in orchestrating metabolic adaptations of adipose tissue. J. Physiol. 2019, 597, 1439–1450. [Google Scholar] [CrossRef] [Green Version]
- Christodoulides, C.; Vidal-Puig, A. PPARs and adipocyte function. Mol. Cell. Endocrinol. 2010, 318, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Rachid, T.L.; Silva-Veiga, F.M.; Graus-Nunes, F.; Bringhenti, I.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. Differential actions of PPAR-alpha and PPAR-beta/delta on beige adipocyte formation: A study in the subcutaneous white adipose tissue of obese male mice. PLoS ONE 2018, 13, e0191365. [Google Scholar] [CrossRef]
- Uldry, M.; Yang, W.; St-Pierre, J.; Lin, J.; Seale, P.; Spiegelman, B.M. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006, 3, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Oliver, W.R., Jr.; Shenk, J.L.; Snaith, M.R.; Russell, C.S.; Plunket, K.D.; Bodkin, N.L.; Lewis, M.C.; Winegar, D.A.; Sznaidman, M.L.; Lambert, M.H.; et al. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc. Natl. Acad. Sci. USA 2001, 98, 5306–5311. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.X.; Lee, C.H.; Tiep, S.; Yu, R.T.; Ham, J.; Kang, H.; Evans, R.M. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell 2003, 113, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Bortolotto, J.W.; Margis, R.; Ferreira, A.C.; Padoin, A.V.; Mottin, C.C.; Guaragna, R.M. Adipose tissue distribution and quantification of PPARbeta/delta and PPARgamma1-3 mRNAs: Discordant gene expression in subcutaneous, retroperitoneal and visceral adipose tissue of morbidly obese patients. Obes. Surg. 2007, 17, 934–940. [Google Scholar] [CrossRef]
- Compher, C.; Badellino, K.O. Obesity and inflammation: Lessons from bariatric surgery. J. Parenter Enteral. Nutr. 2008, 32, 645–647. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef]
- Fain, J.N.; Tichansky, D.S.; Madan, A.K. Transforming growth factor beta1 release by human adipose tissue is enhanced in obesity. Metabolism 2005, 54, 1546–1551. [Google Scholar] [CrossRef]
- Samad, F.; Yamamoto, K.; Pandey, M.; Loskutoff, D.J. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol. Med. 1997, 3, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Sousa-Pinto, B.; Goncalves, L.; Rodrigues, A.R.; Tomada, I.; Almeida, H.; Neves, D.; Gouveia, A.M. Characterization of TGF-beta expression and signaling profile in the adipose tissue of rats fed with high-fat and energy-restricted diets. J. Nutr. Biochem. 2016, 38, 107–115. [Google Scholar] [CrossRef]
- Nam, D.; Guo, B.; Chatterjee, S.; Chen, M.H.; Nelson, D.; Yechoor, V.K.; Ma, K. The adipocyte clock controls brown adipogenesis through the TGF-beta and BMP signaling pathways. J. Cell Sci. 2015, 128, 1835–1847. [Google Scholar] [CrossRef] [Green Version]
- Bremer, A.A.; Devaraj, S.; Afify, A.; Jialal, I. Adipose tissue dysregulation in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E1782–E1788. [Google Scholar] [CrossRef] [Green Version]
- Cancello, R.; Henegar, C.; Viguerie, N.; Taleb, S.; Poitou, C.; Rouault, C.; Coupaye, M.; Pelloux, V.; Hugol, D.; Bouillot, J.L.; et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 2005, 54, 2277–2286. [Google Scholar] [CrossRef] [Green Version]
- Vohl, M.C.; Sladek, R.; Robitaille, J.; Gurd, S.; Marceau, P.; Richard, D.; Hudson, T.J.; Tchernof, A. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes. Res. 2004, 12, 1217–1222. [Google Scholar] [CrossRef]
- Laforest, S.; Labrecque, J.; Michaud, A.; Cianflone, K.; Tchernof, A. Adipocyte size as a determinant of metabolic disease and adipose tissue dysfunction. Crit. Rev. Clin. Lab. Sci. 2015, 52, 301–313. [Google Scholar] [CrossRef]
- Bjorntorp, P.; Grimby, G.; Sanne, H.; Sjostrom, L.; Tibblin, G.; Wilhelmsen, L. Adipose tissue fat cell size in relation to metabolism in weight-stabile, physically active men. Horm. Metab. Res. 1972, 4, 182–186. [Google Scholar] [CrossRef]
- Salans, L.B.; Knittle, J.L.; Hirsch, J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J. Clin. Investig. 1968, 47, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef]
- Verboven, K.; Wouters, K.; Gaens, K.; Hansen, D.; Bijnen, M.; Wetzels, S.; Stehouwer, C.D.; Goossens, G.H.; Schalkwijk, C.G.; Blaak, E.E.; et al. Abdominal subcutaneous and visceral adipocyte size, lipolysis and inflammation relate to insulin resistance in male obese humans. Sci. Rep. 2018, 8, 4677. [Google Scholar] [CrossRef]
- O’Connell, J.; Lynch, L.; Cawood, T.J.; Kwasnik, A.; Nolan, N.; Geoghegan, J.; McCormick, A.; O’Farrelly, C.; O’Shea, D. The relationship of omental and subcutaneous adipocyte size to metabolic disease in severe obesity. PLoS ONE 2010, 5, e9997. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, N.; Cerasi, E.; Leibowitz, G. Diet-induced diabetes in the sand rat (Psammomys obesus). Methods Mol. Biol. 2012, 933, 89–102. [Google Scholar] [CrossRef]
- Levy, E.; Lalonde, G.; Delvin, E.; Elchebly, M.; Precourt, L.P.; Seidah, N.G.; Spahis, S.; Rabasa-Lhoret, R.; Ziv, E. Intestinal and hepatic cholesterol carriers in diabetic Psammomys obesus. Endocrinology 2010, 151, 958–970. [Google Scholar] [CrossRef] [Green Version]
- Spolding, B.; Connor, T.; Wittmer, C.; Abreu, L.L.; Kaspi, A.; Ziemann, M.; Kaur, G.; Cooper, A.; Morrison, S.; Lee, S.; et al. Rapid development of non-alcoholic steatohepatitis in Psammomys obesus (Israeli sand rat). PLoS ONE 2014, 9, e92656. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.T.; Prosser, H.C.; Dunn, L.L.; Vanags, L.Z.; Ridiandries, A.; Tsatralis, T.; Lecce, L.; Clayton, Z.E.; Yuen, S.C.; Robertson, S.; et al. High-Density Lipoproteins Rescue Diabetes-Impaired Angiogenesis via Scavenger Receptor Class B Type I. Diabetes 2016, 65, 3091–3103. [Google Scholar] [CrossRef] [Green Version]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.T.M.; Nankivell, V.A.; Bilu, C.; Shemesh, T.; Nicholls, S.J.; Zimmet, P.; Kronfeld-Schor, N.; Brown, A.; Bursill, C.A. High-Energy Diet and Shorter Light Exposure Drives Markers of Adipocyte Dysfunction in Visceral and Subcutaneous Adipose Depots of Psammomys obesus. Int. J. Mol. Sci. 2019, 20, 6291. https://doi.org/10.3390/ijms20246291
Tan JTM, Nankivell VA, Bilu C, Shemesh T, Nicholls SJ, Zimmet P, Kronfeld-Schor N, Brown A, Bursill CA. High-Energy Diet and Shorter Light Exposure Drives Markers of Adipocyte Dysfunction in Visceral and Subcutaneous Adipose Depots of Psammomys obesus. International Journal of Molecular Sciences. 2019; 20(24):6291. https://doi.org/10.3390/ijms20246291
Chicago/Turabian StyleTan, Joanne T.M., Victoria A. Nankivell, Carmel Bilu, Tomer Shemesh, Stephen J. Nicholls, Paul Zimmet, Noga Kronfeld-Schor, Alex Brown, and Christina A. Bursill. 2019. "High-Energy Diet and Shorter Light Exposure Drives Markers of Adipocyte Dysfunction in Visceral and Subcutaneous Adipose Depots of Psammomys obesus" International Journal of Molecular Sciences 20, no. 24: 6291. https://doi.org/10.3390/ijms20246291