Maternal Protein Restriction Differentially Alters the Expression of AQP1, AQP9 and VEGFr-2 in the Epididymis of Rat Offspring
Abstract
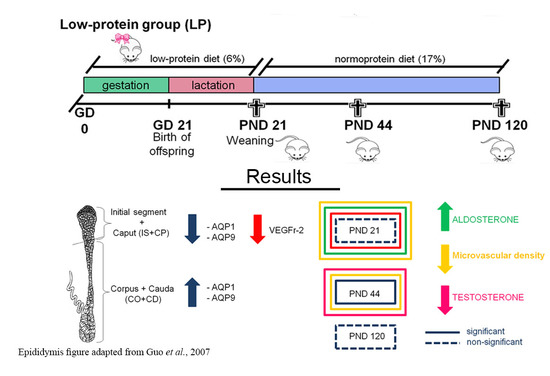
:1. Introduction
2. Results
2.1. A Maternal Low-Protein Diet Promotes Changes in Testosterone and Aldosterone Levels in an Age-Dependent-Manner
2.2. VEGFr-2 but Not VEGFa Expression is Altered by Maternal Protein Restriction
2.3. A Maternal Low-Protein Diet Decreases the Microvascular Density (MVD) on PND 21 and 44
2.4. The Impact of the Maternal Low-Protein Diet on AQP1 and AQP9 Immunolocalization
2.5. The Maternal Low-Protein Diet Changes AQP1 and AQP9 Expression in the Epididymis of the Offspring
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Hormonal Assay
4.4. Immunohistochemistry
4.5. Western Blot
4.6. Microvascular Density (MVD) Determination
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NP | Normoprotein |
| LP | Low-protein |
| PND | Postnatal day |
| AQP | Aquaporins |
| VEGFa | Vascular endothelial growth factor |
| VEGFr-2 | Vascular endothelial growth factor receptor 2 |
| MVD | Microvasculature density |
| IS+CP | Initial segment plus caput |
| CO+CD | Corpus plus cauda |
References
- McArdle, H.J.; Andersen, H.S.; Jones, H.; Gambling, L. Fetal programming: Causes and consequences as revealed by studies of dietary manipulation in rats—A review. Placenta 2006, 27, S56–S60. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; West, A.A.; Caudill, M.A. Maternal choline supplementation: A nutritional approach for improving offspring health? Trends Endocrinol. Metab. 2014, 25, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Zhang, L.B. Fetal programming of cardiac function and disease. Reprod. Sci. 2007, 14, 209–216. [Google Scholar] [CrossRef]
- Masuyama, H.; Hiramatsu, Y. Effects of a high-fat diet exposure in utero on the metabolic syndrome-like phenomenon in mouse offspring through epigenetic changes in adipocytokine gene expression. Endocrinology 2012, 153, 2823–2830. [Google Scholar] [CrossRef]
- Hales, C.N.; Barker, D.J. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. 1992. Int. J. Epidemiol. 2013, 42, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Watkins, A.J.; Sun, C.; Velazquez, M.A.; Smyth, N.R.; Eckert, J.J. Do little embryos make big decisions? How maternal dietary protein restriction can permanently change an embryo’s potential, affecting adult health. Reprod. Fertil. Dev. 2015, 27, 684–692. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Costello, P.M.; Lillycrop, K.A. The developmental environment, epigenetic biomarkers and long-term health. J. Dev. Orig. Health Dis. 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.J.; Reynolds, R.M.; Hardy, D.B. Developmental origins of health and disease: Current knowledge and potential mechanisms. Nutr. Rev. 2017, 75, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.; Bertie, J.; Gray, R.; Bannister, P.; Venkatesan, S.; Johnston, D.; Robinson, S. Increased leucine turnover in women during the third trimester of uncomplicated pregnancy. Metabolism 2004, 53, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gronert, M.S.; Ozanne, S.E. Experimental IUGR and later diabetes. J. Intern. Med. 2007, 261, 437–452. [Google Scholar] [CrossRef] [Green Version]
- Odle, J.; Jacobi, S.K.; Boyd, R.D.; Bauman, D.E.; Anthony, R.V.; Bazer, F.W.; Lock, A.L.; Serazin, A.C. The Potential Impact of Animal Science Research on Global Maternal and Child Nutrition and Health: A Landscape Review. Adv. Nutr. 2017, 8, 362–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zambrano, E.; Rodriguez-Gonzalez, G.L.; Guzman, C.; Garcia-Becerra, R.; Boeck, L.; Diaz, L.; Menjivar, M.; Larrea, F.; Nathanielsz, P.W. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J. Physiol. 2005, 563, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledo, F.C.; Perobelli, J.E.; Pedrosa, F.P.; Anselmo-Franci, J.A.; Kempinas, W.D. In utero protein restriction causes growth delay and alters sperm parameters in adult male rats. Reprod. Biol. Endocrinol. 2011, 9, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Gonzalez, G.L.; Vigueras-Villasenor, R.M.; Millan, S.; Moran, N.; Trejo, R.; Nathanielsz, P.W.; Larrea, F.; Zambrano, E. Maternal protein restriction in pregnancy and/or lactation affects seminiferous tubule organization in male rat offspring. J. Dev. Orig. Health Dis. 2012, 3, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gonzalez, G.L.; Reyes-Castro, L.A.; Vega, C.C.; Boeck, L.; Ibanez, C.; Nathanielsz, P.W.; Larrea, F.; Zambrano, E. Accelerated aging of reproductive capacity in male rat offspring of protein-restricted mothers is associated with increased testicular and sperm oxidative stress. Age 2014, 36, 9721. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, M.J.; Cockett, A.T.K. Structure and Function of the Epididymis. Urol. Res. 1986, 14, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Hermo, L.; Robaire, B. Epididymal cell types and their functions. In The Epididymis: From Molecules to Clinical Pratice; Robaire, B., Hinton, B.T., Eds.; Kluwer Academic/Plenum Publisher: New York, NY, USA, 2002; Volume 1, pp. 81–102. [Google Scholar]
- Gatti, J.L.; Castella, S.; Dacheux, F.; Ecroyd, H.; Metayer, S.; Thimon, V.; Dacheux, J.L. Post-testicular sperm environment and fertility. Anim. Reprod. Sci. 2004, 82–83, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.C.J. Aparelho Reprodutor Masculino. In Histologia Básica; Junqueira, L.C.C.J., Ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2011; Volume 1, pp. 415–431. [Google Scholar]
- Dacheux, J.L.; Dacheux, F. New insights into epididymal function in relation to sperm maturation. Reproduction 2014, 147, R27–R42. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.F.; He, R.H.; Sun, C.C.; Zhang, Y.; Meng, Q.X.; Ma, Y.Y. Function of aquaporins in female and male reproductive systems. Hum. Reprod. Update 2006, 12, 785–795. [Google Scholar] [CrossRef] [Green Version]
- Hermo, L.; Smith, C.E. Thirsty business: Cell, region, and membrane specificity of aquaporins in the testis, efferent ducts, and epididymis and factors regulating their expression. J. Androl. 2011, 32, 565–575. [Google Scholar] [CrossRef]
- Yeste, M.; Morato, R.; Rodriguez-Gil, J.E.; Bonet, S.; Prieto-Martinez, N. Aquaporins in the male reproductive tract and sperm: Functional implications and cryobiology. Reprod. Domest. Anim. 2017, 52 (Suppl. 4), 12–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agre, P. Aquaporin water channels (Nobel Lecture). Angew. Chem. 2004, 43, 4278–4290. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.; Pietrement, C.; Brown, D.; Breton, S. Segmental and cellular expression of aquaporins in the male excurrent duct. BBA-Biomembranes 2006, 1758, 1025–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domeniconi, R.F.; Orsi, A.M.; Justulin, L.A., Jr.; Leme Beu, C.C.; Felisbino, S.L. Immunolocalization of aquaporins 1, 2 and 7 in rete testis, efferent ducts, epididymis and vas deferens of adult dog. Cell Tissue Res. 2008, 332, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.Y.; Lee, W.M. Effects of spironolactone (aldosterone antagonist) on electrolyte and water content of the cauda epididymidis and fertility of male rats. Biol. Reprod. 1982, 27, 771–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, T.T.; Cesarini, D.M. The ability of the rat epididymis to concentrate spermatozoa. Responsiveness to aldosterone. J. Androl. 1983, 4, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Pearce, P.T.; Lipkevicius, O.R.; Funder, J.W. High affinity (type 1) aldosterone-binding sites in rat epididymis. Endocrinology 1986, 118, 2072–2075. [Google Scholar] [CrossRef]
- Setchell, B.P.; Sanchezpartida, L.G.; Chairussyuhur, A. Epididymal Constituents and Related Substances in the Storage of Spermatozoa—A Review. Reprod. Fertil. Dev. 1993, 5, 601–612. [Google Scholar] [CrossRef]
- Setchell, B.P. Anatomy, vasculature, innervation, and fluids of the male reproductive tract. In Knobil and Neill’s Physiology of Reproduction; Neill, J.D., Challis, J.R.G., de Kretser, D.M., Pfaff, D.W., Richards, J.S., Plant, T.M., Wassarman, P.M., Eds.; Elsevier: New York, NY, USA, 2006; Volume 1, pp. 771–825. [Google Scholar]
- Dai, Y.-P.; Gao, X.-Q.; Ma, X.-P.; Yue, Y.-Q. Effects of Chronic Exposure to Sodium Arsenite on Expressions of VEGF and VEGFR2 Proteins in the Epididymis of Rats. BioMed Res. Int. 2017, 2017, 2597256. [Google Scholar] [CrossRef]
- Ergun, S.; Luttmer, W.; Fiedler, W.; Holstein, A.F. Functional expression and localization of vascular endothelial growth factor and its receptors in the human epididymis. Biol. Reprod. 1998, 58, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Koch, S.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb. Perspect. Med. 2012, 2, a006502. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Aznar, A.; Muhl, L.; Gaengel, K. VEGF Receptor Tyrosine Kinases: Key Regulators of Vascular Function. Curr. Top. Dev. Biol. 2017, 123, 433–482. [Google Scholar] [CrossRef] [PubMed]
- Badran, H.H.; Hermo, L.S. Expression and regulation of aquaporins 1, 8, and 9 in the testis, efferent ducts, and epididymis of adult rats and during postnatal development. J. Androl. 2002, 23, 358–373. [Google Scholar]
- Oliveira, C.A.; Carnes, K.; Franca, L.R.; Hermo, L.; Hess, R.A. Aquaporin-1 and -9 are differentially regulated by oestrogen in the efferent ductule epithelium and initial segment of the epididymis. Biol. Cell 2005, 97, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Arrighi, S.; Aralla, M.; Genovese, P.; Picabea, N.; Bielli, A. Undernutrition during foetal to prepubertal life affects aquaporin 9 but not aquaporins 1 and 2 expression in the male genital tract of adult rats. Theriogenology 2010, 74, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.R.; Chuffa, L.G.A.; Martins, O.A.; Kremer, R.; Pinheiro, P.F.F.; de Mello, W.; Martinez, M.; Martinez, F.E.; Domeniconi, R.F. The expression of aquaporins 1 and 9 in adult rat epididymis is perturbed by chronic exposure to ethanol. Tissue Cell 2012, 44, 47–53. [Google Scholar] [CrossRef]
- Hermo, L.; Schellenberg, M.; Liu, L.Y.; Dayanandan, B.; Zhang, T.; Mandato, C.A.; Smith, C.E. Membrane domain specificity in the spatial distribution of aquaporins 5, 7, 9, and 11 in efferent ducts and epididymis of rats. J. Histochem. Cytochem. 2008, 56, 1121–1135. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Barker, D.J. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 2000, 71, 1344S–1352S. [Google Scholar] [CrossRef] [Green Version]
- Console, G.M.; Jurado, S.B.; Oyhenart, E.; Ferese, C.; Pucciarelli, H.; Gomez Dumm, C.L. Morphometric and ultrastructural analysis of different pituitary cell populations in undernourished monkeys. Braz. J. Med. Biol. Res. 2001, 34, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Peixoto-Silva, N.; Frantz, E.D.; Mandarim-de-Lacerda, C.A.; Pinheiro-Mulder, A. Maternal protein restriction in mice causes adverse metabolic and hypothalamic effects in the F1 and F2 generations. Br. J. Nutr. 2011, 106, 1364–1373. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, R.M.; Kerr, J.B.; McKinnell, C.; Millar, M. Temporal relationship between androgen-dependent changes in the volume of seminiferous tubule fluid, lumen size and seminiferous tubule protein secretion in rats. J. Reprod. Fertil. 1994, 101, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Robaire, B.; Hamzeh, M. Androgen action in the epididymis. J. Androl. 2011, 32, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.T.; Ewing, L.L.; Jones, C.E.; Howards, S.S.; Zegeye, B. Androgens in male rat reproductive tract fluids: Hypophysectomy and steroid replacement. Am. J. Physiol. 1985, 248, E274–E280. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Dinh Cat, A.; Jaisser, F. Extrarenal effects of aldosterone. Curr. Opin. Nephrol. Hypertens. 2012, 21, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.J.; Thomas, W. Aldosterone-induced protein kinase signalling and the control of electrolyte balance. Steroids 2018, 133, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Griffin, K.A.; Churchill, P.C.; Picken, M.; Webb, R.C.; Kurtz, T.W.; Bidani, A.K. Differential salt-sensitivity in the pathogenesis of renal damage in SHR and stroke prone SHR. Am. J. Hypertens. 2001, 14, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Rocha, R.; Chander, P.N.; Khanna, K.; Zuckerman, A.; Stier, C.T., Jr. Mineralocorticoid blockade reduces vascular injury in stroke-prone hypertensive rats. Hypertension 1998, 31, 451–458. [Google Scholar] [CrossRef] [PubMed]
- de Lima, M.C.; Scabora, J.E.; Lopes, A.; Mesquita, F.F.; Torres, D.; Boer, P.A.; Gontijo, J.A. Early changes of hypothalamic angiotensin II receptors expression in gestational protein-restricted offspring: Effect on water intake, blood pressure and renal sodium handling. J. Renin-Angiotensin-Aldosterone Syst. 2013, 14, 271–282. [Google Scholar] [CrossRef]
- Reynolds, R.M.; Walker, B.R.; Phillips, D.I.; Dennison, E.M.; Fraser, R.; Mackenzie, S.M.; Davies, E.; Connell, J.M. Programming of hypertension: Associations of plasma aldosterone in adult men and women with birthweight, cortisol, and blood pressure. Hypertension 2009, 53, 932–936. [Google Scholar] [CrossRef]
- Otani, L.; Sugimoto, N.; Kaji, M.; Murai, M.; Chang, S.J.; Kato, H.; Murakami, T. Role of the renin-angiotensin-aldosterone system in the enhancement of salt sensitivity caused by prenatal protein restriction in stroke-prone spontaneously hypertensive rats. J. Nutr. Biochem. 2012, 23, 892–899. [Google Scholar] [CrossRef]
- Gennari-Moser, C.; Khankin, E.V.; Escher, G.; Burkhard, F.; Frey, B.M.; Karumanchi, S.A.; Frey, F.J.; Mohaupt, M.G. Vascular endothelial growth factor-A and aldosterone: Relevance to normal pregnancy and preeclampsia. Hypertension 2013, 61, 1111–1117. [Google Scholar] [CrossRef]
- Ladage, D.; Schutzeberg, N.; Dartsch, T.; Krausgrill, B.; Halbach, M.; Zobel, C.; Muller-Ehmsen, J. Hyperaldosteronism is associated with a decrease in number and altered growth factor expression of endothelial progenitor cells in rats. Int. J. Cardiol. 2011, 149, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Walczak, C.; Gaignier, F.; Gilet, A.; Zou, F.; Thornton, S.N.; Ropars, A. Aldosterone increases VEGF-A production in human neutrophils through PI3K, ERK1/2 and p38 pathways. Biochim. Biophys. Acta 2011, 1813, 2125–2132. [Google Scholar] [CrossRef] [Green Version]
- Hsu, S.J.; Wang, S.S.; Huo, T.I.; Lee, F.Y.; Huang, H.C.; Chang, C.C.; Hsin, I.F.; Ho, H.L.; Lin, H.C.; Lee, S.D. The Impact of Spironolactone on the Severity of Portal-Systemic Collaterals and Hepatic Encephalopathy in Cirrhotic Rats. J. Pharmacol. Exp. Ther. 2015, 355, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Fujii, M.; Inoki, I.; Saga, M.; Morikawa, N.; Arakawa, K.; Inaba, S.; Yoshioka, K.; Konoshita, T.; Miyamori, I. Aldosterone inhibits endothelial morphogenesis and angiogenesis through the downregulation of vascular endothelial growth factor receptor-2 expression subsequent to peroxisome proliferator-activated receptor gamma. J. Steroid Biochem. Mol. Biol. 2012, 129, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.M.; Kirby, J.L.; Hinton, B.T. The Development of the Epididymis. In The Epididymis: From Molecules to Clinical Practice; Robaire, B., Hinton, B.T., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume 1, pp. 251–268. [Google Scholar]
- Pryor, J.L.; Hughes, C.; Foster, W.; Hales, B.F.; Robaire, B. Critical windows of exposure for children’s health: The reproductive system in animals and humans. Environ. Health Perspect. 2000, 108 (Suppl. 3), 491–503. [Google Scholar] [CrossRef]
- Picut, C.A.; Ziejewski, M.K.; Stanislaus, D. Comparative Aspects of Pre- and Postnatal Development of the Male Reproductive System. Birth Defects Res. 2018, 110, 190–227. [Google Scholar] [CrossRef] [PubMed]
- Reckmann, A.N.; Tomczyk, C.U.M.; Davidoff, M.S.; Michurina, T.V.; Arnhold, S.; Muller, D.; Mietens, A.; Middendorff, R. Nestin in the epididymis is expressed in vascular wall cells and is regulated during postnatal development and in case of testosterone deficiency. PLoS ONE 2018, 13, e0194585. [Google Scholar] [CrossRef] [PubMed]
- Elkjaer, M.; Vajda, Z.; Nejsum, L.N.; Kwon, T.; Jensen, U.B.; Amiry-Moghaddam, M.; Frokiaer, J.; Nielsen, S. Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochem. Biophys. Res. Commun. 2000, 276, 1118–1128. [Google Scholar] [CrossRef]
- Pastor-Soler, N.; Bagnis, C.; Sabolic, I.; Tyszkowski, R.; McKee, M.; Van Hoek, A.; Breton, S.; Brown, D. Aquaporin 9 expression along the male reproductive tract. Biol. Reprod. 2001, 65, 384–393. [Google Scholar] [CrossRef]
- Pastor-Soler, N.; Isnard-Bagnis, C.; Herak-Kramberger, C.; Sabolic, I.; Van Hoek, A.; Brown, D.; Breton, S. Expression of aquaporin 9 in the adult rat epididymal epithelium is modulated by androgens. Biol. Reprod. 2002, 66, 1716–1722. [Google Scholar] [CrossRef]
- Turner, T.T. Necessity’s Potion: Inorganic Ions and Small Organic Molecules in the Epididymal Lumen. In The Epididymis: From Molecules to Clinical Practice; Robaire, B., Hinton, B.T., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume 1, pp. 131–150. [Google Scholar]
- Moore, H.D.; Bedford, J.M. Short-term effects of androgen withdrawal on the structure of different epithelial cells in the rat epididymis. Anat. Rec. 1979, 193, 293–311. [Google Scholar] [CrossRef]
- Clulow, J.; Jones, R.C.; Hansen, L.A. Micropuncture and cannulation studies of fluid composition and transport in the ductuli efferentes testis of the rat: Comparisons with the homologous metanephric proximal tubule. Exp. Physiol. 1994, 79, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Dacheux, J.L.; Belleannee, C.; Guyonnet, B.; Labas, V.; Teixeira-Gomes, A.P.; Ecroyd, H.; Druart, X.; Gatti, J.L.; Dacheux, F. The contribution of proteomics to understanding epididymal maturation of mammalian spermatozoa. Syst. Biol. Reprod. Med. 2012, 58, 197–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermo, L.; Oko, R.; Robaire, B. Epithelial cells of the epididymis show regional variations with respect to the secretion of endocytosis of immobilin as revealed by light and electron microscope immunocytochemistry. Anat. Rec. 1992, 232, 202–220. [Google Scholar] [CrossRef]
- Li, Q.; Huang, D.L. Expression of aquaporin 1 in two types of animal model of endolymphatic hydrops]. Chin. J. Otorhinolaryngol. Head Neck Surg. 2007, 42, 181–184. [Google Scholar]
- Li, Q.; Huang, D.L. Effect of aldosterone on aquaporin and ionophorous protein expressions in guinea pig cochlea. J. South. Med. Univ. 2007, 27, 1918–1920. [Google Scholar]
- Chen, Y.C.; Cadnapaphornchai, M.A.; Summer, S.N.; Falk, S.; Li, C.; Wang, W.; Schrier, R.W. Molecular mechanisms of impaired urinary concentrating ability in glucocorticoid-deficient rats. J. Am. Soc. Nephrol. 2005, 16, 2864–2871. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Colombelli, K.T.; Santos, S.A.A.; Camargo, A.C.L.; Constantino, F.B.; Barquilha, C.N.; Rinaldi, J.C.; Felisbino, S.L.; Justulin, L.A. Impairment of microvascular angiogenesis is associated with delay in prostatic development in rat offspring of maternal protein malnutrition. Gen. Comp. Endocrinol. 2017, 246, 258–269. [Google Scholar] [CrossRef]
- Weibel, E.R.; Kistler, G.S.; Scherle, W.F. Practical stereological methods for morphometric cytology. J. Cell Biol. 1966, 30, 23–38. [Google Scholar] [CrossRef] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavariani, M.M.; de Mello Santos, T.; Pereira, D.N.; de Almeida Chuffa, L.G.; Felipe Pinheiro, P.F.; Scarano, W.R.; Domeniconi, R.F. Maternal Protein Restriction Differentially Alters the Expression of AQP1, AQP9 and VEGFr-2 in the Epididymis of Rat Offspring. Int. J. Mol. Sci. 2019, 20, 469. https://doi.org/10.3390/ijms20030469
Cavariani MM, de Mello Santos T, Pereira DN, de Almeida Chuffa LG, Felipe Pinheiro PF, Scarano WR, Domeniconi RF. Maternal Protein Restriction Differentially Alters the Expression of AQP1, AQP9 and VEGFr-2 in the Epididymis of Rat Offspring. International Journal of Molecular Sciences. 2019; 20(3):469. https://doi.org/10.3390/ijms20030469
Chicago/Turabian StyleCavariani, Marilia Martins, Talita de Mello Santos, Dhrielly Natalia Pereira, Luiz Gustavo de Almeida Chuffa, Patricia Fernanda Felipe Pinheiro, Wellerson Rodrigo Scarano, and Raquel Fantin Domeniconi. 2019. "Maternal Protein Restriction Differentially Alters the Expression of AQP1, AQP9 and VEGFr-2 in the Epididymis of Rat Offspring" International Journal of Molecular Sciences 20, no. 3: 469. https://doi.org/10.3390/ijms20030469