Variations in Accumulation of Lignin and Cellulose and Metabolic Changes in Seed Hull Provide Insight into Dehulling Characteristic of Tartary Buckwheat Seeds
Abstract
:1. Introduction
2. Results
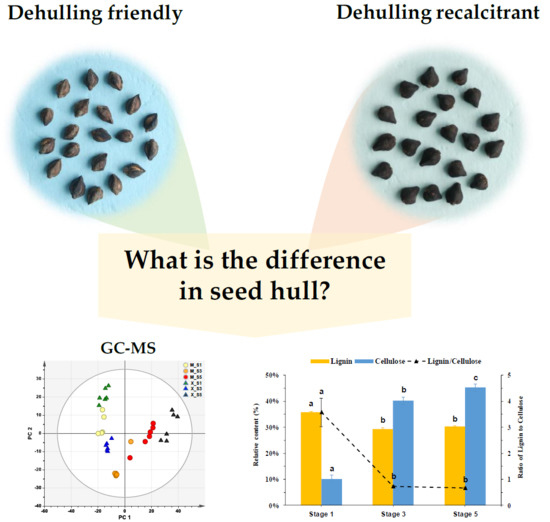
2.1. Dehulling efficiency, Lignin and Cellulose in Tartary Buckwheat Seeds
2.1.1. Dehulling Efficiency in Tartary Buckwheat Seeds
2.1.2. The Lignin and Cellulose Content in Seeds Hull
2.2. Metabolic Changes in the Hull of Developing Seeds
Identification of Differentially Expressed Metabolites
3. Discussion and Conclusion
4. Materials and Methods
4.1. Plant Materials
4.2. The Dehulling Efficiency
4.3. Analysis of Cellulose and Lignin
4.4. Metabolic Profiling
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT, (Statistics division of food and agriculture organization of the united nations). Available online: http://faostat3.fao.org/browse/Q/QC/E (accessed on 4 February 2016).
- Zhang, Z.L.; Zhou, M.L.; Tang, Y.; Li, F.L.; Tang, Y.X.; Shao, J.R.; Xue, W.T.; Wu, Y.M. Bioactive compounds in functional buckwheat food. Food Res. Int. 2012, 49, 389–395. [Google Scholar] [CrossRef]
- Qin, P.; Wang, Q.; Shan, F.; Hou, Z.; Ren, G. Nutritional composition and flavonoids content of flour from different buckwheat cultivars. Int. J. Food Sci. Technol. 2010, 45, 951–958. [Google Scholar] [CrossRef]
- Sytar, O.; Brestic, M.; Zivcak, M.; Phan Tran, L.S. The contribution of buckwheat genetic resources to health and dietary diversity. Curr. genomics 2016, 17, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Morishita, T.; Yamaguchi, H.; Degi, K. The contribution of polyphenols to antioxidative activity in common buckwheat and tartary buckwheat grain. Plant Prod. Sci. 2007, 10, 99–104. [Google Scholar] [CrossRef]
- Brajdes, C.; Vizireanu, C. Sprouted buckwheat an important vegetable source of antioxidants. Ann. Univ. Dun. Jos Galati. Fascicle VI. Food Technol. 2012, 36, 53–60. [Google Scholar]
- Nam, T.G.; Lee, S.M.; Park, J.H.; Kim, D.O.; Baek, N.I.; Eom, S.H. Flavonoid analysis of buckwheat sprouts. Food Chem. 2015, 170, 97–101. [Google Scholar] [CrossRef]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.-C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef]
- Zielińska, D.; Turemko, M.; Kwiatkowski, J.; Zieliński, H. Evaluation of flavonoid contents and antioxidant capacity of the aerial parts of common and tartary buckwheat plants. Molecules 2012, 17, 9668–9682. [Google Scholar] [CrossRef]
- Ahmed, A.; Khalid, N.; Ahmad, A.; Abbasi, N.A.; Latif, M.S.Z.; Randhawa, M.A. Phytochemicals and biofunctional properties of buckwheat: A review. J. Agric. Sci. 2014, 152, 349–369. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Zielinski, H.; Piskula, M.; Zielinska, D.; Szawara-Nowak, D. Buckwheat bioactive compounds, their derived phenolic metabolites and their health benefits. Mol. Nutr. Food Res. 2017, 61, 1600475. [Google Scholar] [CrossRef]
- Bonafaccia, G.; Marocchini, M.; Kreft, I. Composition and technological properties of the flour and bran from common and tartary buckwheat. Food Chem. 2003, 80, 9–15. [Google Scholar] [CrossRef]
- Lee, S.W.; Seo, J.M.; Lee, M.K.; Chun, J.H.; Antonisamy, P.; Arasu, M.V.; Suzuki, T.; Al-Dhabi, N.A.; Kim, S.J. Influence of different LED lamps on the production of phenolic compounds in common and Tartary buckwheat sprouts. Ind. Crops Prod. 2014, 54, 320–326. [Google Scholar] [CrossRef]
- Liu, C.L.; Chen, Y.S.; Yang, J.H.; Chiang, B.H. Antioxidant activity of tartary (Fagopyrum tataricum (L.) Gaertn.) and common (Fagopyrum esculentum Moench) buckwheat sprouts. J. Agric. Food Chem. 2007, 56, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Wijngaard, H.; Arendt, E.K. Buckwheat. Cereal Chem. 2006, 83, 391–401. [Google Scholar] [CrossRef]
- Jia, D.Y.; Yao, K.; Zhang, H.J. Advances in research on nutritional and functional components of tartary buckwheat. Cereal Feed Ind. 2012, 25–27. [Google Scholar]
- Pomeranz, Y.; Lorenz, K. Buckwheat: Structure, composition, and utilization. Crit. Rev. Food Sci. Nutr. 1983, 19, 213–258. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bastida, J.A.; Piskula, M.K.; Zieliñski, H. Recent advances in processing and development of buckwheat derived bakery and non-bakery products–A review. Pol. J. Food Nutri. Sci. 2015, 65, 9–20. [Google Scholar] [CrossRef]
- Chen, W.; Du, W.; Zheng, D.; Liu, G. Experimental study and parameter analysis on buckwheat huller. J. China Agric. Univ. 2017, 7, 107–114. [Google Scholar]
- Liu, Y.H.; Du, W.L.; Wu, Y.S. Experimental study on the optimization of the hulling method and main parameters of tartarian buckwheat. J. Agric. Mech. Res. 2008, 12, 131–133. [Google Scholar]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.-G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef]
- Tsai, H.; Deng, H.; Tsai, S.; Hsu, Y. Bioactivity comparison of extracts from various parts of common and tartary buckwheats: Evaluation of the antioxidant-and angiotensin-converting enzyme inhibitory activities. Chem. Cent. J. 2012, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, Y.; Liu, Q.; Bao, J.; Liu, Q. Identification and quantification of polyphenols in hull, bran and endosperm of common buckwheat (Fagopyrum esculentum) seeds. J. Funct. Foods 2017, 38, 363–369. [Google Scholar] [CrossRef]
- Dziedzic, K.; Górecka, D.; Kucharska, M.; Przybylska, B. Influence of technological process during buckwheat groats production on dietary fibre content and sorption of bile acids. Food Res. Int. 2012, 47, 279–283. [Google Scholar] [CrossRef]
- Biel, W.; Maciorowski, R. Evaluation of chemical composition and nutritional quality of buckwheat groat, bran and hull (Fagopyrum Esculentum Möench L.). Ital. J. Food Sci. 2013, 25, 384–389. [Google Scholar]
- Liu, M.; Zheng, T.; Ma, Z.; Wang, D.; Wang, T.; Sun, R.; He, Z.; Peng, J.; Chen, H. The complete chloroplast genome sequence of Tartary Buckwheat Cultivar Miqiao 1 (Fagopyrum tataricum Gaertn.). Mitochondrial DNA B Resour. 2016, 1, 577–578. [Google Scholar] [CrossRef]
- Song, C.; Xiang, D.-B.; Yan, L.; Song, Y.; Zhao, G.; Wang, Y.-H.; Zhang, B.-L. Changes in seed growth, levels and distribution of flavonoids during tartary buckwheat seed development. Plant Prod. Sci. 2016, 19, 518–527. [Google Scholar] [CrossRef] [Green Version]
- Khan, F.; Pilpel, N.; Ingham, S. The effect of moisture on the density, compaction and tensile strength of microcrystalline cellulose. Powder Technol. 1988, 54, 161–164. [Google Scholar] [CrossRef]
- Dietrych-Szostak, D.; Oleszek, W. Effect of Processing on the flavonoid content in Buckwheat (Fagopyrum e sculentum Möench) grain. J. Agric. Food Chem. 1999, 47, 4384–4387. [Google Scholar] [CrossRef]
- Chabannes, M.; Ruel, K.; Yoshinaga, A.; Chabbert, B.; Jauneau, A.; Joseleau, J.P.; Boudet, A.M. In situ analysis of lignins in transgenic tobacco reveals a differential impact of individual transformations on the spatial patterns of lignin deposition at the cellular and subcellular levels. Plant J. 2001, 28, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Jones, L.; Ennos, A.R.; Turner, S.R. Cloning and characterization of irregular xylem4 (irx4): A severely lignin-deficient mutant of Arabidopsis. The Plant Journal 2001, 26, 205–216. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Chen, X.; Yin, Y.; Lu, K.; Yang, W.; Tang, Y.; Wang, Z. Lodging resistance of winter wheat (Triticum aestivum L.): Lignin accumulation and its related enzymes activities due to the application of paclobutrazol or gibberellin acid. Field Crop. Res. 2014, 157, 1–7. [Google Scholar] [CrossRef]
- Wang, C.; Hu, D.; Liu, X.; She, H.; Ruan, R.; Yang, H.; Yi, Z.; Wu, D. Effects of uniconazole on the lignin metabolism and lodging resistance of culm in common buckwheat (Fagopyrum esculentum M.). Field Crop. Res. 2015, 180, 46–53. [Google Scholar] [CrossRef]
- Genet, M.; Stokes, A.; Salin, F.; Mickovski, S.B.; Fourcaud, T.; Dumail, J.F.; van Beek, R. The influence of cellulose content on tensile strength in tree roots. Plant Soil 2005, 278, 1–9. [Google Scholar] [CrossRef]
- Hu, W.J.; Harding, S.A.; Lung, J.; Popko, J.L.; Ralph, J.; Stokke, D.D.; Tsai, C.J.; Chiang, V.L. Repression of lignin biosynthesis promotes cellulose accumulation and growth in transgenic trees. Nat. Biotechnol. 1999, 17, 808–812. [Google Scholar] [CrossRef]
- Timell, T.E. Compression Wood in Gymnosperms; Springer: Heidelberg, Germany, 1986; pp. 25–27. [Google Scholar] [CrossRef]
- Aloni, R.; Tollier, M.T.; Monties, B. The role of auxin and gibberellin in controlling lignin formation in primary phloem fibers and in xylem of coleus blumei stems. Plant Physiol. 1990, 94, 1743–1747. [Google Scholar] [CrossRef]
- Kärkönen, A.; Koutaniemi, S. Lignin biosynthesis studies in plant tissue cultures. J. Integr. Plant Biol. 2010, 52, 176–185. [Google Scholar] [CrossRef]
- Miao, Z.H.; Fortune, J.A.; Gallagher, J. Anatomical structure and nutritive value of lupin seed coats. Aust. J. Agric. Res. 2001, 52, 985–993. [Google Scholar] [CrossRef]
- Ma, F.; Cholewa, E.; Mohamed, T.; Peterson, C.A.; Gijzen, M. Cracks in the palisade cuticle of soybean seed coats correlate with their permeability to water. Ann. Bot. 2004, 94, 213–228. [Google Scholar] [CrossRef]
- Harris, W.M. Comparative ultrastructure of developing seed coats of ‘hard-seeded’ and ‘soft-seeded’ varieties of soybean, Glycine max (L.) Merr. Int. J. Plant Sci. 1987, 148, 324–331. [Google Scholar] [CrossRef]
- Smýkal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant Sci. 2014, 5, 351. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Ong, R.G.; Mei, C.; Sticklen, M. Lignin down-regulation of Zea mays via dsRNAi and klason lignin analysis. J. Vis. Exp. 2014, 89, 51340. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P. v.; Robertson, J.; Lewis, B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, M.; Zhou, H.; Zhou, X.; Wang, Y. Metabolite profiles of Populus in response to pathogen stress. Biochem. Biophys. Res. Commun. 2015, 465, 421–426. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.; Ma, C.; Xiang, D. Variations in Accumulation of Lignin and Cellulose and Metabolic Changes in Seed Hull Provide Insight into Dehulling Characteristic of Tartary Buckwheat Seeds. Int. J. Mol. Sci. 2019, 20, 524. https://doi.org/10.3390/ijms20030524
Song C, Ma C, Xiang D. Variations in Accumulation of Lignin and Cellulose and Metabolic Changes in Seed Hull Provide Insight into Dehulling Characteristic of Tartary Buckwheat Seeds. International Journal of Molecular Sciences. 2019; 20(3):524. https://doi.org/10.3390/ijms20030524
Chicago/Turabian StyleSong, Chao, Chengrui Ma, and Dabing Xiang. 2019. "Variations in Accumulation of Lignin and Cellulose and Metabolic Changes in Seed Hull Provide Insight into Dehulling Characteristic of Tartary Buckwheat Seeds" International Journal of Molecular Sciences 20, no. 3: 524. https://doi.org/10.3390/ijms20030524