Mechanisms of Toxicity of Industrially Relevant Silicomanganese Dust on Human 1321N1 Astrocytoma Cells: An In Vitro Study
Abstract
:1. Introduction
2. Results
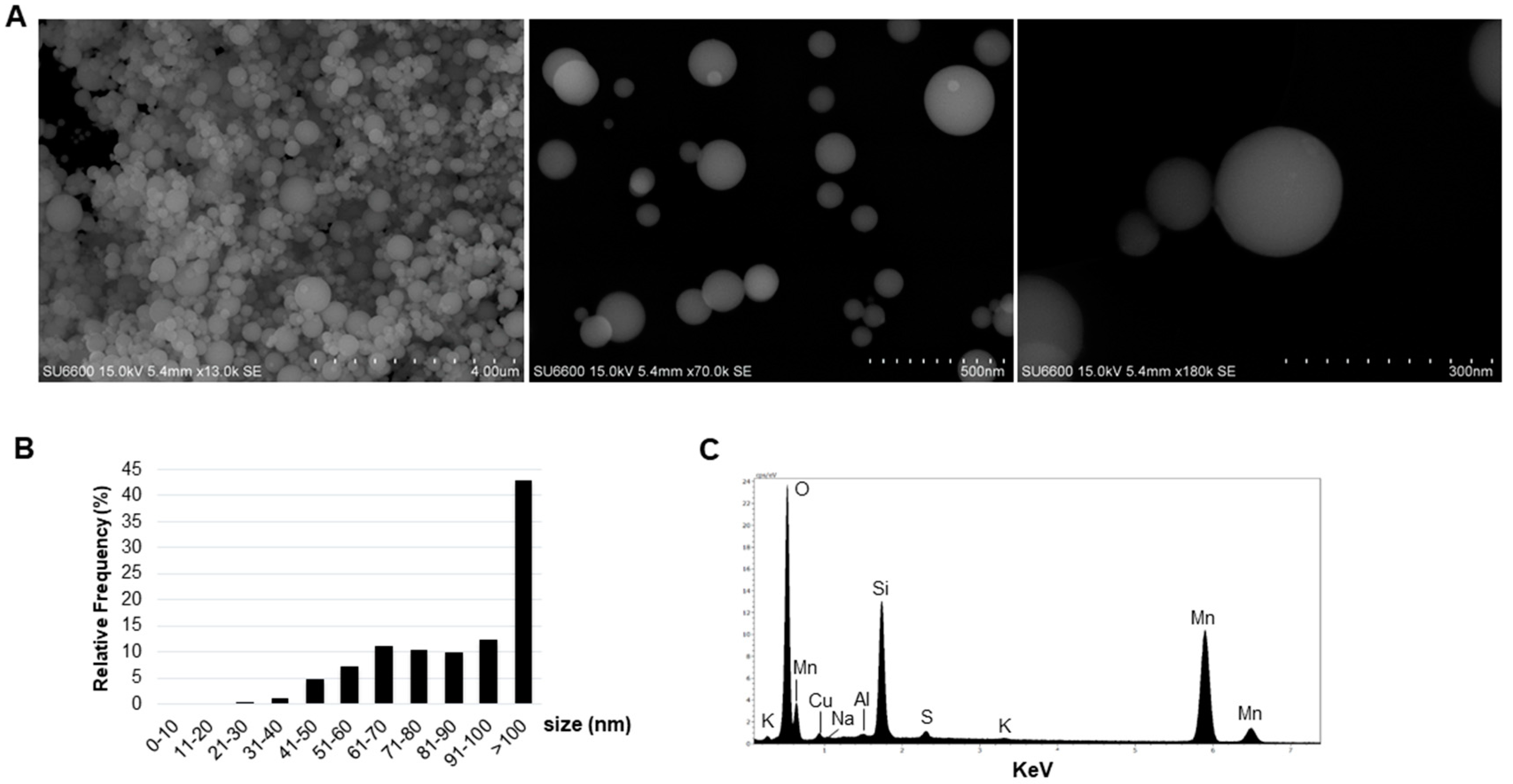
2.1. Characteristics of the Dust
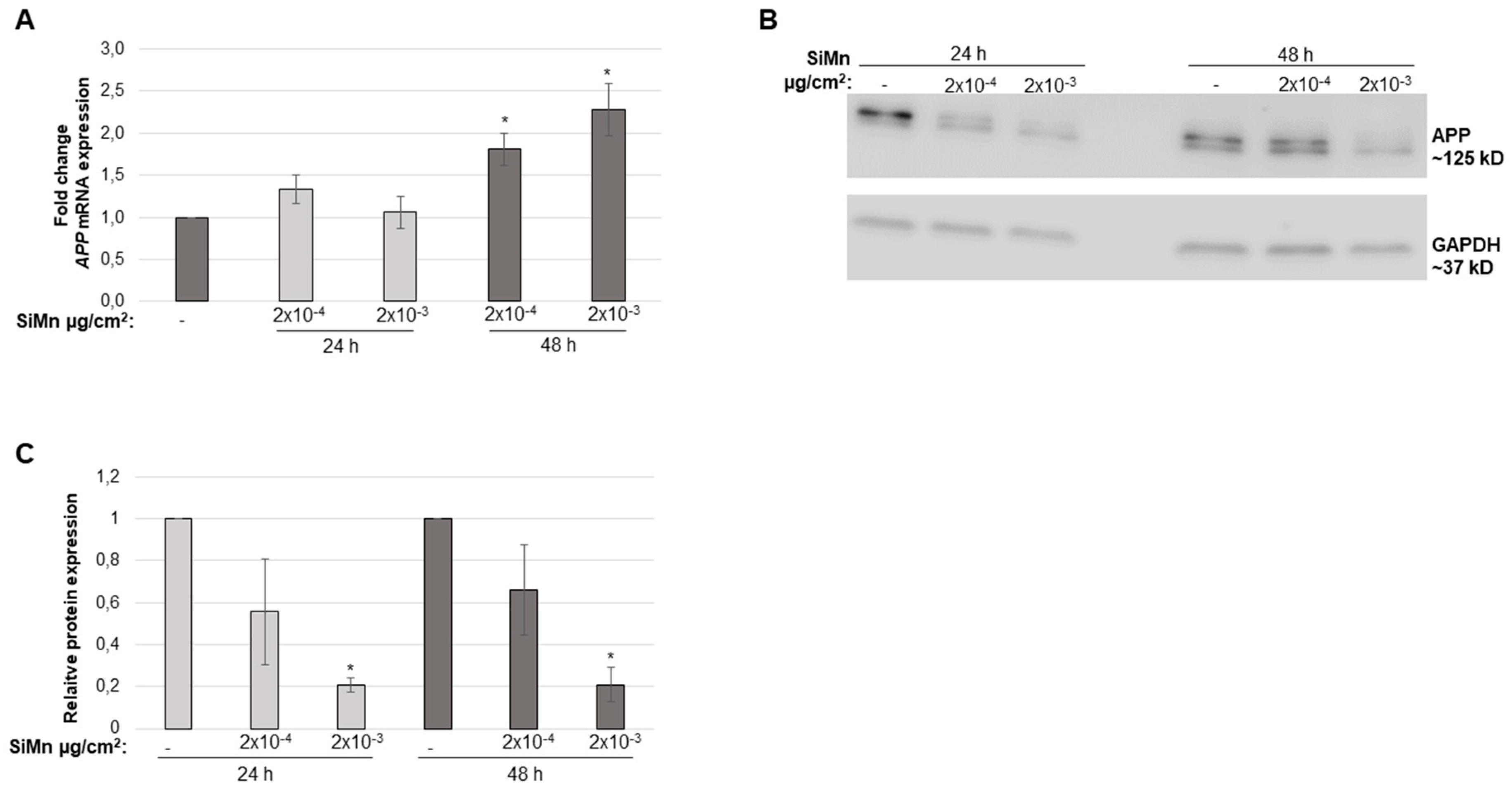
2.2. Cellular Responses of Exposure to SiMn Dust
3. Discussion
4. Materials and Methods
4.1. Generation of the SiMn Dust
4.2. Preparation of the Dust for Characterization and Cell Culture Experiments
4.3. SiMn Dust Characterization
4.3.1. Dynamic Light Scattering
4.3.2. Scanning Electron Microscopy
4.4. Cells and Cell Culture
4.5. Cytotoxicity Assay
4.6. Quantitative PCR (qPCR) for Measurement of APP Gene Expression
4.7. Detection of APP by Western Blot Analysis
4.8. Apoptosis Protein Array
4.9. Functional Assay of Gap Junctional Intercellular Communication (GJIC) by Scrape Loading
4.10. Statistics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kero, I.; Naess, M.K.; Tranell, G. Particle size distributions of particulate emissions from the ferroalloy industry evaluated by electrical low pressure impactor (ELPI). J. Occup. Environ. Hyg. 2015, 12, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kero, I.; Tranell, G. Active Oxidation and Fume Formation from Liquid SiMn. In 7th International Symposium on High-Temperature Metallurgical Processing; Springer International Publishing: Cham, Switzerland, 2016; pp. 77–84. [Google Scholar]
- Ma, Y.; Kero, I.; Tranell, G. Fume Formation from Oxidation of Liquid SiMn Alloy. Oxid. Met. 2018, 89, 211–231. [Google Scholar] [CrossRef]
- Kero, I.; Grådahl, S.; Tranell, G. Airborne Emissions from Si/FeSi Production. JOM 2017, 69, 365–380. [Google Scholar] [CrossRef]
- Kero, I.; Slizovskiy, D.; Wittgens, B.; Tranell, G. Fume Formation from Liquid Ferromanganese. In Sustainable Industrial Processing Summit (SIPS)—Takano International Symposium on Metals and Alloys; Flogen Stars Outreach: Antalya, Turkey, 2015. [Google Scholar]
- Heusinkveld, H.J.; Wahle, T.; Campbell, A.; Westerink, R.H.S.; Tran, L.; Johnston, H.; Stone, V.; Cassee, F.R.; Schins, R.P.F. Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology 2016, 56 (Suppl. C), 94–106. [Google Scholar] [CrossRef] [PubMed]
- Kreyling, W.G. Discovery of unique and ENM- specific pathophysiologic pathways: Comparison of the translocation of inhaled iridium nanoparticles from nasal epithelium versus alveolar epithelium towards the brain of rats. Toxicol. Appl. Pharmacol. 2016, 299, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Oberdorster, G.; Elder, A.; Rinderknecht, A. Nanoparticles and the brain: Cause for concern? J. Nanosci. Nanotechnol. 2009, 9, 4996–5007. [Google Scholar] [CrossRef] [PubMed]
- Cowan, D.M.; Fan, Q.; Zou, Y.; Shi, X.; Chen, J.; Aschner, M.; Rosenthal, F.S.; Zheng, W. Manganese exposure among smelting workers: Blood manganese-iron ratio as a novel tool for manganese exposure assessment. Biomarkers 2009, 14, 3–16. [Google Scholar] [CrossRef]
- Cowan, D.M.; Zheng, W.; Zou, Y.; Shi, X.; Chen, J.; Rosenthal, F.S.; Fan, Q. Manganese exposure among smelting workers: Relationship between blood manganese-iron ratio and early onset neurobehavioral alterations. Neurotoxicology 2009, 30, 1214–1222. [Google Scholar] [CrossRef]
- Dydak, U.; Jiang, Y.M.; Long, L.L.; Zhu, H.; Chen, J.; Li, W.M.; Edden, R.A.; Hu, S.; Fu, X.; Long, Z.; et al. In vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environ. Health Perspect. 2011, 119, 219–224. [Google Scholar] [CrossRef]
- Jiang, Y.; Zheng, W.; Long, L.; Zhao, W.; Li, X.; Mo, X.; Lu, J.; Fu, X.; Li, W.; Liu, S.; et al. Brain magnetic resonance imaging and manganese concentrations in red blood cells of smelting workers: Search for biomarkers of manganese exposure. Neurotoxicology 2007, 28, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Elder, A.; Gelein, R.; Silva, V.; Feikert, T.; Opanashuk, L.; Carter, J.; Potter, J.; Maynard, A.; Ito, Y.; Finkelstein, J.; et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 2006, 114, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Alarifi, S.; Ali, D.; Alkahtani, S. Oxidative Stress-Induced DNA Damage by Manganese Dioxide Nanoparticles in Human Neuronal Cells. BioMed Res. Int. 2017, 2017, 5478790. [Google Scholar] [CrossRef] [PubMed]
- Tuschl, K.; Mills, P.B.; Clayton, P.T. Manganese and the brain. Int. Rev. Neurobiol. 2013, 110, 277–312. [Google Scholar] [PubMed]
- Sarkar, S.; Malovic, E.; Harischandra, D.S.; Ngwa, H.A.; Ghosh, A.; Hogan, C.; Rokad, D.; Zenitsky, G.; Jin, H.; Anantharam, V.; et al. Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes. Neurotoxicology 2018, 64, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, C.; Sun, J.; Xue, Y. Neurotoxicity of silica nanoparticles: Brain localization and dopaminergic neurons damage pathways. ACS Nano 2011, 5, 4476–4489. [Google Scholar] [CrossRef] [PubMed]
- Kretowski, R.; Kusaczuk, M.; Naumowicz, M.; Kotynska, J.; Szynaka, B.; Cechowska-Pasko, M. The Effects of Silica Nanoparticles on Apoptosis and Autophagy of Glioblastoma Cell Lines. Nanomaterials 2017, 7, 230. [Google Scholar] [CrossRef] [PubMed]
- Boulay, A.C.; Gilbert, A.; Oliveira Moreira, V.; Blugeon, C.; Perrin, S.; Pouch, J.; Le Crom, S.; Ducos, B.; Cohen-Salmon, M. Connexin 43 Controls the Astrocyte Immunoregulatory Phenotype. Brain Sci. 2018, 8, 50. [Google Scholar] [CrossRef]
- Pekny, M.; Wilhelmsson, U.; Pekna, M. The dual role of astrocyte activation and reactive gliosis. Neurosci. Lett. 2014, 565, 30–38. [Google Scholar] [CrossRef]
- Liu, X.; Sui, B.; Sun, J. Blood-brain barrier dysfunction induced by silica NPs in vitro and in vivo: Involvement of oxidative stress and Rho-kinase/JNK signaling pathways. Biomaterials 2017, 121, 64–82. [Google Scholar] [CrossRef]
- Ezan, P.; Andre, P.; Cisternino, S.; Saubamea, B.; Boulay, A.C.; Doutremer, S.; Thomas, M.A.; Quenech’du, N.; Giaume, C.; Cohen-Salmon, M. Deletion of astroglial connexins weakens the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1457–1467. [Google Scholar] [CrossRef]
- Thomassen, Y.; Ellingsen, D.G.; Hetland, S.; Sand, G. Chemical speciation and sequential extraction of Mn in workroom aerosols: Analytical methodology and results from a field study in Mn alloy plants. J. Environ. Monit. 2001, 3, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Todd, G.D.; Roney, N.; Crawford, J.; Coles, C.; McClure, P.R.; Garey, J.D.; Zaccaria, K.; Citra, M. Toxicological Profile for Manganese; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012.
- Ordonez-Librado, J.L.; Gutierrez-Valdez, A.L.; Colin-Barenque, L.; Anaya-Martinez, V.; Diaz-Bech, P.; Avila-Costa, M.R. Inhalation of divalent and trivalent manganese mixture induces a Parkinson’s disease model: Immunocytochemical and behavioral evidences. Neuroscience 2008, 155, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Park, R.M.; Bouchard, M.F.; Baldwin, M.; Bowler, R.; Mergler, D. Respiratory manganese particle size, time-course and neurobehavioral outcomes in workers at a manganese alloy production plant. Neurotoxicology 2014, 45, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murugadoss, S.; Lison, D.; Godderis, L.; Van Den Brule, S.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P.H. Toxicology of silica nanoparticles: An update. Arch. Toxicol. 2017, 91, 2967–3010. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.I.; Katayama, T.; Prat, A. Glial influence on the blood brain barrier. Glia 2013, 61, 1939–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banks, W.A. The blood-brain barrier in neuroimmunology: Tales of separation and assimilation. Brain Behav. Immun. 2015, 44, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Campos-Bedolla, P.; Walter, F.R.; Veszelka, S.; Deli, M.A. Role of the blood-brain barrier in the nutrition of the central nervous system. Arch. Med. Res. 2014, 45, 610–638. [Google Scholar] [CrossRef]
- Finsterwald, C.; Magistretti, P.J.; Lengacher, S. Astrocytes: New Targets for the Treatment of Neurodegenerative Diseases. Curr. Pharm. Des. 2015, 21, 3570–3581. [Google Scholar] [CrossRef]
- Guillamon-Vivancos, T.; Gomez-Pinedo, U.; Matias-Guiu, J. Astrocytes in neurodegenerative diseases (I): Function and molecular description. Neurologia 2015, 30, 119–129. [Google Scholar] [CrossRef]
- Phatnani, H.; Maniatis, T. Astrocytes in neurodegenerative disease. Cold Spring Harb. Perspect. Biol. 2015, 7. [Google Scholar] [CrossRef]
- Hanada, S.; Fujioka, K.; Inoue, Y.; Kanaya, F.; Manome, Y.; Yamamoto, K. Cell-based in vitro blood-brain barrier model can rapidly evaluate nanoparticles’ brain permeability in association with particle size and surface modification. Int. J. Mol. Sci. 2014, 15, 1812–1825. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lin, B.; Shao, W.; Zhu, Z.; Ji, T.; Yang, C. In vitro and in vivo studies on the transport of PEGylated silica nanoparticles across the blood-brain barrier. ACS Appl. Mater. Interfaces 2014, 6, 2131–2136. [Google Scholar] [CrossRef]
- Ye, D.; Anguissola, S.; O’Neill, T.; Dawson, K.A. Immunogold labeling reveals subcellular localisation of silica nanoparticles in a human blood-brain barrier model. Nanoscale 2015, 7, 10050–10058. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Liao, S.L. Oxidative stress involves in astrocytic alterations induced by manganese. Exp. Neurol. 2002, 175, 216–225. [Google Scholar] [CrossRef]
- Erikson, K.M.; Dobson, A.W.; Dorman, D.C.; Aschner, M. Manganese exposure and induced oxidative stress in the rat brain. Sci. Total Environ. 2004, 334–335, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Aschner, J.L.; dos Santos, A.P.; Aschner, M. Mitochondrial-dependent manganese neurotoxicity in rat primary astrocyte cultures. Brain Res. 2008, 1203, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Xie, L.; Fang, C.J.; Yang, H.; Wang, Y.J.; Zhen, X.Y.; Yan, C.H.; Wang, Y.; Zhao, M.; Peng, S. Implications for blood-brain-barrier permeability, in vitro oxidative stress and neurotoxicity potential induced by mesoporous silica nanoparticles: Effects of surface modification. RSC Adv. 2016, 6, 2800–2809. [Google Scholar] [CrossRef]
- Arnoldussen, Y.J.; Kringlen Ervik, T.; Baarnes Eriksen, M.; Kero, I.; Skaug, V.; Zienolddiny, S. Cellular reponses of industrially relevant silica dust on human glial cells in vitro. Int. J. Mol. Sci. 2019, 20, 358. [Google Scholar] [CrossRef]
- Almenar-Queralt, A.; Falzone, T.L.; Shen, Z.; Lillo, C.; Killian, R.L.; Arreola, A.S.; Niederst, E.D.; Ng, K.S.; Kim, S.N.; Briggs, S.P.; et al. UV irradiation accelerates amyloid precursor protein (APP) processing and disrupts APP axonal transport. J. Neurosci. 2014, 34, 3320–3339. [Google Scholar] [CrossRef]
- Batarseh, Y.S.; Duong, Q.V.; Mousa, Y.M.; Al Rihani, S.B.; Elfakhri, K.; Kaddoumi, A. Amyloid-beta and Astrocytes Interplay in Amyloid-beta Related Disorders. Int. J. Mol. Sci. 2016, 17, 338. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.R.; Li, Y.M. The role of astrocytes in amyloid production and Alzheimer’s disease. Open Biol. 2017, 7, 170228. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; He, C.; Li, J.; Chen, H.; Ma, Q.; Sui, X.; Tian, S.; Ying, M.; Zhang, Q.; Luo, Y.; et al. Uptake of silica nanoparticles: Neurotoxicity and Alzheimer-like pathology in human SK-N-SH and mouse neuro2a neuroblastoma cells. Toxicol. Lett. 2014, 229, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.; Sin, W.C.; Lozinsky, S.; Bechberger, J.; Vega, J.L.; Guo, X.Q.; Saez, J.C.; Naus, C.C. Gap junction intercellular communication mediated by connexin43 in astrocytes is essential for their resistance to oxidative stress. J. Biol. Chem. 2014, 289, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Sin, W.C.; Aftab, Q.; Bechberger, J.; Leung, J.H.; Chen, H.; Naus, C.C. Astrocytes promote glioma invasion via the gap junction protein connexin43. Oncogene 2016, 35, 1504–1516. [Google Scholar] [CrossRef]
- Soroceanu, L.; Manning, T.J., Jr.; Sontheimer, H. Reduced expression of connexin-43 and functional gap junction coupling in human gliomas. Glia 2001, 33, 107–117. [Google Scholar] [CrossRef]
- Suadicani, S.O.; Flores, C.E.; Urban-Maldonado, M.; Beelitz, M.; Scemes, E. Gap junction channels coordinate the propagation of intercellular Ca2+ signals generated by P2Y receptor activation. Glia 2004, 48, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Arnoldussen, Y.J.; Anmarkrud, K.H.; Skaug, V.; Apte, R.N.; Haugen, A.; Zienolddiny, S. Effects of carbon nanotubes on intercellular communication and involvement of IL-1 genes. J. Cell Commun. Signal. 2016, 10, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Jensen, K.; Kembouche, Y.; Christiansen, E.; Jacobsen, N.; Wallin, H.; Guiot, C. The Generic NANOGENOTOX Dispersion Protocol. Standard Operation Procedure (SOP) and Background Documentation. Final Protocol for Producing Suitable Manufactured Nanomaterial Exposure Media. Available online: https://www.anses.fr/en/system/files/nanogenotox_deliverable_5.pdf. (accessed on 5 February 2019).
- Phuyal, S.; Kasem, M.; Rubio, L.; Karlsson, H.L.; Marcos, R.; Skaug, V.; Zienolddiny, S. Effects on human bronchial epithelial cells following low-dose chronic exposure to nanomaterials: A 6-month transformation study. Toxicol. In Vitro 2017, 44, 230–240. [Google Scholar] [CrossRef]
- Macintyre, E.H.; Wintersgill, C.J.; Thormar, H. The establishment of a line of visna virus-producing human astrocytes (V-1181N1). Med. Res. Eng. 1972, 11, 7–13. [Google Scholar]
- Arnoldussen, Y.J.; Skogstad, A.; Skaug, V.; Kasem, M.; Haugen, A.; Benker, N.; Weinbruch, S.; Apte, R.N.; Zienolddiny, S. Involvement of IL-1 genes in the cellular responses to carbon nanotube exposure. Cytokine 2015, 73, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Opsahl, H.; Rivedal, E. Quantitative determination of gap junction intercellular communication by scrape loading and image analysis. Cell Adhes. Commun. 2000, 7, 367–375. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnoldussen, Y.J.; Kringlen Ervik, T.; Samulin Erdem, J.; Kero, I.; Baarnes Eriksen, M.; Skaug, V.; Zienolddiny, S. Mechanisms of Toxicity of Industrially Relevant Silicomanganese Dust on Human 1321N1 Astrocytoma Cells: An In Vitro Study. Int. J. Mol. Sci. 2019, 20, 740. https://doi.org/10.3390/ijms20030740
Arnoldussen YJ, Kringlen Ervik T, Samulin Erdem J, Kero I, Baarnes Eriksen M, Skaug V, Zienolddiny S. Mechanisms of Toxicity of Industrially Relevant Silicomanganese Dust on Human 1321N1 Astrocytoma Cells: An In Vitro Study. International Journal of Molecular Sciences. 2019; 20(3):740. https://doi.org/10.3390/ijms20030740
Chicago/Turabian StyleArnoldussen, Yke Jildouw, Torunn Kringlen Ervik, Johanna Samulin Erdem, Ida Kero, Mina Baarnes Eriksen, Vidar Skaug, and Shanbeh Zienolddiny. 2019. "Mechanisms of Toxicity of Industrially Relevant Silicomanganese Dust on Human 1321N1 Astrocytoma Cells: An In Vitro Study" International Journal of Molecular Sciences 20, no. 3: 740. https://doi.org/10.3390/ijms20030740




