Vascular Effects of Photodynamic Therapy with Curcumin in a Chorioallantoic Membrane Model
Abstract
:1. Introduction
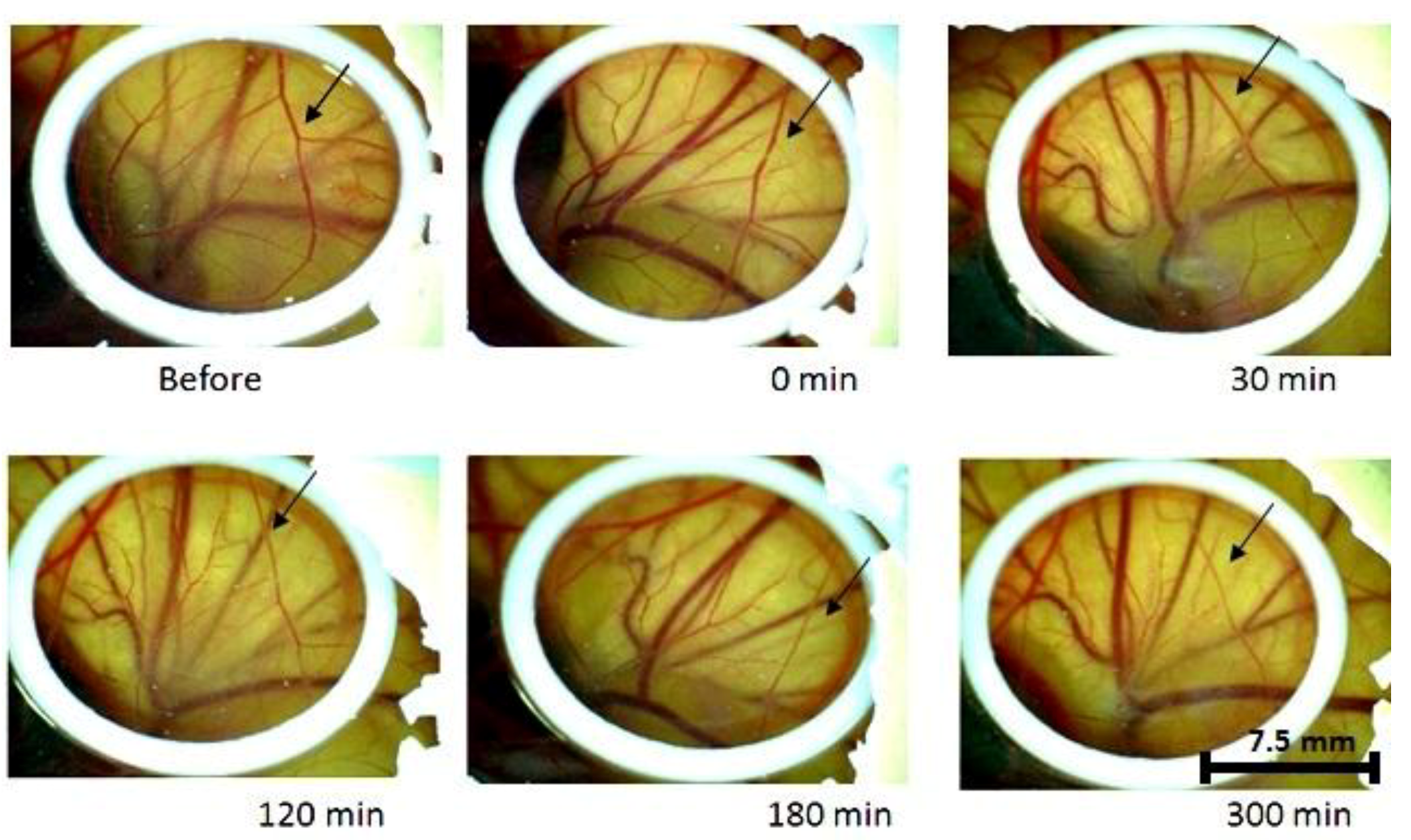
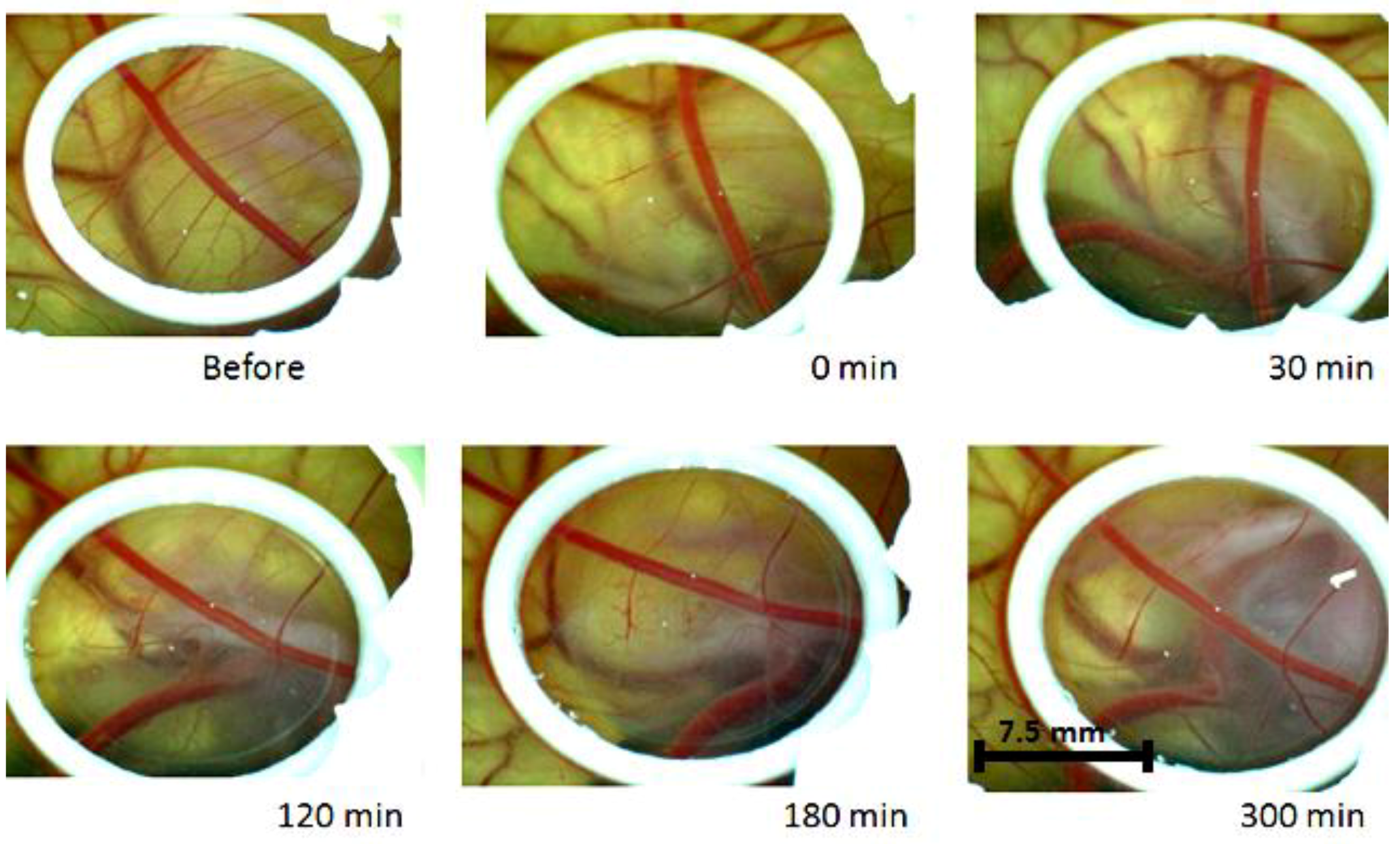
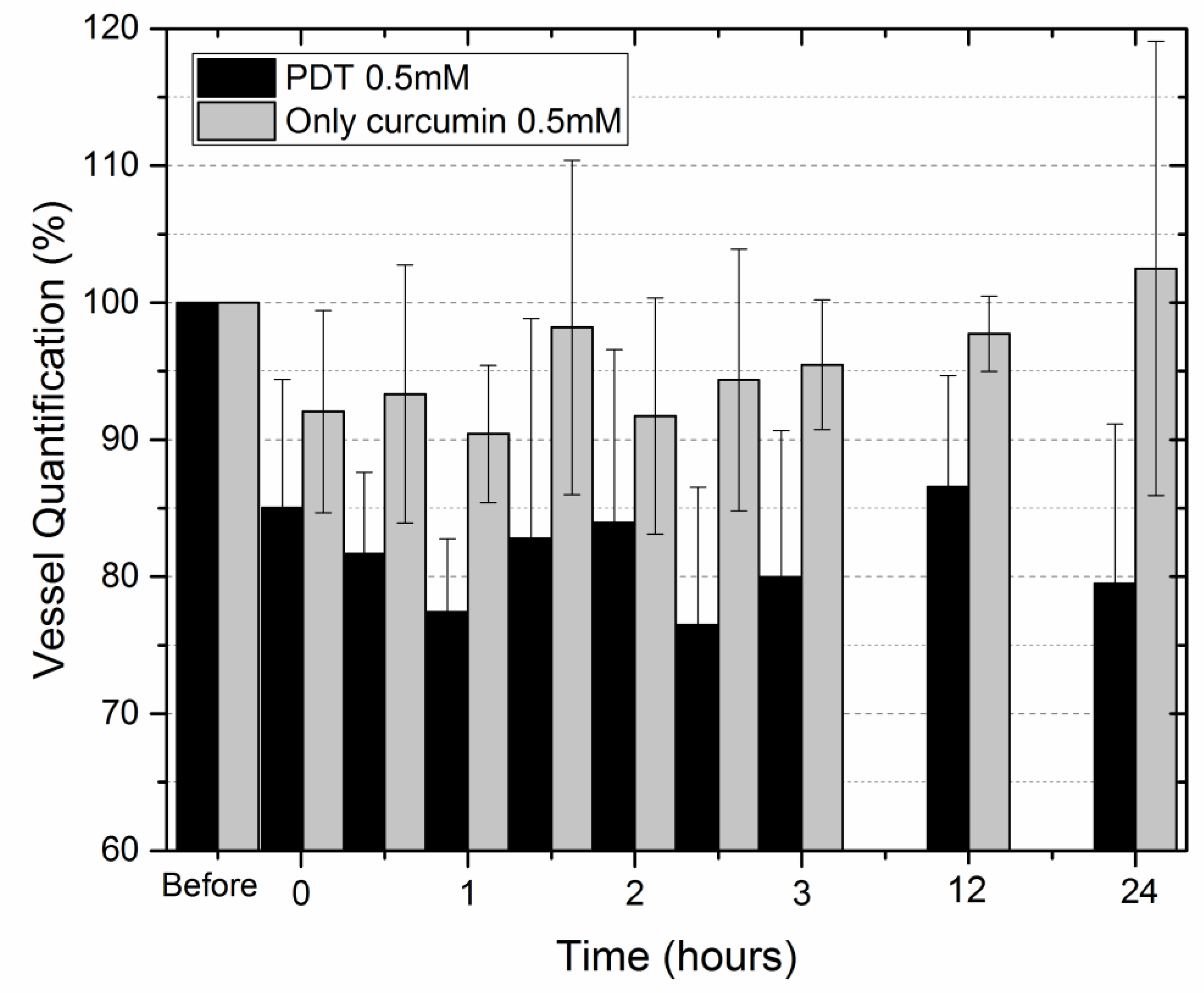
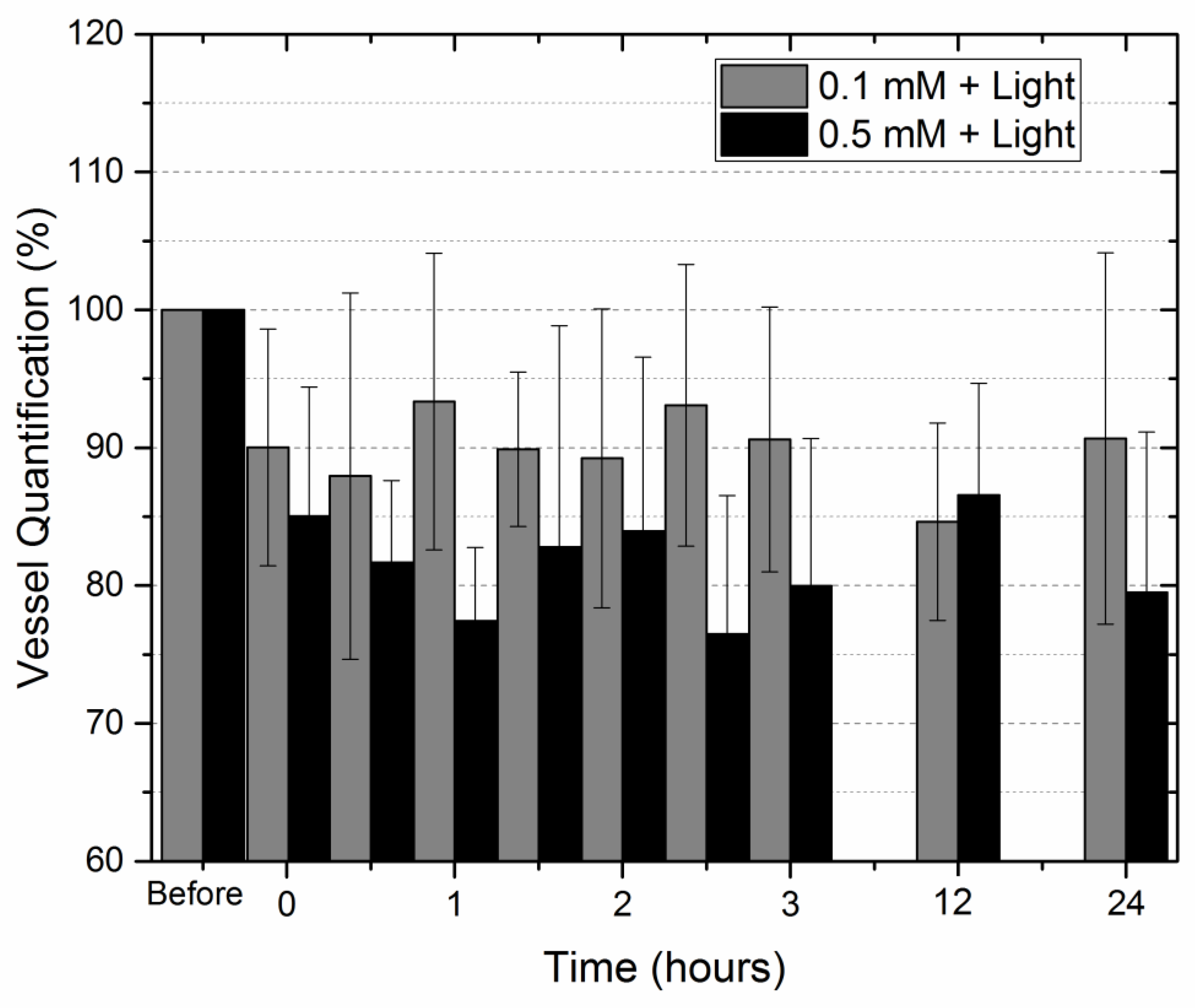
2. Results
3. Discussion
4. Materials and Methods
4.1. CAM Model
4.2. Experimental Groups
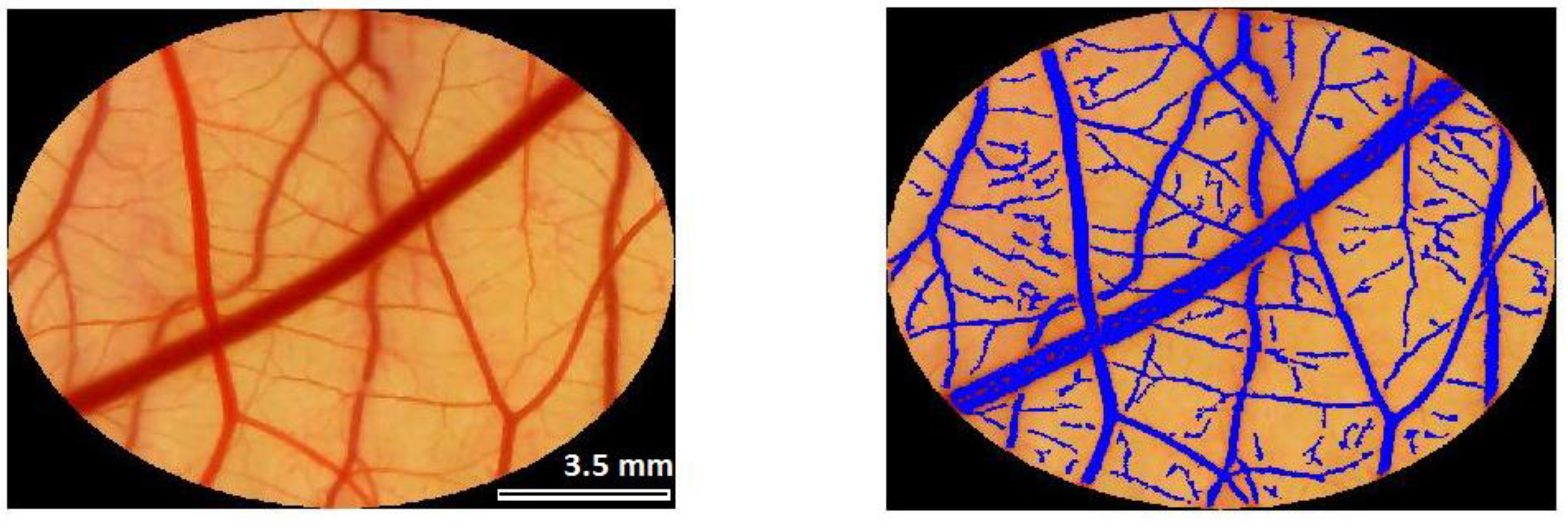
4.3. Evaluation of Vascular Response—Analysis of Images
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shishodia, S.; Chaturvedi, M.M.; Aggarwal, B.B. Role of Curcumin in Cancer Therapy. Curr. Probl. Cancer 2007, 31, 243–305. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dash, J.R.; Parija, S.C. Spasmolytic effect of curcumin on goat ruminal artery is endothelium independent and by activation of sGC. Res. Vet. Sci. 2013, 95, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Rehman, G.; Lee, Y.S. Curcumin in inflammatory diseases. BioFactors 2013, 39, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Purpura, M.; Lowery, R.P.; Wilson, J.M.; Mannan, H.; Münch, G.; Razmovski-Naumovski, V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur. J. Nutr. 2018, 57, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phyther. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Deng, S.; Wu, Q.; Xiang, M.; Wei, X.; Li, L. Improving antiangiogenesis and anti-tumor activity of curcumin by biodegradable polymeric micelles. Biomaterials 2013, 34, 1413–1432. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.P.; Mukerjee, A.; Helson, L.; Gupta, R.; Vishwanatha, J.K. Efficacy of Liposomal Curcumin in a Human Pancreatic Tumor Xenograft Model: Inhibition of Tumor Growth and Angiogenesis. Anticancer Res. 2013, 33, 3603–3609. [Google Scholar] [PubMed]
- Gururaj, A.E.; Belakavadi, M.; Venkatesh, D.A.; Marmé, D.; Salimath, B.P. Molecular mechanisms of anti-angiogenic effect of curcumin. Biochem. Biophys. Res. Commun. 2002, 297, 934–942. [Google Scholar] [CrossRef]
- Nowis, D.; Makowski, M.; Stokłosa, T.; Legat, M.; Issat, T.; Gołąb, J. Direct tumor damage mechanisms of photodynamic therapy. Acta Biochim. Pol. 2005, 52, 339–352. [Google Scholar] [PubMed]
- Triesscheijn, M.; Baas, P.; Schellens, J.H.M.; Stewart, F.A. Photodynamic therapy in oncology. Oncologist 2006, 11, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Fingar, V.H. Vascular effects of photodynamic therapy. J. Clin. Laser Med. Surg. 1996, 14, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, V.; Lindenbaum, E.S.; Kimel, S. Vascular damage during PDT as monitored in the chick chorioallantoic membrane. Int. J. Radiat. Biol. 1991, 60, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Santezi, C.; Reina, B.D.; Dovigo, L.N. Curcumin-Mediated Photodynamic Therapy for the Treatment of Oral Infections—A Review. Photodiagnosis Photodyn. Ther. 2018, 21, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Lagunes, I.; Trigos, Á. Photo-oxidation of ergosterol: Indirect detection of antioxidants photosensitizers or quenchers of singlet oxygen. J. Photochem. Photobiol. B Biol. 2015, 145, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Dovigo, L.N.; Pavarina, A.C.; Ribeiro, A.P.D.; Brunetti, I.L.; Costa, C.A.D.S.; Jacomassi, D.P. Investigation of the photodynamic effects of curcumin against Candida albicans. Photochem. Photobiol. 2011, 87, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Panhóca, V.H.; Geralde, M.C.; Corrêa, T.Q.; Carvalho, M.T.; Souza, W.O.; Bagnato, V.S. Enhancement of the Photodynamic Therapy Effect on Streptococcus Mutans Biofilm. J. Phys. Sci. Appl. 2014, 4, 107–114. [Google Scholar]
- Bernd, A. Visible light and/or UVA offer a strong amplification of the anti-tumor effect of curcumin. Phytochem. Rev. 2014, 13, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Soria-Lozano, P.; Gilaberte, Y.; Paz-Cristobal, M.; Pérez-Artiaga, L.; Lampaya-Pérez, V.; Aporta, J. In vitro effect photodynamic therapy with differents photosensitizers on cariogenic microorganisms. BMC Microbiol. 2015, 15, 187. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, L.; Skupin-mrugalska, P.; Piskorz, J.; Mielcarek, J. Dyes and Pigments Non-porphyrinoid photosensitizers mediated photodynamic inactivation against bacteria. Dye Pigment. 2019, 163, 337–355. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Carmello, J.C.; de Souza Costa, C.A.; Vergani, C.E.; Brunetti, I.L.; Bagnato, V.S. Curcumin-mediated photodynamic inactivation of Candida albicans in a murine model of oral candidiasis. Med. Mycol. 2013, 51, 243–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aurelio, M.; Dds, P.; Tonon, C.C.; Spolidório, D.M.P.; Bagnato, V.S. Photodynamic potential of curcumin and blue LED against Streptococcus mutans in a planktonic culture. Photodiagnosis Photodyn. Ther. 2013, 10, 313–319. [Google Scholar]
- Pathogens, C.; Arau, C.; Fontana, C.R.; Ph, D.; Bagnato, V.S.; Ph, D. Photodynamic Effects of Curcumin Against. Photomed. Laser Surg. 2012, 30, 393–399. [Google Scholar]
- Silva, A.P.; Chiandrone, D.J.; Tinta, J.W.R.; Kurachi, C.; Inada, N.M.; Bagnato, V.S. Development and comparison of two devices for treatment of onychomycosis by photodynamic therapy. J. Biomed. Opt. 2015, 20, 061109. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.P.; Carbinatto, F.M.; Bagnato, V.S.; Inada, N.M. A Promising Strategy for the Treatment of Onychomycosis with Curcumin and Photodynamic Therapy. J. Pharm. Pharmacol. 2015, 3, 434–437. [Google Scholar]
- Chen, B.; Pogue, B.W.; Hoopes, P.J.; Hasan, T. Vascular and cellular targeting for photodynamic therapy. Crit. Rev. Eukaryot. Gene Expr. 2006, 16, 279–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, T.H.; Huang, Z.; Li, Q.; Yuan, K.H.; Hu, Z.Q. Comparison of pulsed dye laser (PDL) and photodynamic therapy (PDT) for treatment of facial port-wine stain (PWS) birthmarks in pediatric patients. Photodiagnosis Photodyn. Ther. 2014, 11, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis 2014, 4, 779–804. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Vacca, A.; Roncali, L.; Dammacco, F. The chick embryo chorioallantoic membrane as a model for in vivo research on anti-angiogenesis. Curr. Pharm. Biotechnol. 1996, 1, 73–82. [Google Scholar] [CrossRef]
- Vargas, A.; Zeisser-Labouèbe, M.; Lange, N.; Gurny, R.; Delie, F. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv. Drug Deliv. Rev. 2007, 59, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.A.; Jones, S.M.; Paggett, K.C. Emergence of hearing in the chicken embryo. J. Neurophysiol. 2006, 96, 128141. [Google Scholar] [CrossRef] [PubMed]
- Corazza, A.V.; Jorge, J.; Kurachi, C.; Bagnato, V.S. Photobiomodulation on the angiogenesis of skin wounds in rats using different light sources. Photomed. Laser Surg. 2007, 25, 102–106. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, A.P.C.; Paraguassú, G.M.; Silveira, N.T.T.; De Souza, J.; Cangussú, M.C.T.; Dos Santos, J.N.; Cangussú, M.C.; dos Santos, J.N.; Pinheiro, A.L. Laser and LED phototherapies on angiogenesis. Lasers Med. Sci. 2013, 28, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Thivierge, C.; Nowak-Sliwinska, P.; Han, J.; Van Den Bergh, H.; Wagnières, G.; Burgess, K.; Lee, H.B. In vitro and in vivo photocytotoxicity of boron dipyrromethene derivatives for photodynamic therapy. J. Med. Chem. 2010, 53, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Buzzá, H.H.; Silva, L.V.; Moriyama, L.T.; Bagnato, V.S.; Kurachi, C. Evaluation of vascular effect of Photodynamic Therapy in chorioallantoic membrane using different photosensitizers. J. Photochem. Photobiol. B Biol. 2014, 138, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, P.; Zhang, W.; Yuan, S.; Chen, Z.; Yang, Q.; Yuan, D.; Wang, F.; Liu, Q. Suppression of experimental choroidal neovascularization by curcumin in mice. PLoS ONE 2012, 7, e53329. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.P.; Zhu, S.; Ko, J.E.; Ashritha, N.; Kandimalla, R.; Snyder, J.P.; Shoji, M.; El-Rayes, B.F. Antiangiogenic effects of a novel synthetic curcumin analogue in pancreatic cancer. Cancer Lett. 2015, 357, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Moy, W.J.; Kelly, K.M.; Choi, B.; Clinic, M.; States, U.; States, U. Hemoporfin-mediated photodynamic therapy on normal vasculature: Implications for phototherapy of port-wine stain birthmarks. J. Clin. Transl. Res. 2017, 2, 107–111. [Google Scholar]
- Jalde, S.S.; Chauhan, A.K.; Lee, J.H.; Chaturvedi, P.K.; Park, J.S.; Kim, Y.W. Synthesis of novel Chlorin e6-curcumin conjugates as photosensitizers for photodynamic therapy against pancreatic carcinoma. Eur. J. Med. Chem. 2018, 147, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kang, S.M.; Jeong, S.H.; Chung, K.H.; Kim, B.I. Antibacterial photodynamic therapy with curcumin and Curcuma xanthorrhiza extract against Streptococcus mutans. Photodiagnosis Photodyn. Ther. 2017, 20, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Debefve, E.; Pegaz, B.; Van Den Bergh, H.; Wagnières, G.; Lange, N.; Ballini, J.P. Video monitoring of neovessel occlusion induced by photodynamic therapy with verteporfin (Visudyne®®), in the CAM model. Angiogenesis 2008, 11, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Chin, W.W.L.; Heng, P.W.S.; Lim, P.L.; Lau, W.K.O.; Olivo, M. Membrane transport enhancement of chlorin e6-polyvinylpyrrolidone and its photodynamic efficacy on the chick chorioallantoic model. J. Biophotonics 2008, 1, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Nowak-Sliwinska, P.; Kamarulzaman, F.A.; Van Den Bergh, H.; Wagnières, G.; Lee, H.B. The neovessel occlusion efficacy of 151-hydroxypurpurin-7-lactone dimethyl ester induced with photodynamic therapy. Photochem. Photobiol. 2010, 86, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, G.; Jedelská, J.; Omar, S.M.; Strehlow, B.; Schneider, M.; Bakowsky, U. Stabilized tetraether lipids based particles guided prophyrins photodynamic therapy. Drug Deliv. 2018, 25, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Eid, M.; Fanchaouy, M.; Gurny, R.; Delie, F. In vivo photodynamic activity of photosensitizer-loaded nanoparticles: Formulation properties, administration parameters and biological issues involved in PDT outcome. Eur. J. Pharm. Biopharm. 2008, 69, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Duse, L.; Pinnapireddy, S.R.; Strehlow, B.; Jedelská, J.; Bakowsky, U. Low level LED photodynamic therapy using curcumin loaded tetraether liposomes. Eur. J. Pharm. Biopharm. 2018, 16, 233–241. [Google Scholar] [CrossRef] [PubMed]

| Group | [Curcumin] (mM/cm2) | Light Irradiance (mW/cm2) | Light Fluence (J/cm2) |
|---|---|---|---|
| L1 | - | 10 | 6 |
| L2 | - | 12 | 7.2 |
| L3 | - | 50 | 15 |
| L4 | - | 50 | 30 |
| L5 | - | 60 | 36 |
| C1 | 0.1 | - | - |
| C2 | 0.33 | ||
| C3 | 0.5 | ||
| C4 | 1 | ||
| C5 | 5 | ||
| C6 | 10 | ||
| PDT1 | 0.1 | 50 | 30 |
| PDT2 | 0.33 | ||
| PDT3 | 0.5 | ||
| PDT4 | 1 | ||
| PDT5 | 5 | ||
| PDT6 | 10 | ||
| PDT7 | 0.1 | 50 | 15 |
| PDT8 | 0.5 | ||
| PDT9 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzzá, H.H.; Fialho de Freitas, L.C.; Moriyama, L.T.; Teixeira Rosa, R.G.; Bagnato, V.S.; Kurachi, C. Vascular Effects of Photodynamic Therapy with Curcumin in a Chorioallantoic Membrane Model. Int. J. Mol. Sci. 2019, 20, 1084. https://doi.org/10.3390/ijms20051084
Buzzá HH, Fialho de Freitas LC, Moriyama LT, Teixeira Rosa RG, Bagnato VS, Kurachi C. Vascular Effects of Photodynamic Therapy with Curcumin in a Chorioallantoic Membrane Model. International Journal of Molecular Sciences. 2019; 20(5):1084. https://doi.org/10.3390/ijms20051084
Chicago/Turabian StyleBuzzá, Hilde Harb, Lucas Cruz Fialho de Freitas, Lilian Tan Moriyama, Ramon Gabriel Teixeira Rosa, Vanderlei Salvador Bagnato, and Cristina Kurachi. 2019. "Vascular Effects of Photodynamic Therapy with Curcumin in a Chorioallantoic Membrane Model" International Journal of Molecular Sciences 20, no. 5: 1084. https://doi.org/10.3390/ijms20051084




