Expression of Transcripts in Marmoset Oocytes Retrieved during Follicle Isolation Without Gonadotropin Induction
Abstract
:1. Introduction
2. Results
2.1. In Vitro Maturation of Marmoset Oocytes at Various Concentrations of Factors
2.2. Fertilization of in vitro Matured Oocytes
2.3. Localization of Specific Protein in in vitro Matured Oocytes
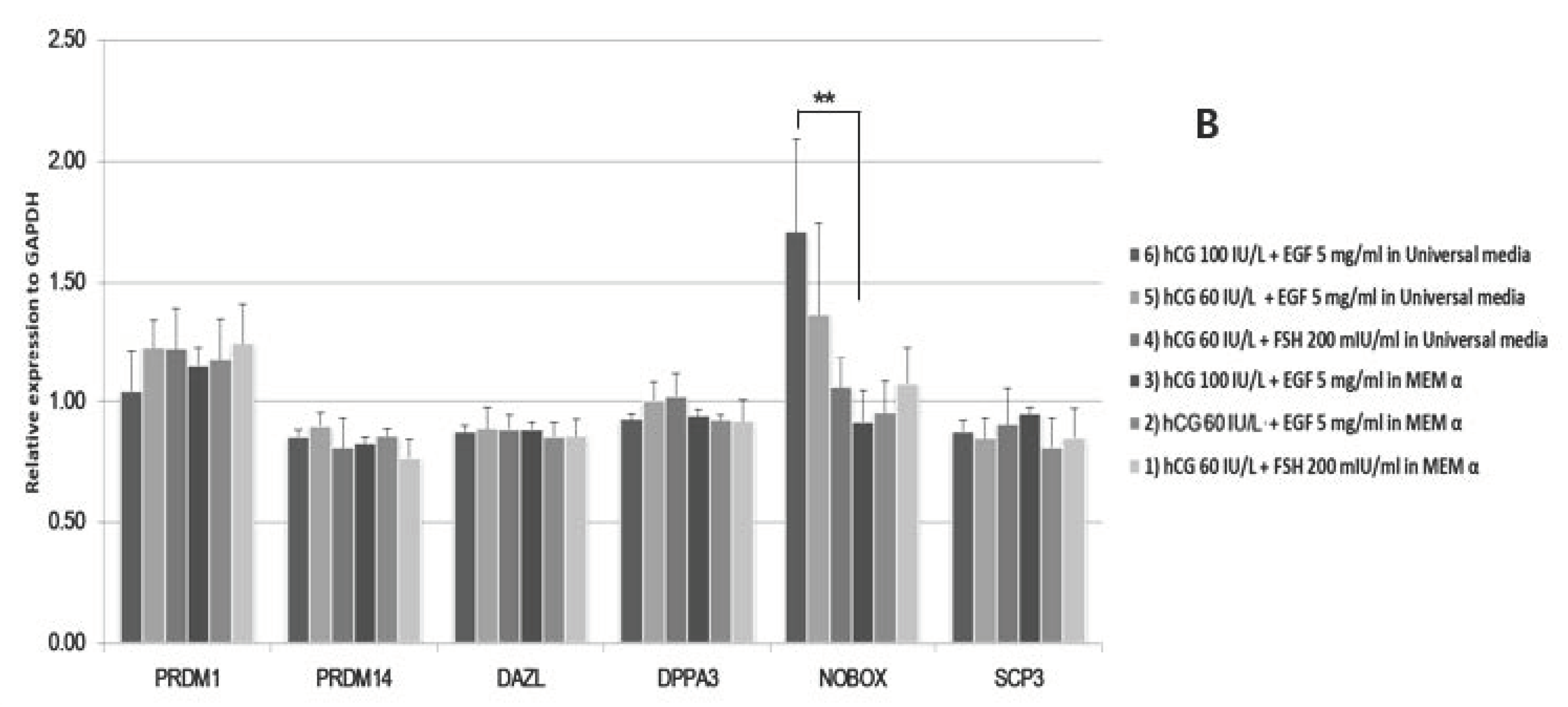
2.4. Differential Expression of miRNA and mRNA in Matured Oocytes
2.5. Annotation of miRNAs Using Database Set
3. Discussion
4. Materials and Methods
4.1. Ethics and Animal Anesthesia
4.2. Isolation of Ovaries and in vitro Maturation of Oocytes
4.3. Sperm Collection
4.4. Fertilization of in vitro Matured Oocytes
4.5. Single Cell Reverse Transcription of Oocyte
4.6. Evaluation of miRNAs Using qPCR
4.7. qRT-PCR to Evaluate the Levels of Candidate Gene Expression
4.8. Immunostaining of Oocytes
4.9. Database Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pfeffer, P.L.; Sisco, B.; Donnison, M.; Somers, J.; Smith, C. Isolation of genes associated with developmental competency of bovine oocytes. Theriogenology 2007, 68, S84–S90. [Google Scholar] [CrossRef] [PubMed]
- Espey, L.L. The distribution of collagenous connective tissue in rat ovarian follicles. Biol. Reprod. 1976, 14, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Espey, L.L.; Coons, P.J. Factors which influence ovulatory regradation of rabbit ovarian follicles. Biol. Reprod. 1976, 14, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Park, K.E.; Kim, Y.Y.; Kim, H.; Ku, S.Y.; Suh, C.S.; Kim, S.H.; Choi, Y.M. Effects of Estradiol on the Paracrine Regulator Expression of In Vitro Maturated Murine Ovarian Follicles. Tissue Eng. Regen. Med. 2017, 14, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Park, K.E.; Ku, S.Y.; Jung, K.C.; Liu, H.C.; Kim, Y.Y.; Kim, Y.J.; Kim, S.H.; Choi, Y.M.; Kim, J.G.; Moon, S.Y. Effects of urinary and recombinant gonadotropins on in vitro maturation outcomes of mouse preantral follicles. Reprod. Sci. 2013, 20, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.H.; Leask, R.; Srsen, V.; Riley, S.C.; Spears, N.; Telfer, E.E. Effect of ascorbic acid on health and morphology of bovine preantral follicles during long-term culture. Reproduction 2001, 122, 487–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.Y.; Min, H.; Kim, H.; Choi, Y.M.; Liu, H.C.; Ku, S.Y. Differential MicroRNA Expression Profile of Human Embryonic Stem Cell-Derived Cardiac Lineage Cells. Tissue Eng. Regen. Med. 2017, 14, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki-Horimoto, K.; Nakajima, N.; Kiso, M.; Takahashi, K.; Ito, M.; Inoue, T.; Horiuchi, M.; Okahara, N.; Sasaki, E.; Hasegawa, H.; et al. The Marmoset as an Animal Model of Influenza: Infection With A(H1N1)pdm09 and Highly Pathogenic A(H5N1) Viruses via the Conventional or Tracheal Spray Route. Front. Microbiol. 2018, 9, 844. [Google Scholar] [CrossRef] [PubMed]
- Hanazawa, K.; Mueller, T.; Becker, T.; Heistermann, M.; Behr, R.; Sasaki, E. Minimally invasive transabdominal collection of preimplantation embryos from the common marmoset monkey (Callithrix jacchus). Theriogenology 2012, 78, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. MicroRNA pathways in flies and worms: Growth, death, fat, stress, and timing. Cell 2003, 113, 673–676. [Google Scholar] [CrossRef]
- Lai, E.C. microRNAs: Runts of the genome assert themselves. Curr. Biol. 2003, 13, R925–R936. [Google Scholar] [CrossRef] [PubMed]
- Tay, Y.; Zhang, J.; Thomson, A.M.; Lim, B.; Rigoutsos, I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature 2008, 455, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Baskerville, S.; Shenoy, A.; Babiarz, J.E.; Baehner, L.; Blelloch, R. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat. Genet. 2008, 40, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Mondou, E.; Dufort, I.; Gohin, M.; Fournier, E.; Sirard, M.A. Analysis of microRNAs and their precursors in bovine early embryonic development. Mol. Hum. Reprod. 2012, 18, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarty, A.; Tranguch, S.; Daikoku, T.; Jensen, K.; Furneaux, H.; Dey, S.K. MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc. Natl. Acad. Sci. USA 2007, 104, 15144–15149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCallie, B.; Schoolcraft, W.B.; Katz-Jaffe, M.G. Aberration of blastocyst microRNA expression is associated with human infertility. Fertil. Steril. 2010, 93, 2374–2382. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Ku, S.Y.; Kim, Y.Y.; Liu, H.C.; Chi, S.W.; Kim, S.H.; Choi, Y.M.; Kim, J.G.; Moon, S.Y. MicroRNAs transfected into granulosa cells may regulate oocyte meiotic competence during in vitro maturation of mouse follicles. Hum. Reprod. 2013, 28, 3050–3061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Ku, S.Y.; Rosenwaks, Z.; Liu, H.C.; Chi, S.W.; Kang, J.S.; Lee, W.J.; Jung, K.C.; Kim, S.H.; Choi, Y.M.; et al. MicroRNA expression profiles are altered by gonadotropins and vitamin C status during in vitro follicular growth. Reprod. Sci. 2010, 17, 1081–1089. [Google Scholar] [PubMed]
- Kim, Y.Y.; Kim, Y.J.; Cho, K.M.; Kim, S.H.; Park, K.E.; Kang, B.C.; Jung, K.C.; Kim, M.S.; Ku, S.Y. The expression profile of angiotensin system on thawed murine ovaries. Tissue Eng. Regen. Med. 2016, 13, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Tamadon, A.; Ku, S.Y. Potential Use of Antiapoptotic Proteins and Noncoding RNAs for Efficient In Vitro Follicular Maturation and Ovarian Bioengineering. Tissue Eng. Part B Rev. 2017, 23, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Amanai, M.; Brahmajosyula, M.; Perry, A.C. A restricted role for sperm-borne microRNAs in mammalian fertilization. Biol. Reprod. 2006, 75, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Kishi, N.; Sato, K.; Sasaki, E.; Okano, H. Common marmoset as a new model animal for neuroscience research and genome editing technology. Dev. Growth Differ. 2014, 56, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Kuroki, Y.; Kumita, W.; Fujiyama, A.; Toyoda, A.; Kawai, J.; Iriki, A.; Sasaki, E.; Okano, H.; Sakakibara, Y. Resequencing of the common marmoset genome improves genome assemblies and gene-coding sequence analysis. Sci. Rep. 2015, 5, 16894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Liu, Y.; Dong, D.; Zhang, Z. Evolution of an X-linked primate-specific micro RNA cluster. Mol. Biol. Evol. 2010, 27, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Lagana, A.S.; Vitale, S.G.; Ban Frangez, H.; Vrtacnik-Bokal, E.; D’Anna, R. Vitamin D in human reproduction: The more, the better? An evidence-based critical appraisal. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4243–4251. [Google Scholar] [PubMed]
- Nandi, A.; Sinha, N.; Ong, E.; Sonmez, H.; Poretsky, L. Is there a role for vitamin D in human reproduction? Horm. Mol. Biol. Clin. Investig. 2016, 25, 15–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, S.G.; Rossetti, P.; Corrado, F.; Rapisarda, A.M.; La Vignera, S.; Condorelli, R.A.; Valenti, G.; Sapia, F.; Lagana, A.S.; Buscema, M. How to Achieve High-Quality Oocytes? The Key Role of Myo-Inositol and Melatonin. Int. J. Endocrinol. 2016, 2016, 4987436. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Munoz, E.; Sathyapalan, T.; Rossetti, P.; Shah, M.; Long, M.; Buscema, M.; Valenti, G.; La Rosa, V.L.; Cianci, S.; Vitale, S.G. Polycystic Ovary Syndrome: Implication for Drug Metabolism on Assisted Reproductive Techniques-A Literature Review. Adv. Ther. 2018, 35, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Fazleabas, A.T.; Shikanov, A.; Jackson, E.; Barrett, S.L.; Hirshfeld-Cytron, J.; Kiesewetter, S.E.; Shea, L.D.; Woodruff, T.K. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol. Reprod. 2011, 84, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Uehara, S.; Uno, Y.; Yuki, Y.; Inoue, T.; Sasaki, E.; Yamazaki, H. A New Marmoset P450 4F12 Enzyme Expressed in Small Intestines and Livers Efficiently Metabolizes Antihistaminic Drug Ebastine. Drug Metab. Dispos. 2016, 44, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.; Nunomura, S.; Mori, S.; Suemizu, H.; Itoh, T.; Takabayashi, S.; Okada, Y.; Yahata, T.; Shiina, T.; Katoh, H.; et al. Common marmoset CD117+ hematopoietic cells possess multipotency. Int. Immunol. 2015, 27, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Sadakane, O.; Watakabe, A.; Ohtsuka, M.; Takaji, M.; Sasaki, T.; Kasai, M.; Isa, T.; Kato, G.; Nabekura, J.; Mizukami, H.; et al. In Vivo Two-Photon Imaging of Dendritic Spines in Marmoset Neocortex. eNeuro 2015, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.Y.; Hikabe, O.; Suzuki, S.; Hirano, T.; Siomi, H.; Sasaki, E.; Imamura, M.; Okano, H. Sphere-formation culture of testicular germ cells in the common marmoset, a small New World monkey. Primates 2016, 57, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Debowski, K.; Drummer, C.; Lentes, J.; Cors, M.; Dressel, R.; Lingner, T.; Salinas-Riester, G.; Fuchs, S.; Sasaki, E.; Behr, R. The transcriptomes of novel marmoset monkey embryonic stem cell lines reflect distinct genomic features. Sci. Rep. 2016, 6, 29122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogonuki, N.; Inoue, H.; Matoba, S.; Kurotaki, Y.K.; Kassai, H.; Abe, Y.; Sasaki, E.; Aiba, A.; Ogura, A. Oocyte-activating capacity of fresh and frozen-thawed spermatids in the common marmoset (Callithrix jacchus). Mol. Reprod. Dev. 2018, 85, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.B.; Nayudu, P.L.; Nowshari, M.A.; Hodges, J.K. Meiotic competence of marmoset monkey oocytes is related to follicle size and oocyte-somatic cell associations. Biol. Reprod. 1995, 52, 1234–1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grupen, C.G.; Gilchrist, R.B.; Nayudu, P.L.; Barry, M.F.; Schulz, S.J.; Ritter, L.J.; Armstrong, D.T. Effects of ovarian stimulation, with and without human chorionic gonadotrophin, on oocyte meiotic and developmental competence in the marmoset monkey (Callithrix jacchus). Theriogenology 2007, 68, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, O.Y.; Delimitreva, S.; Heistermann, M.; Scheerer-Bernhard, J.U.; Wedi, E.; Nayudu, P.L. Critical estradiol dose optimization for oocyte in vitro maturation in the common marmoset. Theriogenology 2015, 83, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W.; Kim, Y.Y.; Ahn, J.H.; Kang, B.C.; Ku, S.Y. Use of nonhuman primates for the development of bioengineered female reproductive organs. Tissue Eng. Regen. Med. 2016, 13, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, Y.Y.; Kang, B.C.; Kim, M.S.; Ko, I.K.; Liu, H.C.; Rosenwaks, Z.; Ku, S.Y. Induction of multiple ovulation via modulation of angiotensin II receptors in in vitro ovarian follicle culture models. J. Tissue Eng. Regen. Med. 2017, 11, 3100–3110. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Yun, J.W.; Kim, J.M.; Park, C.G.; Rosenwaks, Z.; Liu, H.C.; Kang, B.C.; Ku, S.Y. Gonadotropin ratio affects the in vitro growth of rhesus ovarian preantral follicles. J. Investig. Med. 2016, 64, 888–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacovicova, K.; Awadova, T.; Mikel, P.; Anger, M. In Vitro Maturation of Mouse Oocytes Increases the Level of Kif11/Eg5 on Meiosis II Spindles. Biol. Reprod. 2016, 95, 18. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, L.; Nogueira, D.; Dumortier, F.; De Sutter, P. Assessment of a new in vitro maturation system for mouse and human cumulus-enclosed oocytes: Three-dimensional prematuration culture in the presence of a phosphodiesterase 3-inhibitor. Hum. Reprod. 2009, 24, 1946–1959. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, O.Y.; Delimitreva, S.; Isachenko, E.; Valle, R.R.; Michelmann, H.W.; Berenson, A.; Nayudu, P.L. Epidermal growth factor effects on marmoset monkey (Callithrix jacchus) oocyte in vitro maturation, IVF and embryo development are altered by gonadotrophin concentration during oocyte maturation. Hum. Reprod. 2010, 25, 2047–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delimitreva, S.; Zhivkova, R.; Isachenko, E.; Umland, N.; Nayudu, P.L. Meiotic abnormalities in in vitro-matured marmoset monkey (Callithrix jacchus) oocytes: Development of a non-human primate model to investigate causal factors. Hum. Reprod. 2006, 21, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Kanda, A.; Nobukiyo, A.; Yoshioka, M.; Hatakeyama, T.; Sotomaru, Y. Quality of common marmoset (Callithrix jacchus) oocytes collected after ovarian stimulation. Theriogenology 2018, 106, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Ringuette, M.J.; Sobieski, D.A.; Chamow, S.M.; Dean, J. Oocyte-specific gene expression: Molecular characterization of a cDNA coding for ZP-3, the sperm receptor of the mouse zona pellucida. Proc. Natl. Acad. Sci. USA 1986, 83, 4341–4345. [Google Scholar] [CrossRef] [PubMed]
- Philpott, C.C.; Ringuette, M.J.; Dean, J. Oocyte-specific expression and developmental regulation of ZP3, the sperm receptor of the mouse zona pellucida. Dev. Biol. 1987, 121, 568–575. [Google Scholar] [CrossRef]
- Murayama, Y.; Mizuno, J.; Kamakura, H.; Fueta, Y.; Nakamura, H.; Akaishi, K.; Anzai, K.; Watanabe, A.; Inui, H.; Omata, S. Mouse zona pellucida dynamically changes its elasticity during oocyte maturation, fertilization and early embryo development. Hum. Cell 2006, 19, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Newport, A.; Carroll, J. Structure and composition of the zona pellucida of the mouse oocyte. Biochem. Soc. Trans. 1976, 4, 896–897. [Google Scholar] [CrossRef] [PubMed]
- Topper, E.K.; Kruijt, L.; Calvete, J.; Mann, K.; Topfer-Petersen, E.; Woelders, H. Identification of bovine zona pellucida glycoproteins. Mol. Reprod. Dev. 1997, 46, 344–350. [Google Scholar] [CrossRef]
- Kolluri, S.K.; Kaul, R.; Banerjee, K.; Gupta, S.K. Nucleotide sequence of cDNA encoding bonnet monkey (Macaca radiata) zona pellucida glycoprotein-ZP3. Reprod. Fertil. Dev. 1995, 7, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Sharma, M.; Behera, A.K.; Bisht, R.; Kaul, R. Sequence of complementary deoxyribonucleic acid encoding bonnet monkey (Macaca radiata) zona pellucida glycoprotein-ZP1 and its high-level expression in Escherichia coli. Biol. Reprod. 1997, 57, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Bogner, K.; Hinsch, K.D.; Nayudu, P.; Konrad, L.; Cassara, C.; Hinsch, E. Localization and synthesis of zona pellucida proteins in the marmoset monkey (Callithrix jacchus) ovary. Mol. Hum. Reprod. 2004, 10, 481–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, M.L.; Fontenot, G.K.; Harris, J.D. The expression and localization of zona pellucida glycoproteins and mRNA in cynomolgus monkeys (Macaca fascicularis). J. Reprod. Fertil. Suppl. 1996, 50, 35–41. [Google Scholar] [PubMed]
- Konrad, L.; Kuhnert, S.; Nayudu, P.L.; Einspanier, R.; Hinsch, K.D.; Hinsch, E. Quantification of ZP1, ZP2 and ZP3 mRNA of marmoset monkey (Callithrix jacchus) oocytes from periantral and antral follicles. Andrologia 2012, 44, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.P.; Glasner, M.E.; Yekta, S.; Burge, C.B.; Bartel, D.P. Vertebrate microRNA genes. Science 2003, 299, 1540. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.M.; Matzuk, M.M. Oocyte-somatic cell communication and microRNA function in the ovary. Ann. Endocrinol. 2010, 71, 144–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Xue, R.; Yuan, H.J.; Wang, T.Y.; Lin, J.; Zhang, J.; Liang, B.; Tan, J.H. MicroRNA-21 plays a pivotal role in the oocyte-secreted factor-induced suppression of cumulus cell apoptosis. Biol. Reprod. 2017, 96, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Sheng, Y.; Wang, Z. MicroRNA-224 delays oocyte maturation through targeting Ptx3 in cumulus cells. Mech. Dev. 2017, 143, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Toms, D.; Li, J. MicroRNA-574 suppresses oocyte maturation via targeting hyaluronan synthase 2 in porcine cumulus cells. Am. J. Physiol. Cell Physiol. 2018, 314, C268–C277. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Toms, D.; Shen, W.; Li, J. MicroRNA-378 regulates oocyte maturation via the suppression of aromatase in porcine cumulus cells. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E525–E534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, J.R.; McDaneld, T.G.; Wiedmann, R.T.; Cushman, R.A.; Echternkamp, S.E.; Vallet, J.L.; Smith, T.P. MicroRNA expression profile in bovine cumulus-oocyte complexes: Possible role of let-7 and miR-106a in the development of bovine oocytes. Anim. Reprod. Sci. 2012, 130, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Wu, W.J.; Zhou, X.L.; Xiao, P.; Wang, Y.; Liu, H.L. Expression and preliminary functional profiling of the let-7 family during porcine ovary follicle atresia. Mol. Cells 2015, 38, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, M.; Sun, J.; Jia, L.; Ma, S.; Gao, J.; Xu, Y.; Zhang, H.; Tsang, S.Y.; Li, X. MicroRNA-27a-3p affects estradiol and androgen imbalance by targeting Creb1 in the granulosa cells in mouse polycytic ovary syndrome model. Reprod. Biol. 2017, 17, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, J.; Xu, B.; Chrusciel, M.; Gao, J.; Bazert, M.; Stelmaszewska, J.; Xu, Y.; Zhang, H.; Pawelczyk, L.; et al. Functional Characterization of MicroRNA-27a-3p Expression in Human Polycystic Ovary Syndrome. Endocrinology 2018, 159, 297–309. [Google Scholar] [CrossRef] [PubMed]







| Media Composition | Number of Matured (MII) Oocytes | |
|---|---|---|
| MEM α | (1) hCG 60 IU/L + FSH 200 mIU/mL | 4 |
| (2) hCG 60 IU/L + EGF 5 mg/mL | 8 | |
| (3) hCG 100 IU/L + EGF 5 mg/mL | 4 | |
| Universal IVF | (4) hCG 60 IU/L + FSH 200 mIU/mL | 9 |
| (5) hCG 60 IU/L + EGF 5 mg/mL | 3 | |
| (6) hCG 100 IU/L + EGF 5 mg/mL | 11 |
| microRNA | Mature Sequence | Accession ID |
|---|---|---|
| Cja-let-7b | ugagguaguagguugugugguu | MIMAT0039325 |
| Cja-let-7c | ugagguaguagguuguaugguu | MIMAT0049314 |
| Cja-mir-27a | uucacaguggcuaaguuccgc | MIMAT0039518 |
| Cja-miR-224 | caagucacuagugguuccauuu | MIMAT0039393 |
| Genes | Forward | Reverse |
|---|---|---|
| Cja_GAPDH | TGCTGGCGCTGAGTATGTG | AGCCCCAGCCTTCTCCAT |
| Cja_OCT4 | GGAACAAAACACGGAGGAGTC | CAGGGTGATCCTCTTCTGCTTC |
| Cja_BMP15 | CATTCACTGCGGTACATGCT | TAGTTGGAGATGATGGCGGT |
| Cja_VASA | TGGACATGATGCACCACCAGCA | TGGGCCAAAATTGGCAGGAGAAA |
| Cja_ZP1 | CACAGAACAGACCCCCACCTAG | CGCTGGTGGTGTGAGGGAAATG |
| Cja_ZP2 | ACTCCCCTCTGTGTTCTGTG | CTGCCTCCTCCCTTGTTT |
| Cja_ZP3 | TGTGGCACTCCAAGCCATGC | AGGGCGAGCCACAGGAACCAATG |
| Cja_SALL4 | AAGGCAACTTGAAGGTTCACTACA | GATGGCCAGCTTCCTTCCA |
| Cja_LIN28A | GACGTCTTTGTGCACCAGAGTAA | CGGCCTCACCTTCCTTCAA |
| Cja_PRDM1 | ATGAAGTTGCCTCCCAGCAA | TTCCTACAGGCACCCTGACT |
| Cja_PRDM14 | CGGGGAGAAGCCCTTCAAAT | CTCCTTGTGTGAACGTCGGA |
| Cja_DAZL | GAAGAAGTCGGGCAGTGCTT | AACGAGCAACTTCCCATGAA |
| Cja_DPPA3 | GCGGATGGGATCCTTCTGAG | GAGTAGCTTTCTCGGTCTGCT |
| Cja_NOBOX | GAAGACCACTATCCTGACAGTG | TCAGAAGTCAGCAGCATGGGG |
| Cja_SCP3 | TGGAAAACACAACAAGATCA | GCTATCTCTTGCTGCTGAGT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.Y.; Kang, B.-C.; Yun, J.W.; Ahn, J.H.; Kim, Y.J.; Kim, H.; Rosenwaks, Z.; Ku, S.-Y. Expression of Transcripts in Marmoset Oocytes Retrieved during Follicle Isolation Without Gonadotropin Induction. Int. J. Mol. Sci. 2019, 20, 1133. https://doi.org/10.3390/ijms20051133
Kim YY, Kang B-C, Yun JW, Ahn JH, Kim YJ, Kim H, Rosenwaks Z, Ku S-Y. Expression of Transcripts in Marmoset Oocytes Retrieved during Follicle Isolation Without Gonadotropin Induction. International Journal of Molecular Sciences. 2019; 20(5):1133. https://doi.org/10.3390/ijms20051133
Chicago/Turabian StyleKim, Yoon Young, Byeong-Cheol Kang, Jun Won Yun, Jae Hun Ahn, Yong Jin Kim, Hoon Kim, Zev Rosenwaks, and Seung-Yup Ku. 2019. "Expression of Transcripts in Marmoset Oocytes Retrieved during Follicle Isolation Without Gonadotropin Induction" International Journal of Molecular Sciences 20, no. 5: 1133. https://doi.org/10.3390/ijms20051133





