Transcriptomic Changes in Medicago truncatula and Lotus japonicus Root Nodules during Drought Stress
Abstract
:1. Introduction
2. Results and Discussion
2.1. Two- and Four-Day Long Water Deprivation Results in Decreased Nitrogenase Activity and Increased Expression of Drought Stress Markers in M. truncatula and L. japonicus Root Nodules
2.2. Symbiosis with Rhizobia Drastically Changes Plant Transcriptome in both M. truncatula and L. japonicus
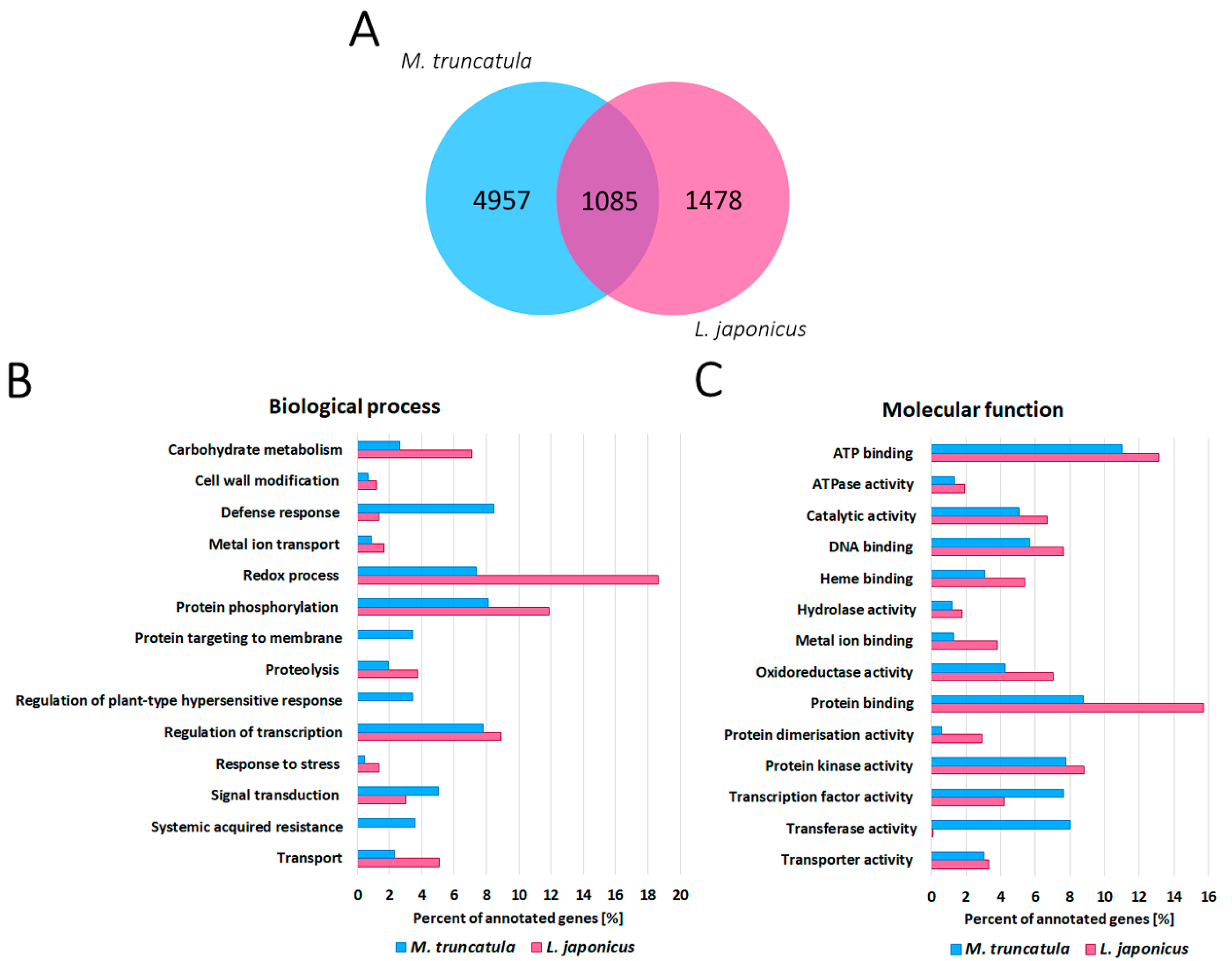
2.3. RNA-Seq Analysis Revealed a High Number of Differentially Expressed Plant Genes during Water Deprivation Stress in the M. truncatula and L. japonicus Root Nodules
2.4. RNA-Seq Analysis Revealed Differentially Expressed Bacterial Genes during Water Deprivation Stress in the M. truncatula and L. japonicus Root Nodules
3. Materials and Methods
3.1. Plant Material, Inoculation with Rhizobia, Growth Conditions and Drought Stress Induction
3.2. Estimation of Nitrogenase Activity
3.3. RNA Isolation and Sequencing
3.4. cDNA Synthesis and Real-Time qPCR
3.5. Microscopic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ferguson, B.J.; Indrasumunar, A.; Hayashi, S.; Lin, M.-H.; Lin, Y.-H.; Reid, D.E.; Gresshoff, P.M. Molecular analysis of legume nodule development and autoregulation. J. Integr. Plant Biol. 2010, 52, 61–76. [Google Scholar] [CrossRef]
- Sprent, J.I.; Sprent, P. Nitrogen Fixing Organisms: Pure and Applied Aspects; Chapman and Hall: London, UK, 1990. [Google Scholar]
- Vasse, J.; de Billy, F.; Camut, S.; Truchet, G. Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J. Bacteriol. 1990, 172, 4295–4306. [Google Scholar] [CrossRef] [PubMed]
- Szczyglowski, K.; Shaw, R.S.; Wopereis, J.; Copeland, S.; Hamburger, D.; Kasiborski, B.; Dazzo, F.B.; de Bruijn, F.J. Nodule organogenesis and symbiotic mutants of the model legume Lotus japonicus. Mol. Plant Microbe Interact. 1998, 11, 684–697. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Global synthesis of drought effects on food legume production. PLoS ONE 2015, 10, e0127401. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.; Frendo, P.; Ladrera, R.; Zabalza, A.; Puppo, A.; Arrese-Igor, C.; González, E.M. Nitrogen fixation control under drought stress. Localized or systemic? Plant Physiol. 2007, 143, 1968–1974. [Google Scholar] [CrossRef]
- Larrainzar, E.; Wienkoop, S.; Scherling, C.; Kempa, S.; Ladrera, R.; Arrese-Igor, C.; Weckwerth, W.; González, E.M. Carbon metabolism and bacteroid functioning are involved in the regulation of nitrogen fixation in Medicago truncatula under drought and recovery. Mol. Plant Microbe Interact. 2009, 22, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. ISBN 978-90-481-2666-8. [Google Scholar] [Green Version]
- Zhang, J.-Y.; Cruz, D.E.; Carvalho, M.H.; Torres-Jerez, I.; Kang, Y.; Allen, S.N.; Huhman, D.V.; Tang, Y.; Murray, J.; Sumner, L.W.; et al. Global reprogramming of transcription and metabolism in Medicago truncatula during progressive drought and after rewatering. Plant Cell Environ. 2014, 37, 2553–2576. [Google Scholar] [CrossRef]
- Filippou, P.; Antoniou, C.; Fotopoulos, V. Effect of drought and rewatering on the cellular status and antioxidant response of Medicago truncatula plants. Plant Signal Behav. 2011, 6, 270–277. [Google Scholar] [CrossRef]
- Nunes, C.; de Sousa Araújo, S.; da Silva, J.M.; Fevereiro, M.P.S.; da Silva, A.B. Physiological responses of the legume model Medicago truncatula cv. Jemalong to water deficit. Environ. Exp. Bot. 2008, 63, 289–296. [Google Scholar] [CrossRef]
- Amede, T.; Schubert, S.; Stahr, K. Mechanisms of drought resistance in grain legumes I: Osmotic adjustment. Sinet Ethiop J. Sci. 2003, 26, 37–46. [Google Scholar] [CrossRef]
- Dhanushkodi, R.; Matthew, C.; McManus, M.T.; Dijkwel, P.P. Drought-induced senescence of Medicago truncatula nodules involves serpin and ferritin to control proteolytic activity and iron levels. New Phytol. 2018, 220, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Sun, Y.; Peng, X.; Wang, Q.; Harris, M.; Ge, F. Up-regulation of abscisic acid signaling pathway facilitates aphid xylem absorption and osmoregulation under drought stress. J. Exp. Bot. 2016, 67, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.; Betti, M.; Sánchez, D.H.; Udvardi, M.K.; Monza, J.; Márquez, A.J. Deficiency in plastidic glutamine synthetase alters proline metabolism and transcriptomic response in Lotus japonicus under drought stress. New Phytol. 2010, 188, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S.; Siddique, K.H.M. Drought stress in grain legumes during reproduction and grain filling. J. Agron. Crop Sci. 2017, 203, 81–102. [Google Scholar] [CrossRef]
- Sanchez, D.H.; Schwabe, F.; Erban, A.; Udvardi, M.K.; Kopka, J. Comparative metabolomics of drought acclimation in model and forage legumes. Plant Cell Environ. 2012, 35, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Betti, M.; Pérez-Delgado, C.; García-Calderón, M.; Díaz, P.; Monza, J.; Márquez, A.J. Cellular stress following water deprivation in the model legume Lotus japonicus. Cells 2012, 1, 1089–1106. [Google Scholar] [CrossRef] [PubMed]
- Gil-Quintana, E.; Lyon, D.; Staudinger, C.; Wienkoop, S.; González, E.M. Medicago truncatula and Glycine max: Different drought tolerance and similar local response of the root nodule proteome. J. Proteome Res. 2015, 14, 5240–5251. [Google Scholar] [CrossRef]
- López-Gómez, M.; Tejera, N.A.; Iribarne, C.; Herrera-Cervera, J.A.; Lluch, C. Different strategies for salt tolerance in determined and indeterminate nodules of Lotus japonicus and Medicago truncatula. Arch. Agron. Soil Sci. 2012, 58, 1061–1073. [Google Scholar] [CrossRef]
- Staudinger, C.; Mehmeti-Tershani, V.; Gil-Quintana, E.; Gonzalez, E.M.; Hofhansl, F.; Bachmann, G.; Wienkoop, S. Evidence for a rhizobia-induced drought stress response strategy in Medicago truncatula. J. Proteom. 2016, 136, 202–213. [Google Scholar] [CrossRef]
- Hardy, R.W.F.; Holsten, R.D.; Jackson, E.K.; Burns, R.C. The acetylene-ethylene assay for N2 fixation: Laboratory and field evaluation 1. Plant Physiol. 1968, 43, 1185–1207. [Google Scholar] [CrossRef] [PubMed]
- Vessey, J.K. Measurement of nitrogenase activity in legume root nodules: In defense of the acetylene reduction assay. Plant Soil 1994, 158, 151–162. [Google Scholar] [CrossRef]
- Durand, J.L.; Sheehy, J.E.; Minchin, F.R. Nitrogenase activity, photosynthesis and nodule water potential in soyabean plants experiencing water deprivation. J. Exp. Bot. 1987, 38, 311–321. [Google Scholar] [CrossRef]
- Gil-Quintana, E.; Larrainzar, E.; Arrese-Igor, C.; González, E.M. Is N-feedback involved in the inhibition of nitrogen fixation in drought-stressed Medicago truncatula? J. Exp. Bot. 2013, 64, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo, L.D.; Hunt, S.; Layzell, D.B. The role of oxygen in the regulation of nitrogenase activity in drought-stressed soybean nodules. Plant Physiol. 1994, 106, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Gil-Quintana, E.; Larrainzar, E.; Seminario, A.; Díaz-Leal, J.L.; Alamillo, J.M.; Pineda, M.; Arrese-Igor, C.; Wienkoop, S.; González, E.M. Local inhibition of nitrogen fixation and nodule metabolism in drought-stressed soybean. J. Exp. Bot. 2013, 64, 2171–2182. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, W.; Zehirov, G.; Szatmari, A.; Debreczeny, M.; Ishihara, H.; Kevei, Z.; Farkas, A.; Mikulass, K.; Nagy, A.; Tiricz, H.; et al. Plant peptides govern terminal differentiation of bacteria in symbiosis. Science 2010, 327, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Monahan-Giovanelli, H.; Pinedo, C.A.; Gage, D.J. Architecture of infection thread networks in developing root nodules induced by the symbiotic bacterium Sinorhizobium meliloti on Medicago truncatula. Plant Physiol. 2006, 140, 661–670. [Google Scholar] [CrossRef]
- Eckardt, N.A. The role of flavonoids in root nodule development and auxin transport in Medicago truncatula. Plant Cell 2006, 18, 1539–1540. [Google Scholar] [CrossRef]
- Wasson, A.P.; Pellerone, F.I.; Mathesius, U. Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell 2006, 18, 1617–1629. [Google Scholar] [CrossRef]
- Sańko-Sawczenko, I.; Łotocka, B.; Czarnocka, W. Expression analysis of PIN genes in root tips and nodules of Medicago truncatula. Int. J. Mol. Sci. 2016, 17, 1197. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.W.; Fisher, K.; Dean, D.R. Nitrogenase structure and function: A biochemical-genetic perspective. Annu. Rev. Microbiol. 1995, 49, 335–366. [Google Scholar] [CrossRef] [PubMed]
- Udvardi, M.; Poole, P.S. Transport and Metabolism in Legume-Rhizobia Symbioses. Annu. Rev. Plant Biol. 2013, 64, 781–805. [Google Scholar] [CrossRef] [PubMed]
- Crespi, M.; Gálvez, S. Molecular mechanisms in root nodule development. J. Plant Growth Regul. 2000, 19, 155–166. [Google Scholar] [PubMed]
- Evans, P.J.; Gallesi, D.; Mathieu, C.; Hernandez, M.J.; de Felipe, M.; Halliwell, B.; Puppo, A. Oxidative stress occurs during soybean nodule senescence. Planta 1999, 208, 73–79. [Google Scholar] [CrossRef]
- Morris, P.C. MAP kinase signal transduction pathways in plants. New Phytol. 2001, 151, 67–89. [Google Scholar] [CrossRef] [Green Version]
- Vinardell, J.M.; Fedorova, E.; Cebolla, A.; Kevei, Z.; Horvath, G.; Kelemen, Z.; Tarayre, S.; Roudier, F.; Mergaert, P.; Kondorosi, A.; et al. Endoreduplication mediated by the anaphase-promoting complex activator CCS52A is required for symbiotic cell differentiation in Medicago truncatula nodules. Plant Cell 2003, 15, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Brewin, N.J. Plant Cell Wall Remodelling in the rhizobium–legume symbiosis. Crit. Rev. Plant Sci. 2004, 23, 293–316. [Google Scholar] [CrossRef]
- Hungria, M.; Stacey, G. Molecular signals exchanged between host plants and rhizobia: Basic aspects and potential application in agriculture. Soil Biol. Bioch. 1997, 29, 819–830. [Google Scholar] [CrossRef]
- Zhu, J.-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Wi, S.J.; Park, K.Y. Antisense expression of carnation cDNA encoding ACC synthase or ACC oxidase enhances polyamine content and abiotic stress tolerance in transgenic tobacco plants. Mol Cells 2002, 13, 209–220. [Google Scholar] [PubMed]
- Waizenegger, I.; Lukowitz, W.; Assaad, F.; Schwarz, H.; Jürgens, G.; Mayer, U. The Arabidopsis KNOLLE and KEULE genes interact to promote vesicle fusion during cytokinesis. Curr. Biol. 2000, 10, 1371–1374. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.K.; Bracker, C.A.; Hasegawa, P.M.; Handa, A.K.; Buckel, S.; Hermodson, M.A.; Pfankoch, E.; Regnier, F.E.; Bressan, R.A. Characterization of osmotin: A thaumatin-like protein associated with osmotic adaptation in plant cells. Plant Physiol. 1987, 85, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.C.; Lee, H.J.; Yum, S.S.; Soh, W.Y.; Cho, D.Y.; Auh, C.K.; Lee, T.K.; Soh, H.C.; Kim, Y.S.; Lee, S.C. Drought-inducible-but ABA-independent-thaumatin-like protein from carrot (Daucus carota L.). Plant Cell Rep. 2005, 24, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Akune, M.; Kogiso, M.; Imagama, Y.; Osuki, K.; Uchiumi, T.; Higashi, S.; Han, S.-Y.; Yoshida, S.; Asami, T.; et al. Control of nodule number by the phytohormone abscisic acid in the roots of two leguminous species. Plant Cell Physiol. 2004, 45, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, T.; Sugiyama, N.; Mizoguchi, M.; Hayashi, S.; Myouga, F.; Yamaguchi-Shinozaki, K.; Ishihama, Y.; Hirayama, T.; Shinozaki, K. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 17588–17593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong-Bo, S.; Zong-Suo, L.; Ming-An, S. LEA proteins in higher plants: Structure, function, gene expression and regulation. Colloids Surf. B Biointerfaces 2005, 45, 131–135. [Google Scholar] [CrossRef]
- Duque, P. A role for SR proteins in plant stress responses. Plant Signal Behav. 2011, 6, 49–54. [Google Scholar] [CrossRef]
- Abe, H.; Yamaguchi-Shinozaki, K.; Urao, T.; Iwasaki, T.; Hosokawa, D.; Shinozaki, K. Role of arabidopsis MYC and MYB homologs in drought- and abscisic acid-regulated gene expression. Plant Cell 1997, 9, 1859–1868. [Google Scholar]
- Jakoby, M.; Weisshaar, B.; Dröge-Laser, W.; Vicente-Carbajosa, J.; Tiedemann, J.; Kroj, T.; Parcy, F. bZIP Research Group bZIP transcription factors in Arabidopsis. Trends Plant Sci. 2002, 7, 106–111. [Google Scholar] [CrossRef]
- De Zélicourt, A.; Diet, A.; Marion, J.; Laffont, C.; Ariel, F.; Moison, M.; Zahaf, O.; Crespi, M.; Gruber, V.; Frugier, F. Dual involvement of a Medicago truncatula NAC transcription factor in root abiotic stress response and symbiotic nodule senescence. Plant J. 2012, 70, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Licausi, F.; Ohme-Takagi, M.; Perata, P. APETALA2/Ethylene Responsive Factor (AP2/ERF) transcription factors: Mediators of stress responses and developmental programs. New Phytol. 2013, 199, 639–649. [Google Scholar] [CrossRef]
- Solomon, M.; Belenghi, B.; Delledonne, M.; Menachem, E.; Levine, A. The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell 1999, 11, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Khanna-Chopra, R.; Srivalli, B.; Ahlawat, Y.S. Drought induces many forms of cysteine proteases not observed during natural senescence. Biochem. Biophys. Res. Commun. 1999, 255, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, S.; Boex-Fontvieille, E.; Reinbothe, C.; von Wettstein, D.; Reinbothe, S. The complex world of plant protease inhibitors: Insights into a Kunitz-type cysteine protease inhibitor of Arabidopsis thaliana. Commun. Integr. Biol. 2018, 11, e1368599. [Google Scholar] [CrossRef] [PubMed]
- Otegui, M.S.; Noh, Y.-S.; Martínez, D.E.; Petroff, M.G.V.; Staehelin, L.A.; Amasino, R.M.; Guiamet, J.J. Senescence-associated vacuoles with intense proteolytic activity develop in leaves of Arabidopsis and soybean. Plant J. 2005, 41, 831–844. [Google Scholar] [CrossRef] [Green Version]
- Kirkegaard, K.; Wang, J.C. Bacterial DNA topoisomerase I can relax positively supercoiled DNA containing a single-stranded loop. J. Mol. Biol. 1985, 185, 625–637. [Google Scholar] [CrossRef]
- Wang, J.C. Cellular roles of DNA topoisomerases: A molecular perspective. Nat. Rev. Mol. Cell Biol. 2002, 3, 430–440. [Google Scholar] [CrossRef]
- Okubo, M.; Murase, T. Glycogen-debranching enzyme. In Wiley Encyclopedia of Molecular Medicine; American Cancer Society; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; ISBN 978-0-471-20307-0. [Google Scholar]
- Christensen, S.K.; Mikkelsen, M.; Pedersen, K.; Gerdes, K. RelE, a global inhibitor of translation, is activated during nutritional stress. Proc. Natl. Acad. Sci. USA 2001, 98, 14328–14333. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, K.; Zavialov, A.V.; Pavlov, M.Y.; Elf, J.; Gerdes, K.; Ehrenberg, M. The Bacterial toxin RelE displays codon-specific cleavage of mRNAs in the ribosomal A site. Cell 2003, 112, 131–140. [Google Scholar] [CrossRef]
- Gerdes, K.; Christensen, S.K.; Løbner-Olesen, A. Prokaryotic toxin–antitoxin stress response loci. Nat. Rev. Microbiol. 2005, 3, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Miller, P.F.; Willard, J.; Olson, E.R. Functional and biochemical characterization of Escherichia coli sugar efflux transporters. J. Biol. Chem. 1999, 274, 22977–22984. [Google Scholar] [CrossRef]
- Mascher, T.; Helmann, J.D.; Unden, G. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol. Mol. Biol. Rev. 2006, 70, 910–938. [Google Scholar] [CrossRef] [PubMed]
- Hunte, C.; Screpanti, E.; Venturi, M.; Rimon, A.; Padan, E.; Michel, H. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature 2005, 435, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Sulavik, M.C.; Gambino, L.F.; Miller, P.F. The MarR repressor of the multiple antibiotic resistance (mar) operon in Escherichia coli: Prototypic member of a family of bacterial regulatory proteins involved in sensing phenolic compounds. Mol. Med. 1995, 1, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.P.; Grove, A. Ligand-responsive transcriptional regulation by members of the MarR family of winged helix proteins. Curr. Issues Mol. Biol. 2006, 8, 51–62. [Google Scholar]
- Schell, M.A. Molecular Biology of the LysR Family of transcriptional regulators. Annu. Rev. Microbiol. 1993, 47, 597–626. [Google Scholar] [CrossRef]
- Luo, L.; Yao, S.-Y.; Becker, A.; Rüberg, S.; Yu, G.-Q.; Zhu, J.-B.; Cheng, H.-P. Two New Sinorhizobium meliloti LysR-type transcriptional regulators required for nodulation. J. Bacteriol. 2005, 187, 4562–4572. [Google Scholar] [CrossRef]
- Milcamps, A.; de Bruijn, F.J. Identification of a novel nutrient-deprivation-induced Sinorhizobium meliloti gene (hmgA) involved in the degradation of tyrosine. Microbiology 1999, 145, 935–947. [Google Scholar] [CrossRef]
- Mandelstam, J. Protein turnover and its function in the economy of the cell. Ann. N. Y. Acad. Sci. 1963, 102, 621–636. [Google Scholar] [CrossRef]
- Lodwig, E.M.; Hosie, A.H.F.; Bourdès, A.; Findlay, K.; Allaway, D.; Karunakaran, R.; Downie, J.A.; Poole, P.S. Amino-acid cycling drives nitrogen fixation in the legume–Rhizobium symbiosis. Nature 2003, 422, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Fournier, J.; Timmers, A.C.J.; Sieberer, B.J.; Jauneau, A.; Chabaud, M.; Barker, D.G. Mechanism of infection thread elongation in root hairs of Medicago truncatula and dynamic interplay with associated rhizobial colonization. Plant Physiol. 2008, 148, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Vesper, S.J.; Bauer, W.D. Role of pili (fimbriae) in attachment of Bradyrhizobium japonicum to soybean roots. Appl. Environ. Microbiol. 1986, 52, 134–141. [Google Scholar] [PubMed]
- Zatakia, H.M.; Nelson, C.E.; Syed, U.J.; Scharf, B.E. ExpR coordinates the expression of symbiotically important, bundle-forming Flp pili with quorum sensing in Sinorhizobium meliloti. Appl. Environ. Microbiol. 2014, 80, 2429–2439. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Sung, J.-M. Effect of water stress on the reduction of nitrate and nitrite by soybean nodules. Plant Physiol. 1983, 73, 1065–1066. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.C.; Fink, G.R. Life and death in a macrophage: Role of the glyoxylate cycle in virulence. Eukaryot Cell 2002, 1, 657–662. [Google Scholar] [CrossRef]
- Plancke, C.; Vigeolas, H.; Höhner, R.; Roberty, S.; Emonds-Alt, B.; Larosa, V.; Willamme, R.; Duby, F.; Dhali, D.O.; Thonart, P.; et al. Lack of isocitrate lyase in Chlamydomonas leads to changes in carbon metabolism and in the response to oxidative stress under mixotrophic growth. Plant J. 2014, 77, 404–417. [Google Scholar] [CrossRef]
- Hamel, R.; Appanna, V.D.; Viswanatha, T.; Puiseux-Dao, S. Overexpression of isocitrate lyase is an important strategy in the survival of Pseudomonas fluorescens exposed to aluminum. Biochem. Biophys. Res. Commun. 2004, 317, 1189–1194. [Google Scholar] [CrossRef]
- Nandakumar, M.; Nathan, C.; Rhee, K.Y. Isocitrate lyase mediates broad antibiotic tolerance in Mycobacterium tuberculosis. Nat. Commun. 2014, 5, 4306. [Google Scholar] [CrossRef]
- Popov, V.O.; Lamzin, V.S. NAD(+)-dependent formate dehydrogenase. Biochem. J. 1994, 301, 625–643. [Google Scholar] [CrossRef] [PubMed]
- Manav, M.C.; Sofos, N.; Hove-Jensen, B.; Brodersen, D.E. The abc of phosphonate breakdown: A mechanism for bacterial survival. BioEssays 2018, 40, 1800091. [Google Scholar] [CrossRef] [PubMed]
- Fineran, P.C.; Blower, T.R.; Foulds, I.J.; Humphreys, D.P.; Lilley, K.S.; Salmond, G.P.C. The phage abortive infection system, ToxIN, functions as a protein–RNA toxin–antitoxin pair. Proc. Natl. Acad. Sci. USA 2009, 106, 894–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brissette, J.L.; Russel, M.; Weiner, L.; Model, P. Phage shock protein, a stress protein of Escherichia coli. Proc. Natl. Acad. Sci. USA 1990, 87, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Csonka, L.N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Mol. Biol. Rev. 1989, 53, 121–147. [Google Scholar]
- Kung, C.; Martinac, B.; Sukharev, S. Mechanosensitive channels in microbes. Annu. Rev. Microbiol. 2010, 64, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Romantsov, T.; Guan, Z.; Wood, J.M. Cardiolipin and the osmotic stress responses of bacteria. Biochim. Biophys. Acta 2009, 1788, 2092–2100. [Google Scholar] [CrossRef] [Green Version]
- Suárez, R.; Wong, A.; Ramírez, M.; Barraza, A.; Orozco, M.D.C.; Cevallos, M.A.; Lara, M.; Hernández, G.; Iturriaga, G. Improvement of drought tolerance and grain yield in common bean by overexpressing trehalose-6-phosphate synthase in rhizobia. Mol. Plant Microbe Interact. 2008, 21, 958–966. [Google Scholar] [CrossRef]
- Elsheikh, E.A.E.; Wood, M. Rhizobia and bradyrhizobia under salt stress: Possible role of trehalose in osmoregulation. Lett. Appl. Microbiol. 1990, 10, 127–129. [Google Scholar] [CrossRef]
- Benaroudj, N.; Lee, D.H.; Goldberg, A.L. Trehalose Accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J. Biol. Chem. 2001, 276, 24261–24267. [Google Scholar] [CrossRef]
- Hérouart, D.; Sigaud, S.; Moreau, S.; Frendo, P.; Touati, D.; Puppo, A. Cloning and characterization of the katA gene of Rhizobium meliloti encoding a hydrogen peroxide-inducible catalase. J. Bacteriol. 1996, 178, 6802–6809. [Google Scholar] [CrossRef] [PubMed]
- Storz, G.; Tartaglia, L.A.; Farr, S.B.; Ames, B.N. Bacterial defenses against oxidative stress. Trends Genet. 1990, 6, 363–368. [Google Scholar] [CrossRef]
- Panaretou, B.; Siligardi, G.; Meyer, P.; Maloney, A.; Sullivan, J.K.; Singh, S.; Millson, S.H.; Clarke, P.A.; Naaby-Hansen, S.; Stein, R.; et al. Activation of the ATPase activity of Hsp90 by the stress-regulated cochaperone Aha1. Mol. Cell. 2002, 10, 1307–1318. [Google Scholar] [CrossRef]
- Sauviac, L.; Philippe, H.; Phok, K.; Bruand, C. An extracytoplasmic function sigma factor acts as a general stress response regulator in Sinorhizobium meliloti. J. Bacteriol. 2007, 189, 4204–4216. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Salazar, J.M.; Salazar, E.; Encarnación, S.; Ramírez-Romero, M.A.; Rivera, J. Role of the extracytoplasmic function sigma factor RpoE4 in oxidative and osmotic stress responses in Rhizobium etli. J. Bacteriol. 2009, 191, 4122–4132. [Google Scholar] [CrossRef] [PubMed]
- Doherty, A.J.; Jackson, S.P.; Weller, G.R. Identification of bacterial homologues of the Ku DNA repair proteins. FEBS Lett. 2001, 500, 186–188. [Google Scholar] [CrossRef] [Green Version]
- Pearl, L.H. Structure and function in the uracil-DNA glycosylase superfamily. Mutat. Res./DNA Repair 2000, 460, 165–181. [Google Scholar] [CrossRef]
- Loza-Correa, M.; Gomez-Valero, L.; Buchrieser, C. Circadian clock proteins in Prokaryotes: Hidden rhythms? Front. Microbiol. 2010, 1, 130. [Google Scholar] [CrossRef]
- Vincent, J.M. A Manual for the Practical Study of Root Nodule Bacteria; Blackwell Scientific Publications Ltd.: Oxford, UK, 1970. [Google Scholar]
- Bergersen, F.J. Measurement of nitrogen fixation by direct means. In Methods for Evaluating Biological Nitrogen Fixation; Bergersen, F.J., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 1980; pp. 65–110. ISBN 978-0471277590. [Google Scholar]
- Sańko-Sawczenko, I.; Dmitruk, D.; Łotocka, B.; Różańska, E.; Czarnocka, W. Expression analysis of PIN genes in root tips and nodules of Lotus japonicus. Int. J. Mol. Sci. 2019, 20, 235. [Google Scholar] [CrossRef]
- Thimm, O.; Bläsing, O.; Gibon, Y.; Nagel, A.; Meyer, S.; Krüger, P.; Selbig, J.; Müller, L.A.; Rhee, S.Y.; Stitt, M. MAPMAN: A user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 2004, 37, 914–939. [Google Scholar] [CrossRef]
- Fujisawa, T.; Okamoto, S.; Katayama, T.; Nakao, M.; Yoshimura, H.; Kajiya-Kanegae, H.; Yamamoto, S.; Yano, C.; Yanaka, Y.; Maita, H.; et al. CyanoBase and RhizoBase: Databases of manually curated annotations for cyanobacterial and rhizobial genomes. Nucleic Acids Res. 2014, 42, D666–D670. [Google Scholar] [CrossRef] [PubMed]
- Mun, T.; Bachmann, A.; Gupta, V.; Stougaard, J.; Andersen, S.U. Lotus Base: An integrated information portal for the model legume Lotus japonicus. Sci. Rep. 2016, 6, 39447. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dai, X.; Liu, T.; Zhao, P.X. LegumeIP: An integrative database for comparative genomics and transcriptomics of model legumes. Nucleic Acids Res. 2012, 40, D1221–D1229. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A.M. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.L.; Moorman, A.F.M. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]
- Shirasawa, K.; Isobe, S.; Tabata, S.; Hirakawa, H. Kazusa Marker DataBase: A database for genomics, genetics, and molecular breeding in plants. Breed Sci. 2014, 64, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Nakamura, Y.; Kaneko, T.; Asamizu, E.; Kato, T.; Nakao, M.; Sasamoto, S.; Watanabe, A.; Ono, A.; Kawashima, K.; et al. Genome structure of the legume, Lotus japonicus. DNA Res. 2008, 15, 227–239. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kalita, M.; Stępkowski, T.; Łotocka, B.; Małek, W. Phylogeny of nodulation genes and symbiotic properties of Genista tinctoria bradyrhizobia. Arch. Microbiol. 2006, 186, 87–97. [Google Scholar] [CrossRef]
- Trump, B.F.; Smuckler, E.A.; Benditt, E.P. A method for staining epoxy sections for light microscopy. J. Ultrastruct. Res. 1961, 5, 343–348. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sańko-Sawczenko, I.; Łotocka, B.; Mielecki, J.; Rekosz-Burlaga, H.; Czarnocka, W. Transcriptomic Changes in Medicago truncatula and Lotus japonicus Root Nodules during Drought Stress. Int. J. Mol. Sci. 2019, 20, 1204. https://doi.org/10.3390/ijms20051204
Sańko-Sawczenko I, Łotocka B, Mielecki J, Rekosz-Burlaga H, Czarnocka W. Transcriptomic Changes in Medicago truncatula and Lotus japonicus Root Nodules during Drought Stress. International Journal of Molecular Sciences. 2019; 20(5):1204. https://doi.org/10.3390/ijms20051204
Chicago/Turabian StyleSańko-Sawczenko, Izabela, Barbara Łotocka, Jakub Mielecki, Hanna Rekosz-Burlaga, and Weronika Czarnocka. 2019. "Transcriptomic Changes in Medicago truncatula and Lotus japonicus Root Nodules during Drought Stress" International Journal of Molecular Sciences 20, no. 5: 1204. https://doi.org/10.3390/ijms20051204
APA StyleSańko-Sawczenko, I., Łotocka, B., Mielecki, J., Rekosz-Burlaga, H., & Czarnocka, W. (2019). Transcriptomic Changes in Medicago truncatula and Lotus japonicus Root Nodules during Drought Stress. International Journal of Molecular Sciences, 20(5), 1204. https://doi.org/10.3390/ijms20051204






