1. Introduction
Neuro traumatology can impact the whole nervous system. It is mainly caused by neural traumata, which itself is caused by traffic accidents, tumor damages, and side effects from neurosurgery [
1]. Neural damages always lead to the physical and psychological incapacitation of patients and reduce their ability to work [
2]. Thus, the regeneration of damaged neurons is a fundamental goal of neuroscience and clinical medicine.
Electrical stimulation (ES) is considered a potentially useful therapeutic for the nervous system and for wound healing [
3,
4]. Electro-excitable tissues (such as peripheral nerves, the central nervous system, the deep brain, and muscles) are considered useful candidates for clinical treatment via ES. When their threshold is reached under an electrical field, portions of the axon membranes of electro-excitable tissues are artificially depolarized. This causes action potential across axons [
5]. Although the structural and functional recovery of a damaged nervous system depends upon a variety of factors, reports elsewhere support ES use for the promotion of the neurite growth of treated nerve cells. They reported improved survival rates and better functional preservation of the central nervous system after injury [
6].
A fundamental issue in the development of electrically stimulated regeneration of neurons is the correct selection of electrodes that have enough of a safe-charge-injection limit (Q
inj, i.e., electrochemical capacitance). However, the regular clinical use of ES was thwarted because of the absence of suitable stimulation electrodes with a high Q
inj, good biocompatibility, and long-term stability [
7]. For example, ES of peripheral nerves requests a relatively high charge per pulse, i.e., high charge storage capacity across a relatively small sized electrode [
8]. Conventional metal-based stimulation electrodes, such as platinum or metal oxide (i.e., iridium oxide) coated electrodes [
9], were found to be not suitable for neural stimulation due to their poor electroactivity. To this end, electrically conducting polymers (CPs) were incorporated in reports elsewhere, as they had better electroactivity properties than conventional neural electrodes [
10]. Recently, graphene based nanoelectrodes were described that provided promising electrical, mechanical, and biocompatible properties [
11,
12]. The two-dimensional (2D) π–π conjugated plane of the graphene matrix enables a fast electron-delivery channel between the electrode and the cells. This increases the cell response to an applied electrical field during cell regeneration. The large surface area of graphene helps to integrate it with cells. Unfortunately, graphene-only electrodes have a low electroactivity and so are not suitable for use in ES. Using nitrogen-doping of graphene was also observed to improve the electroactivity of graphene-based nanoelectrodes [
13]. The incorporation of a range of nitrogen-containing polymers (including conducting polymers) with graphene was reported as an efficient way to enhance the electroactivity of graphene [
14].
In this work, we demonstrate a facile but efficient way to prepare a conducting polymer (e.g., polyaniline), functionalized with graphene via a polymerization enhanced edge-functionalization ball-milling (EFBM) method. Along with other groups from reports elsewhere [
15,
16], we discovered that the EFBM method can provide an eco-friendly, low cost, facile, and efficient technique for preparing chemical functional groups (such as carboxyl- and carbonyl-) modified graphene for multifunctional applications. We also reported that nitrogen-containing polymers, such as chitosan, can be added to form highly electroactive nitrogen-doped graphene via the EFBM method [
14]. In the present research, the EFBM technique was further improved using a monomer polymerization process. Typically, a nitrogen-containing monomer, such as aniline, was ball milled together with graphite powder. Using this process, the edge-functionalization of aniline with graphite layers (via active nitrogen sites) is formed first. Then, polymerization of aniline occurs because of the heat from the milling friction. The formation of polymer chains increases the layered distance of the graphite and further helps the exfoliation of graphene nanosheets during milling. The formed polyaniline functionalized graphene (PANI-G) has various useful properties. These include high electroactivity (because of its edge nitrogen-doped graphene and conducting polymers), excellent mechanical and electrical properties (because of its graphene matrix), and a high biocompatibility. These properties make the PANI-G a potentially useful nanoelectrode for ES.
The as-synthesized PANI-G was then applied here to the growth of PC12 cells. PC12 is a cell line derived from the pheochromocytoma of the rat adrenal medulla. PC12 cells are frequently used as a cell model for neuronal studies since PC12 cells can easily differentiate into neuron-like cells [
17]. In this work, an alternating electrical field was applied to the PC12 cell-cultured PANI-G nanoelectrode. This significantly increased the axon length, by 60%, with no adverse impact on the cell density and enhanced the wound regeneration ability of PC12 cells. This may show that the as-synthesized nanoscale ES system could be useful for the regeneration of neurons.
3. Discussion
Improvements to conventional ES techniques are highly needed since there is a demand for better nerve damage therapies [
18]. As described in our previous reports and other reports elsewhere, the lack of high-electroactive stimulation electrodes is a key obstacle for the use of ES in clinics. In this work, we synthesized nitrogen edge-functionalized graphene (PANI-G) using aniline and graphite via an efficient polymerization-enhanced EFBM method. The PANI-G made with this technique exhibited high electroactivity and biocompatibility, important for the effective application of ES. To prepare scaffold nanoelectrodes for the ES on PC12 cells, PANI-G was cast onto flexible ITO films. A 60% increase in axon length and an increase in the number of regenerated PC12 cells that were damaged were observed.
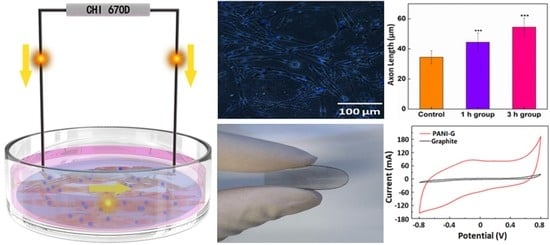
During the application of ES, the extracellular region is driven to a more negative potential while the intracellular compartment is driven to a more positive potential. A charge is thus transferred across the cell membranes due to passive cell membrane properties and active ion channels. The Qinj of the stimulation electrode is a limiting factor for the ES efficiency. The PANI-G reported here had a high electrochemical capacitance and electroactivity, which are attractive for the efficient application of ES. Particularly, the nitrogen edge-functionalized graphene preparation method reported here was an eco-friendly, facile, and efficient technique. Furthermore, good biocompatibility (more than 80% cell viability) of the PANI-G is also important for the ES application.
The ES process parameters used impacted the stimulation effects. Modifiable parameters include the applied potential waveform type, stimulation frequency, stimulation duration, and intensity of the applied electric field. (1) For example, the use of monophasic pulsingstimulation can be effective, but it causes bio-damage during long-term stimulation due to the great negative overpotentialscaused during pulsing. Compared to monophasic pulsing, biphasic pulsing creates lower negative overpotentials. During biphasic pulsing, the first stimulating phase elicits the initiation of an action potential, while the secondreversal phase reverses the direction of electrochemical processes occurring from the first phase. (2) It was observed that 1 h per day of ES was effective in promoting both motor and sensory axon regeneration [
18]. Stimulation effects, however, were not dependent onits frequency. For an instance, a longer stimulation frequency had no impact on accelerating the outgrowth of sensory nerve [
19]. In this study, we found that the cell density of PC12 cells remained unchanged, but the average axon length increased an extra 30%, when ES frequency was increased from 1 to 3 h per day. This work may provide how various stimulation goals can be meet, especially those focused on the axon length of neurons. (3) This work illustrated that stimulation duration played a greater role in nerve regeneration than previously thought. A significant improvement in the axon length of PC12 cells was obtained when the ES time was increased from 3 to 5 days. The axon length decreased when the ES time was shortened from 5 to 7 days. This may be attributed to the apoptosis of PC12 cells. Generally, most PC12 cells went through apoptosis and necrosis after culturing for 7 days [
20]. In this work, however, PC12 cells maintained healthy cell morphology with significantly enhanced axon length, when compared to the control. This suggests that the ES treatment may improve the anti-aging ability of PC12 cells. (4) The intensity of the applied electrical field was also observed to influence the outcomes of stimulation. This work described that ±500 mV/cm was the best step-potential-range for the stimulated improvement of axon length of PC12 cells on a PANI-G nanoelectrode. Reports elsewhere suggest that 500 mV/cm can significantly enhance the neurite outgrowth of PC12 cells on conductive MEH-PPV: PCL electrospun nanofibers [
21]. The neuronal differentiation of PC12 cells was reported elsewhere to be enhanced in an electric field intensity of 30 to 80 mV/mm [
22]. Thus, the intensity of the applied electric field varied depending upon the types of both the ES electrodes used and the cells tested.
The function of the nervous system is dependent on the highly specific connections between neurons, achieved by neurite extension to correct targets [
23]. The regeneration of axons is thus improved through functional recovery after nerve injury [
24]. The changes to cells were mediated by the autocrine or paracrine action of neurotrophins, released by neurons during ES [
25]. In this work, ES using PANI-G nanoelectrodes could repair a mechanically damaged PC12 cell model. This suggests that the ES technique described here also enhanced the autocrine or paracrine action of neurotrophins.
4. Materials and Methods
4.1. Synthesis and Characterization of PANI-G
The PANI-G was prepared via a polymerization-enhanced edge-functional ball-milling method. In a typical preparation, 10 mg graphite powders (Qingdao Haida Corporation, Qingdao, China) were placed in 10 µL aniline (Sigma-Aldrich, St. Louis, CA, USA). The mixture was milled in a planetary ball-milling machine (Nanjing NanDa Instrument Plant, Nanjing, China) set at a spin rate of 400 to 500 rpm for 6 to 8 h. The formed material was then transferred to a centrifuge tube containing 50 mL dehydrated alcohol. It was then centrifuged at 1000 rpm for 10 min to remove any remaining graphite and large sized-particles left in the precipitate. The supernatant was collected and re-centrifuged at 6000 rpm for 10 min to remove the remaining monomer left in the supernatant. The as-processed precipitate was then placed in a dialysis bag (molecular weight cutoff: 8000–14,000, Ye’yuan Biological Ltd., Shanghai, China). It was soaked in dehydrated alcohol for 72 h to further remove any remaining impurities. The final PANI-G materials were dispersed in HBSS (pH = 7.3, Gibco, Waltham, MA, USA) before testing it on biological samples.
The physiochemical properties of the as-prepared PANI-G nanosheets were characterized using Raman spectroscopy (Nicolet 6700, Thermo Scientific, Waltham, MA, USA) and atomic force microscopy (Digital Instruments Mutimode 8, Bruker, Billerica, MA, USA). The electrochemical properties and ES were performed using a CHI 760D electrochemical work station. Cyclic voltammetry of nanomaterials was carried out on a PANI-G coated glassy carbon (GC) electrode by dropping PANI-G onto a GC electrode (3 mm i.d.) and allowing it to be dried in air.
4.2. Fabrication of the PANI-G Nanoelectrodes
Indium tin oxide (ITO) coated conducting film (Zhuhai Kaiwei Technology Ltd., Zhuhai, China) was used as the supporting substrate for the PANI-G membrane. The ITO film was pre-treated by applying a radio-frequency glow discharge plasma [
26] in an oxygen atmosphere for 10 s to clean its surface and improve its hydrophilicity. A total of 40 µg/mL PANI-G dispersion in HBSS was then spin-coated onto the ITO film. The resulting film remained in a 37 °C incubator overnight to remove the solvent. The amount of PANI-G in the as-prepared PANI-G/ITO electrode was approximately 20 µg/cm
2.
4.3. Cell Culture
PC12 cells (Wuhan Procell Life Technology, Wuhan, China) were cultured in RPMI 1640 Medium (Gibco, Waltham, USA) supplemented with 10% heat-inactivated qualified fetal bovine serum (FBS, Gibco) and 0.2% gentamicin solution (50 mg/mL, Gibco). The cells were then incubated at 37 °C with 5% CO2 in a humid atmosphere. The PANI-G/ITO membrane was cut into circular pieces to fit into the culture wells. The membrane was sterilized by a 75% ethanol solution and exposed to ultraviolet light for 1 h, prior to its use in cell culturing. The biocompatibility of PANI-G was tested using a Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan) assay. This assay measures cell viability in cell proliferation and cytotoxicity using a sensitive colorimetric reaction. Dehydrogenase activities within cells reduce a highly water-soluble tetrazolium salt, WST-8 (developed by Dojindo). This forms a yellow-colored formazan dye soluble in the culture media. The amount of this dissolved dye can be used to count the number of living cells, which is proportional to the amount of dissolved dye. In this work, PC12 cells were seeded in a 96-well plate at a density of 5000 cells/well. Cells were pre-incubated for 24 h in a humidified incubator (at 37 °C, 5% CO2). PANI-G at various concentrations was added to the cell wells and co-cultured with nanomaterials over 24 to 72 h, before washing the cells with HBSS. A total of 10 µL CCK-8 solution was added into each well. The culture plate was then placed in the incubator over 0.5 to 2 h. The culture media absorbance was measured at 450 nm with a microplate reader (SpectraMax M5, Molecular Devices, San Jose, CA, USA).
4.4. Electrical Stimulation
PC12 cells were seeded on the PANI-G/ITO electrode to a density of 0.25 × 10
3/mm
2 and pre-cultured for 24 h. A platinum (Pt) electrode (ϕ = 0.5 mm) was contacted with the PANI-G/ITO electrode, while another Pt electrode was placed in the cell culture medium. The distance between these electrodes remained at 1 cm. An electrical field was applied to these cells using a double-pulsed potential chronoamperometry. A schematic set-up of the electrical stimulation is shown in
Figure S11.
A forward 500 mV/cm potential and a reverse −500 mV/cm potential were applied. ES was carried out for 1 or 3 h each day. The stimulation was run for 3, 5, and 7 days, respectively. After this treatment, the medium was refreshed immediately to avoid its contamination. PC12 cells were then maintained in the incubator at 37 °C under 5% CO2. The properties of PC12 cells were measured after ES. As a negative control, PC12 cells were also cultured on pristine ITO film (that did not have a PANI-G coating) before testing with ES. Negative controls were also tested. PC12 cells were cultured in plates with no electrical stimulation.
4.5. Cell Morphology and Nucleus Staining
PC12 cells attached to PANI-G membranes were fixed by applying 4% paraformaldehyde (Aladdin, Shanghai, China) for 10 min and then permeabilized with 0.1% Triton-X 100 solution (Beyotime, Shanghai China) for 5 min. A total of 1 µg/mL 4’,6-diamidino-2-phenylindole (DAPI) was used to visualize nuclei. Superfluous dye stuff was washed away using PBS. A drop of antifade medium (Invitrogen, Waltham, MA, USA) was added to the cells, which was sealed with transparent nail polish. Images of samples were captured using fluorescent microscopy (Olympus, Shinjuku, Japan). The axon length of the PC12 cells was measured along the linear distance between the brink of a nucleus and the tip of an axon. The mean axon length was calculated by averaging the length of 300 measurements in each sample. These averages were then statistically analyzed across different samples. The mean cell density was calculated by imaging 3 fields in each sample and statistically analyzed. The cell density was countered using DAPI-stained fluorescence images that were analyzed with an Image-J software.
4.6. Statistical Analysis
A one-way analysis of variance (ANOVA) and post-hoc test statistically compared the changes in the axon length and cell density after electrical stimulation (
Figure 2 and
Figure 3). A
t-test for each variable tested was calculated by comparing the wound healing ability of the ES group to a negative control. Statistical differences were calculated using a SPSS 16.0 software (Version 23, Armonk, New York, NY, USA). A *
p < 0.05 was predetermined as significant difference in these calculations. A **
p < 0.01 indicates a higher significance and a ***
p < 0.005 represents a very high significance.