Xenopus Oocyte’s Conductance for Bioactive Compounds Screening and Characterization
Abstract
:1. Introduction
2. Results
2.1. Pharmacological Characterization of ATX Activity on Xenopus Oocytes Endogenous Currents
2.2. Pharmacological Characterization of SFE-Extract on Xenopus Oocytes’ Endogenous Currents
2.3. Comparison of ATX Versus SFE-Extract Effects on Endogenous Amiloride-Sensitive Sodium Current
2.4. Electrophysiological Characterization of ATX Versus SFE-Extract on Endogenous Amiloride-Sensitive Sodium Channels
3. Discussion
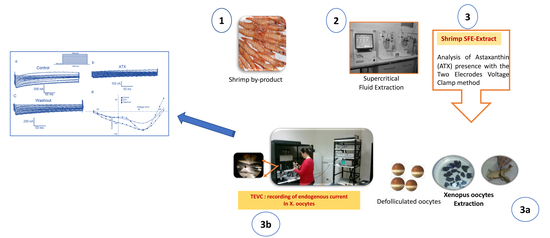
4. Materials and Methods
4.1. Extract Preparation from Shrimp By-Products
4.2. Isolation and Culture of Oocytes
4.3. Oocyte Treatment
4.4. Electrophysiological Measurements
4.5. Electrophysiological Results Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications. Mar. Drugs. 2014, 12, 128–152. [Google Scholar] [CrossRef]
- The state of world fisheries and aquaculture (FAO): Opportunities and challenges. FAO Rom. 2014, 223. Available online: http://www.fao.org/3/a-i3720e.pdf (accessed on 10 April 2019).
- Mao, X.; Guo, N.; Sun, J.; Xue, C. Comprehensive utilization of shrimp waste based on biotechnological methods: A review. J. Cleaner. Product. 2017, 143, 814–823. [Google Scholar] [CrossRef]
- Baccouche, B.; Mbarek, S.; Dellaa, A.; Hammoum, I.; Messina, C.M.; Santulli, A.; Ben Chaouacha-Chekir, R. Protective Effect of Astaxanthin on Primary Retinal Cells of the Gerbil Psammomys Obesus Cultured in Diabetic Milieu. J. Food Biochem. 2016. [Google Scholar] [CrossRef]
- Dose, J.; Matsugo, S.; Yokokawa, H.; Koshida, Y.; Okazaki, S.; Seidel, U.; Eggersdorfer, M.; Rimbach, G.; Esatbeyoglu, T. Free Radical Scavenging and Cellular Antioxidant Properties of Astaxanthin. Int. J. Mol. Sci. 2016, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Lobos, P.; Bruna, B.; Cordova, A.; Barattini, P.; Galáz, J.L.; Adasme, T.; Hidalgo, C.; Muñoz, P.; Paula-Lima, A. Astaxanthin Protects Primary Hippocampal Neurons against Noxious Effects of Aβ-Oligomers. Neural Plast. 2016. [Google Scholar] [CrossRef]
- Nazıroğlu, M.; Dikici, D.M.; Dursun, S. Role of oxidative stress and Ca2+ signaling on molecular pathways of neuropathic pain in diabetes: Focus on TRP channels. Neurochem. Res. 2012, 37, 2065–2075. [Google Scholar] [CrossRef]
- Brawek, B.; Garaschuk, O. Network-wide dysregulation of calcium homeostasis in Alzheimer’s disease. Cell Tissue Res. 2014, 357, 427–438. [Google Scholar] [CrossRef]
- Afify, E.A.; Khedr, M.M.; Omar, A.G.; Nasser, S.A. The involvement of K(ATP) channels in morphine-induced antinociception and hepatic oxidative stress in acute and inflammatory pain in rats. Fundam. Clin. Pharmacol. 2013, 27, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Sesti, F.; Liu, S.; Cai, S.Q. Oxidation of potassium channels by ROS: A general mechanism of aging and neurodegeneration? Trends Cell Biol. 2010, 20, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, J.I. Oxidative stress fine-tunes the dance of hERG K+ channels. J. Physiol. 2010, 588, 2975. [Google Scholar] [CrossRef] [PubMed]
- Pardo, L.A.; Stühmer, W. The roles of K(+) channels in cancer. Nat. Rev. Cancer. 2014, 14, 39–48. [Google Scholar] [CrossRef]
- Fedele, F.; Mancone, M.; Chilian, W.M.; Severino, P.; Canali, E.; Logan, S.; De Marchis, M.L.; Volterrani, M.; Palmirotta, R.; Guadagni, F. Role of genetic polymorphisms of ion channels in the pathophysiology of coronary microvascular dysfunction and ischemic heart disease. Basic Res Cardiol. 2013, 108, 387. [Google Scholar] [CrossRef] [Green Version]
- Averaimo, S.; Milton, R.H.; Duchen, M.R.; Mazzanti, M. Chloride intracellular channel 1 (CLIC1): Sensor and effector during oxidative stress. FEBS Lett. 2010, 584, 2076–2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miledi, R.; Woodward, R.M. Effects of defolliculation on membrane current responses of Xenopus oocytes. J. Physiol. 1989, 416, 601–621. [Google Scholar] [CrossRef] [PubMed]
- Baud, C.; Kado, R.T. Induction and disappearance of excitability in the oocyte of Xenopus laevis: A voltage-clamp study. J. Physiol. 1984, 356, 275–289. [Google Scholar] [CrossRef]
- Miledi, R.; Parker, I.; Sumikawa, K. Recording of single gamma-amino-butyrate- and acetylcholine-activated receptor channels translated by exogenous mRNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1983, 218, 481–484. [Google Scholar] [PubMed]
- Miledi, R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc. R. Soc. Lond. B. 1982, 215, 491–497. [Google Scholar]
- Parker, I.; Miledi, R. A calcium-independent chloride current activated by hyperpolarization in Xenopus oocytes. Proc. R. Soc. London. 1988, 233, 191–199. [Google Scholar]
- Parodi, J.; Romero, F.; Miledi, R.; Martínez-Torres, A. Some effects of the venom of the Chilean spider Latrodectus mactans on endogenous ion-currents of Xenopus laevis oocytes. Biochem. Biophys. Res. Commun. 2008, 375, 571–575. [Google Scholar] [CrossRef]
- Cheikh, A.; Maatoug, S.; El Ayeb, M.; Benkhalifa, R. Caractérisation des activités présentes dans le venin d’Androctonus australis hector sur les courants endogènes de l’ovocyte de Xénope. Arch Inst Past Tunis 2015, 3, 37–46. [Google Scholar]
- Weber, W.M. Ion currents of Xenopus laevis oocytes: State of the art. Biochimica et Biophysica Acta. 1999, 1421, 213–233. [Google Scholar] [CrossRef]
- Baud, C.; Kado, R.T.; Marcher, K. Sodium channels induced by depolarization of the Xenopus laevis oocyte. Proc. Natl. Acad. Sci. USA 1982, 79, 3188–3192. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.H.R.; Baczko, I.; Jones, L.; Fercho, M.; Light, P.E. Inhibition of cardiac voltage-gated sodium channels by grape polyphenols. Br. J. Pharmacol. 2006, 149, 657–665. [Google Scholar] [CrossRef] [Green Version]
- Kado, R.T.; Marcher, K.; Ozon, R. Demonstration of a long depolarization in the oocytes of Xenopus laevis. C. R. Seances Acad. Sci. D. 1979, 288, 1187–1189. [Google Scholar] [PubMed]
- Kado, R.T.; Baud, C. The rise and fall of electrical excitability in the oocyte of Xenopus laevis. J. Physiol. 1981, 77, 1113–1117. [Google Scholar]
- Li, H.F.; Chen, S.A.; Wu, S.N. Evidence for the stimulatory effect of resveratrol on Ca2+-activated K+ current in vascular endothelial cells. Cardiovasc. Res. 2000, 45, 1035–1045. [Google Scholar] [CrossRef] [Green Version]
- Dobrydneva, Y.; Williams, R.L.; Morris, G.Z.; Blackmore, P.F. Dietary phytoestrogens and their synthetic structural analogues as calcium channel blockers in human platelets. J. Cardiovasc. Pharmacol. 2002, 40, 399–410. [Google Scholar] [CrossRef]
- Wallerath, T.; Deckert, G.; Ternes, T.; Anderson, H.; Li, H.; Witte, K.; Förstermann, U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 2002, 106, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Li, Y. The chemical inducibility of mouse cardiac antioxidants and phase 2 enzymes in vivo. Biochem Biophys Res Commun. 2004, 317, 1080–1088. [Google Scholar] [CrossRef]
- Orsini, F.; Verotta, L.; Lecchi, M.; Restano, R.; Curia, G.; Redaelli, E.; Wanke, E. Resveratrol derivatives and their role as potassium channels modulators. J. Nat. Prod. 2004, 67, 421–426. [Google Scholar] [CrossRef]
- Kim, H.I.; Kim, T.H.; Song, J.H. Resveratrol inhibits Na+ currents in rat dorsal root ganglion neurons. Brain Res. 2005, 1045, 134–141. [Google Scholar] [CrossRef]
- López, M.; Arce, L.; Garrido, J.; Ríos, A.; Valcárcel, M. Selective extraction of astaxanthin from crustaceans by use of supercritical carbon dioxide. Talanta 2004, 20, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, H.; Dudley, S.C. Reactive oxygen species originating from mitochondria regulate the cardiac sodium channel. Circ. Res. 2010, 107, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.L.; Sanyal, S.; Pfahnl, A.E.; Jiao, Z.; Allen, J.; Liu, H.; Dudley, S.C., Jr. NF-κB-dependent transcriptional regulation of the cardiac scn5a sodium channel by angiotensin II. Am.J. Physiol. Cell Physiol. 2008, 294, C372–C379. [Google Scholar] [CrossRef] [PubMed]
- Lazrak, A.; Chen, L.; Jurkuvenaite, A.; Doran, S.F.; Liu, G.; Li, Q.; Lancaster, J.R.; Matalon, S. Regulation of alveolar epithelial Na+ channels by ERK1/2 in chlorine-breathing mice. Am. J. Respir. Cell Mol. Biol. 2012, 46, 342–354. [Google Scholar] [CrossRef]
- Sauer, H.; Wartenberg, M.; Hescheler, J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol. Biochem. 2001, 11, 173–186. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.N.; Zhao, D.; Wang, Q.S.; Gu, Y.C.; Ma, H.P.; Zhang, Z.R. Role of the epithelial sodium channel in salt-sensitive hypertension. Acta Pharmacol. Sin. 2011, 32, 789–797. [Google Scholar] [CrossRef] [Green Version]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- De la Fuente, J.C.; Oyarzún, B.; Quezada, N.; Del Valle, J.M. Solubility of carotenoid pigments (lycopene and astaxanthin) in supercritical carbon dioxide. Fluid Phase Equilibria 2006, 247, 90–95. [Google Scholar] [CrossRef]
- Simpson, B.K.; Haard, N.F. The use of enzymes to extract carotenoprotein from shrimp waste. J. Appl. Biochem. 1985, 7, 212–222. [Google Scholar]
- Dumont, J.N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J. Morphol. 1972, 136, 153–179. [Google Scholar] [CrossRef] [PubMed]






| ATX (µg) | 0.1 | 0.25 | 0.5 | 1 |
|---|---|---|---|---|
| xINa inhibition % | 34.83 ± 0.06 | 40.25 ± 0.10 | 55.75 ± 0.14 | 65.99 ± 0.17 |
| Product | Concentration (µg) | xINa Inhibition% |
|---|---|---|
| SFE-Extract | 10 | 36.46 ± 0.05% |
| SFE-Extract/ATX | 10/1 | 66.51 ± 0.07% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheikh, A.; Tabka, H.; Tlili, Y.; Santulli, A.; Bouzouaya, N.; Bouhaouala-Zahar, B.; Benkhalifa, R. Xenopus Oocyte’s Conductance for Bioactive Compounds Screening and Characterization. Int. J. Mol. Sci. 2019, 20, 2083. https://doi.org/10.3390/ijms20092083
Cheikh A, Tabka H, Tlili Y, Santulli A, Bouzouaya N, Bouhaouala-Zahar B, Benkhalifa R. Xenopus Oocyte’s Conductance for Bioactive Compounds Screening and Characterization. International Journal of Molecular Sciences. 2019; 20(9):2083. https://doi.org/10.3390/ijms20092083
Chicago/Turabian StyleCheikh, Amani, Hager Tabka, Yassine Tlili, Andrea Santulli, Noureddine Bouzouaya, Balkiss Bouhaouala-Zahar, and Rym Benkhalifa. 2019. "Xenopus Oocyte’s Conductance for Bioactive Compounds Screening and Characterization" International Journal of Molecular Sciences 20, no. 9: 2083. https://doi.org/10.3390/ijms20092083