DI-5-Cuffs: Lumbar Intervertebral Disc Proteoglycan and Water Content Changes in Humans after Five Days of Dry Immersion to Simulate Microgravity
Abstract
:1. Introduction
2. Objectives
3. Results
3.1. Plasma Volume
3.2. Blood Osmolality (mOsmol/kgH20)
3.3. Spine Height and Lumbar Lordosis
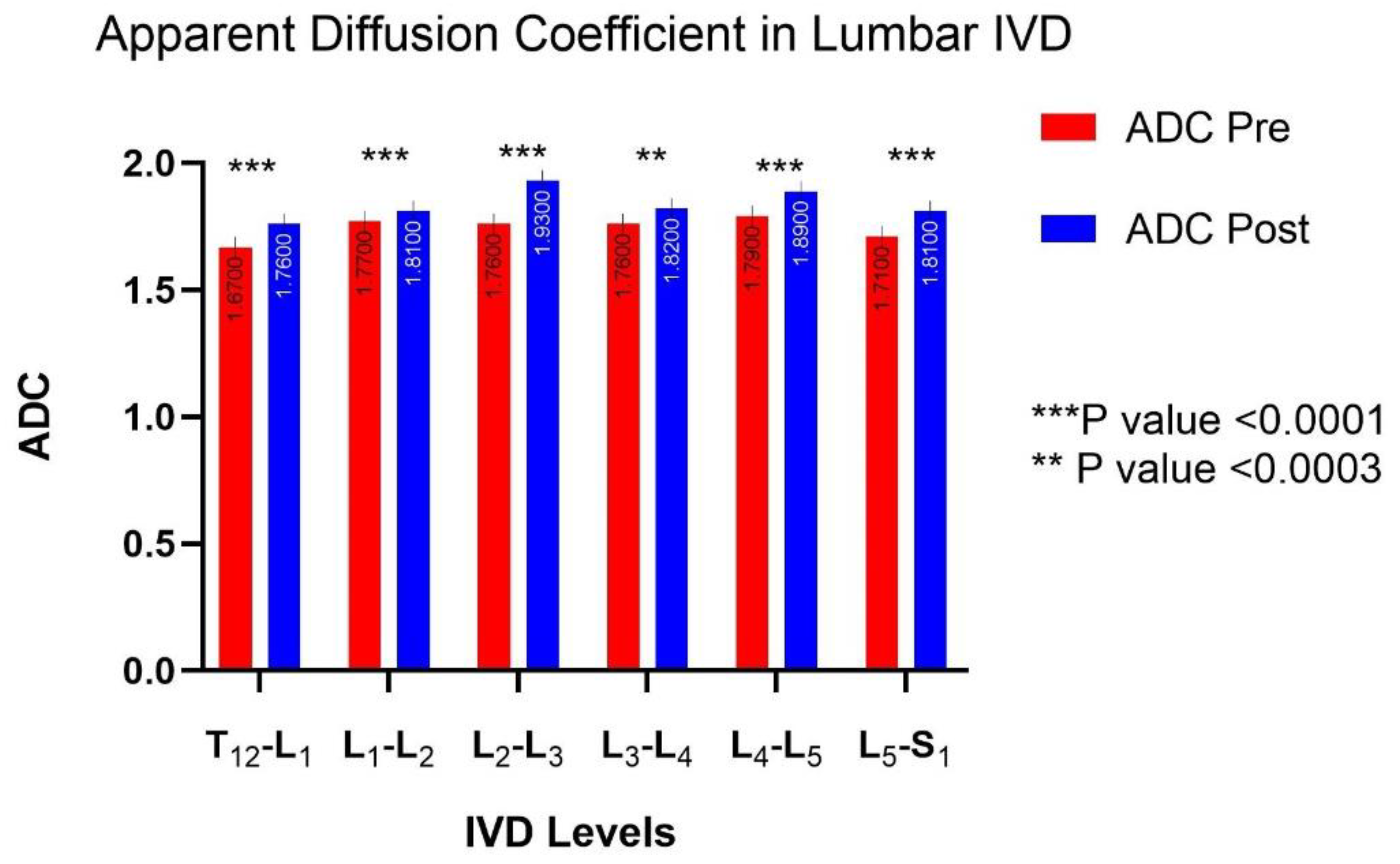
3.4. MRI Diffusion: Lumbar Vertebral Disc Water Content
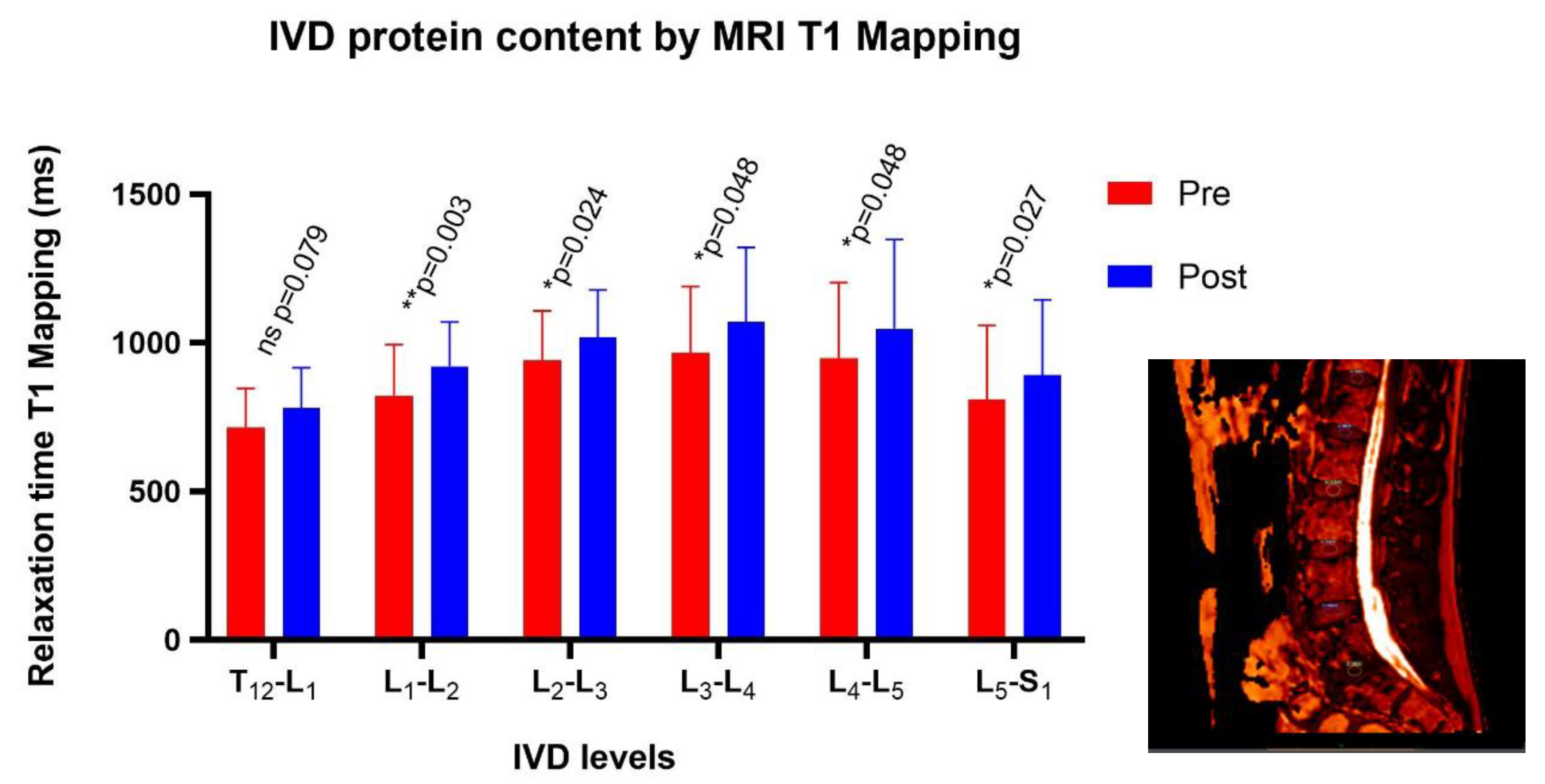
3.5. MRI T1-Mapping: Protein Content Inside the Nucleus Pulposus
3.6. Back Pain
4. Discussion
4.1. Spaceflight Countermeasures
4.2. Limitations
5. Methods
5.1. Subjects
5.2. General Protocol
5.3. Magnetic Resonance Imaging
5.3.1. Disc Water Content
5.3.2. Protein Content in IVD
5.3.3. MRI Spinal Height and Lumbar Curvature
5.3.4. Plasma Volume Variations
5.4. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Apparent diffusion coefficient |
| AF | Annulus fibrosus |
| ANOVA | Analysis of variance |
| B | Before dry immersion |
| BDC | Base data collection |
| CI | Confidence interval |
| DI | Dry immersion, day (D1 to D5) |
| DWI | Diffusion-weighted imaging |
| ECF | Extracellular fluid |
| FCD | Fixed charge density |
| IVD | Intervertebral disc |
| L | Lumbar vertebra (L1 to L5) |
| MRI | Magnetic resonance imaging |
| MRS | Magnetic resonance spectroscopy |
| NP | Nucleus pulposus |
| PV | Plasma volume |
| R | Recovery |
| SD | Standard deviation |
| TE | Echo time |
| Tx | Thoracic vertebra (T1 to T12) |
| TR | Repetition time |
References
- Kerstman, E.L.; Scheuring, R.A.; Barnes, M.G.; DeKorse, T.B.; Saile, L.G. Space Adaptation Back Pain: A Retrospective Study. Aviat. Space Environ. Med. 2012, 83, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, S.L.; Campbell, M.R.; Scheuring, R.; Feiveson, A.H. Risk of herniated nucleus pulposus among U.S. astronauts. Aviat. Space Environ. Med. 2010, 81, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Belavy, D.L.; Adams, M.; Brisby, H.; Cagnie, B.; Danneels, L.; Fairbank, J.; Hargens, A.R.; Judex, S.; Scheuring, R.A.; Sovelius, R.; et al. Disc herniations in astronauts: What causes them, and what does it tell us about herniation on earth? Eur. Spine J. 2016, 25, 144–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, D.A.; Scott, J.P.R. Spinal Health during Unloading and Reloading Associated with Spaceflight. Front. Physiol. 2018, 8, 1126. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.P.R.; Weber, T.; Green, D.A. Introduction to the Frontiers Research Topic: Optimization of Exercise Countermeasures for Human Space Flight—Lessons From Terrestrial Physiology and Operational Considerations. Front. Physiol. 2019, 10, 173. [Google Scholar] [CrossRef] [Green Version]
- Navasiolava, N.M.; Custaud, M.-A.; Tomilovskaya, E.S.; Larina, I.M.; Mano, T.; Gauquelin-Koch, G.; Gharib, C.; Kozlovskaya, I.B. Long-term dry immersion: Review and prospects. Eur. J. Appl. Physiol. 2011, 111, 1235–1260. [Google Scholar] [CrossRef] [Green Version]
- De Abreu, S.; Amirova, L.; Murphy, R.; Wallace, R.; Twomey, L.; Gauquelin-Koch, G.; Raverot, V.; Larcher, F.; Custaud, M.-A.; Navasiolava, N. Multi-System Deconditioning in 3-Day Dry Immersion without Daily Raise. Front. Physiol. 2017, 8, 799. [Google Scholar] [CrossRef] [Green Version]
- Treffel, L.; Dmitrieva, L.; Gauquelin-Koch, G.; Custaud, M.-A.; Blanc, S.; Gharib, C.; Millet, C. Craniomandibular System and Postural Balance after 3-Day Dry Immersion. PLoS ONE 2016, 11, e0150052. [Google Scholar] [CrossRef] [Green Version]
- Arbeille, P.; Avan, P.; Treffel, L.; Zuj, K.; Normand, H.; Denise, P. Jugular and Portal Vein Volume, Middle Cerebral Vein Velocity, and Intracranial Pressure in Dry Immersion. Aerosp. Med. Hum. Perform. 2017, 88, 457–462. [Google Scholar] [CrossRef]
- Treffel, L.; Mkhitaryan, K.; Gellee, S.; Gauquelin-Koch, G.; Gharib, C.; Blanc, S.; Millet, C. Intervertebral Disc Swelling Demonstrated by 3D and Water Content Magnetic Resonance Analyses after a 3-Day Dry Immersion Simulating Microgravity. Front. Physiol. 2016, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Treffel, L.; Massabuau, N.; Zuj, K.; Custaud, M.-A.; Gauquelin-Koch, G.; Blanc, S.; Gharib, C.; Millet, C. Pain and Vertebral Dysfunction in Dry Immersion: A Model of Microgravity Simulation Different from Bed Rest Studies. Pain Res. Manag. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treffel, L. Dysfonctions Vertébrales et Posturales après Simulations de la Microgravité. Ph.D. Thesis, Université de Strasbourg, Strasbourg, France, 2017. [Google Scholar]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef] [PubMed]
- Baldoni, M.; Gu, W. Effect of fixed charge density on water content of IVD during bed rest: A numerical analysis. Med. Eng. Phys. 2019, 70, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hou, J.; Lv, D.; Liang, W.; Jiang, X.; Han, H.; Quan, X. Multimodal quantitative magnetic resonance imaging for lumbar intervertebral disc degeneration. Exp. Ther. Med. 2017, 14, 2078–2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loerch, L.H. Exercise Countermeasures on ISS: Summary and Future Directions. Aerosp. Med. Hum. Perform. 2015, 86, A92–A94. [Google Scholar] [CrossRef]
- Winnard, A.; Nasser, M.; Debuse, D.; Stokes, M.; Evetts, S.; Wilkinson, M.; Hides, J.; Caplan, N. Systematic review of countermeasures to minimise physiological changes and risk of injury to the lumbopelvic area following long-term microgravity. Musculoskelet. Sci. Pract. 2017, 27, S5–S14. [Google Scholar] [CrossRef]
- Carvil, P.A.; Attias, J.; Evetts, S.N.; Waldie, J.M.; Green, D.A. The Effect of the Gravity Loading Countermeasure Skinsuit Upon Movement and Strength. J. Strength Cond. Res. 2017, 31, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Wood, K.N.; Murray, K.R.; Greaves, D.K.; Hughson, R.L. Inflight leg cuff test does not identify the risk for orthostatic hypotension after long-duration spaceflight. NPJ Microgravity 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Hackney, K.J.; Everett, M.; Scott, J.M.; Ploutz-Snyder, L. Blood flow-restricted exercise in space. Extreme Physiol. Med. 2012, 1, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind-Holst, M.; Cotter, J.D.; Helge, J.W.; Boushel, R.; Augustesen, H.; Van Lieshout, J.J.; Pott, F.C. Cerebral autoregulation dynamics in endurance-trained individuals. J. Appl. Physiol. 2011, 110, 1327–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J. Crew Height Measurement. The Apollo-Soyuz Test Project: Medical Report, NASA SP411; National Aeronautics and Space Administration: Washington, DC, USA, 1977. [Google Scholar]
- Gunga, H.-C. Human Physiology in Extreme Environments; Academic Press Inc.: San Diego, CA, USA, 2015. [Google Scholar]
- Bailey, J.F.; Miller, S.L.; Khieu, K.; O’Neill, C.W.; Healey, R.M.; Coughlin, D.G.; Sayson, J.V.; Chang, D.G.; Hargens, A.R.; Lotz, J.C. From the international space station to the clinic: How prolonged unloading may disrupt lumbar spine stability. Spine J. 2018, 18, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Styf, J.R.; Ballard, R.E.; Fechner, K.; Watenpaugh, D.E.; Kahan, N.J.; Hargens, A.R. Height increase, neuromuscular function, and back pain during 6 degrees head-down tilt with traction. Aviat. Space Environ. Med. 1997, 68, 24–29. [Google Scholar] [PubMed]
- Tomilovskaya, E.; Shigueva, T.; Sayenko, D.; Rukavishnikov, I.; Kozlovskaya, I. Dry Immersion as a Ground-Based Model of Microgravity Physiological Effects. Front. Physiol. 2019, 10, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilke, H.J.; Neef, P.; Caimi, M.; Hoogland, T.; Claes, L.E. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine 1999, 24, 755–762. [Google Scholar] [CrossRef]
- Schmidt, H.; Schilling, C.; Reyna, A.L.P.; Shirazi-Adl, A.; Dreischarf, M. Fluid-flow dependent response of intervertebral discs under cyclic loading: On the role of specimen preparation and preconditioning. J. Biomech. 2016, 49, 846–856. [Google Scholar] [CrossRef]
- Sayson, J.V.; Hargens, A.R. Pathophysiology of low back pain during exposure to microgravity. Aviat. Space Environ. Med. 2008, 79, 365–373. [Google Scholar] [CrossRef]
- Plomp, K.A.; Viðarsdóttir, U.S.; Weston, D.A.; Dobney, K.; Collard, M. The ancestral shape hypothesis: An evolutionary explanation for the occurrence of intervertebral disc herniation in humans. BMC Evol. Biol. 2015, 15, 68. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.G.; Healey, R.M.; Snyder, A.J.; Sayson, J.V.; Macias, B.R.; Coughlin, D.G.; Bailey, J.F.; Parazynski, S.E.; Lotz, J.C.; Hargens, A.R. Lumbar Spine Paraspinal Muscle and Intervertebral Disc Height Changes in Astronauts after Long-duration Spaceflight on the International Space Station. Spine 2016, 41, 1917. [Google Scholar] [CrossRef] [Green Version]
- Nachemson, A.L. Disc pressure measurements. Spine 1981, 6, 93–97. [Google Scholar] [CrossRef]
- Theodoridis, G.C.; Lee, J.S. Blood volume change and redistribution after change in posture. Aviat. Space Environ. Med. 1995, 66, 1097–1102. [Google Scholar]
- Robin, A.; Auvinet, A.; Degryse, B.; Murphy, R.; Bareille, M.-P.; Beck, A.; Gharib, C.; Gauquelin-Koch, G.; Daviet, A.; Larcher, F.; et al. DI-5-CUFFS: Venoconstrictive Thigh Cuffs Limit Body Fluid Changes but Not Orthostatic Intolerance Induced by a 5-Day Dry Immersion. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef]
- Vergroesen, P.-P.A.; Emanuel, K.S.; Peeters, M.; Kingma, I.; Smit, T.H. Are axial intervertebral disc biomechanics determined by osmosis? J. Biomech. 2018, 70, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.; Urban, J.P.; Roberts, S.; Menage, J. The fluid content of the human intervertebral disc. Comparison between fluid content and swelling pressure profiles of discs removed at surgery and those taken postmortem. Spine 1992, 17, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Campana, S. Evaluation of the Relationship between Biomechanical Properties and Imaging: In vitro Study of Intervertebral Disc. Ph.D. Thesis, Arts et Métiers ParisTech, Paris, France, 2004. [Google Scholar]
- Gaspar, A.M.; Appavou, M.-S.; Busch, S.; Unruh, T.; Doster, W. Dynamics of well-folded and natively disordered proteins in solution: A time-of-flight neutron scattering study. Eur. Biophys. J. EBJ 2008, 37, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Vernikos, J.; Schneider, V.S. Space, Gravity and the Physiology of Aging: Parallel or Convergent Disciplines? A Mini-Review. Gerontology 2010, 56, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Roughley, P.J.; Alini, M.; Antoniou, J. The role of proteoglycans in aging, degeneration and repair of the intervertebral disc. Biochem. Soc. Trans. 2002, 30, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Fontes, R.B.V.; Baptista, J.S.; Rabbani, S.R.; Traynelis, V.C.; Liberti, E.A. Normal aging in human lumbar discs: An ultrastructural comparison. PLoS ONE 2019, 14, e0218121. [Google Scholar] [CrossRef] [Green Version]
- De Vasconcellos Fontes, R.B.; Baptista, J.S.; Rabbani, S.R.; Traynelis, V.C.; Liberti, E.A. Structural and Ultrastructural Analysis of the Cervical Discs of Young and Elderly Humans. PLoS ONE 2015, 10, e0139283. [Google Scholar] [CrossRef]
- Wade, K.R.; Robertson, P.A.; Thambyah, A.; Broom, N.D. How healthy discs herniate: A biomechanical and microstructural study investigating the combined effects of compression rate and flexion. Spine 2014, 39, 1018–1028. [Google Scholar] [CrossRef]
- Murray, K.J.; Le Grande, M.R.; Ortega de Mues, A.; Azari, M.F. Characterisation of the correlation between standing lordosis and degenerative joint disease in the lower lumbar spine in women and men: A radiographic study. BMC Musculoskelet. Disord. 2017, 18, 330. [Google Scholar] [CrossRef]
- Bailey, J.F.; Hargens, A.R.; Cheng, K.K.; Lotz, J.C. Effect of microgravity on the biomechanical properties of lumbar and caudal intervertebral discs in mice. J. Biomech. 2014, 47, 2983–2988. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.F.; Garcia, K.M.; Sargsyan, A.E.; Ebert, D.; Riascos-Castaneda, R.F.; Dulchavsky, S.A. Preflight, In-Flight, and Postflight Imaging of the Cervical and Lumbar Spine in Astronauts. Aerosp. Med. Hum. Perform. 2018, 89, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Bogduk, N. Chapter 32—Functional anatomy of the spine. In Handbook of Clinical Neurology; Masdeu, J.C., González, R.G., Eds.; Neuroimaging Part II; Elsevier: Amsterdam, The Netherlands, 2016; Volume 136, pp. 675–688. [Google Scholar]
- Cao, P.; Kimura, S.; Macias, B.R.; Ueno, T.; Watenpaugh, D.E.; Hargens, A.R. Exercise within lower body negative pressure partially counteracts lumbar spine deconditioning associated with 28-day bed rest. J. Appl. Physiol. Bethesda Md 1985 2005, 99, 39–44. [Google Scholar] [CrossRef]
- Loehr, J.A.; Lee, S.M.C.; English, K.L.; Sibonga, J.; Smith, S.M.; Spiering, B.A.; Hagan, R.D. Musculoskeletal adaptations to training with the advanced resistive exercise device. Med. Sci. Sports Exerc. 2011, 43, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.; Bruce-Low, S.; Smith, D.; Osborne, N.; Thorkeldsen, A. Can specific loading through exercise impart healing or regeneration of the intervertebral disc? Spine J. 2015, 15, 2117–2121. [Google Scholar] [CrossRef] [PubMed]
- Pechenkova, E.; Nosikova, I.; Rumshiskaya, A.; Litvinova, L.; Rukavishnikov, I.; Mershina, E.; Sinitsyn, V.; Van Ombergen, A.; Jeurissen, B.; Jillings, S.; et al. Alterations of Functional Brain Connectivity After Long-Duration Spaceflight as Revealed by fMRI. Front. Physiol. 2019, 10, 761. [Google Scholar] [CrossRef]
- Shen, S.; Wang, H.; Zhang, J.; Wang, F.; Liu, S.-R. Diffusion Weighted Imaging, Diffusion Tensor Imaging, and T2* Mapping of Lumbar Intervertebral Disc in Young Healthy Adults. Iran. J. Radiol. 2016, 13. [Google Scholar] [CrossRef] [Green Version]
- Noguerol, T.M.; Luna, A.; Cabrera, M.G.; Riofrio, A.D. Clinical applications of advanced magnetic resonance imaging techniques for arthritis evaluation. World J. Orthop. 2017, 8, 660. [Google Scholar] [CrossRef]
- Schleich, C.; Müller-Lutz, A.; Eichner, M.; Schmitt, B.; Matuschke, F.; Bittersohl, B.; Zilkens, C.; Wittsack, H.-J.; Antoch, G.; Miese, F. Glycosaminoglycan Chemical Exchange Saturation Transfer of Lumbar Intervertebral Discs in Healthy Volunteers. Spine 2016, 41, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Schleich, C.; Müller-Lutz, A.; Zimmermann, L.; Boos, J.; Schmitt, B.; Wittsack, H.-J.; Antoch, G.; Miese, F. Biochemical imaging of cervical intervertebral discs with glycosaminoglycan chemical exchange saturation transfer magnetic resonance imaging: Feasibility and initial results. Skeletal Radiol. 2016, 45, 79–85. [Google Scholar] [CrossRef]
- Galley, J.; Balagué, F. Revisiting Radiographic L5-S1 Parallelism Using MRI T1 Mapping. J. Belg. Soc. Radiol. 2018, 102. [Google Scholar] [CrossRef] [PubMed]
- Andersson, G.B.; Murphy, R.W.; Ortengren, R.; Nachemson, A.L. The influence of backrest inclination and lumbar support on lumbar lordosis. Spine 1979, 4, 52–58. [Google Scholar] [CrossRef] [PubMed]




| D1_Evening | D3_Morning | D5_Morning | D5_Evening | R0_Morning | |
|---|---|---|---|---|---|
| Control | −8.9 ± 4.6 * | −22.1 ± 6 * | −19.2 ± 8.5 * | −11.8 ± 6.4 * | −15.3 ± 6.7 * |
| Cuffs | −6.8 ± 5 * | −19.1 ± 5.4 * | −14.6 ± 4.3 * | −6.8 ± 5 * | −10 ± 4.4 * |
| Control | Cuffs | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | B-1_m | DI-1_e | DI-3_m | DI-5_m | B-1_m | DI-1_e | DI-3_m | DI-5_m |
| Sodium, mmol/L | 141 ± 2 | 140 ± 2 | 139 ± 2 | 139 ± 2 | 141 ± 2 | 139 ± 3 | 138 ± 4 * | 138 ± 5 * |
| Potassium, mmol/L | 3.8 ± 0.2 | 4 ± 0.3 * | 3.9 ± 0.2 | 3.9 ± 0.2 | 3.9 ± 0.2 | 4.0 ± 0.2 | 4.0 ± 0.2 | 4.0 ± 0.2 |
| Proteins, g/L | 67 ± 4 | 68 ± 2 | 73 ± 3 * | 71 ± 4 * | 67 ± 3 | 66 ± 3 | 68 ± 4 # | 65 ± 4 # |
| Osmolality, mOsmol/kg | 292 ± 5 | 294 ± 3 | 293 ± 4 | 293 ± 5 | 293 ± 4 | 294 ± 7 | 293 ± 5 | 292 ± 6 |
| Age (y) | Height (cm) | Spine Height by MRI | Lumbar lordosis (°) | Weight (kg) | BMI (kg/m2) | VO2max (ml/min/kg) | HR (bpm) | T (°C) | SBP (mmHg) | DBP (mmHg) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 9) | 33.9 ± 7.1 | 176 ± 6 | 59.28 ± 0.37 | 46.82 ± 3.66 | 73.9 ± 7.5 | 23.9 ± 1.7 | 46.5 ± 8.1 | 57 ± 6 | 36.4 ± 0.3 | 115 ± 11 | 68 ± 5 |
| Cuffs (n = 9) | 34.1 ± 3.7 | 180 ± 4 | 61.45 ± 0.40 | 41.45 ± 11.17 | 74.3 ± 8.8 | 22.7 ± 1.8 | 46.9 ± 5.8 | 58 ± 8 | 36.4 ± 0.5 | 117 ± 10 | 68 ± 9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treffel, L.; Navasiolava, N.; Mkhitaryan, K.; Jouan, E.; Zuj, K.; Gauquelin-Koch, G.; Custaud, M.-A.; Gharib, C. DI-5-Cuffs: Lumbar Intervertebral Disc Proteoglycan and Water Content Changes in Humans after Five Days of Dry Immersion to Simulate Microgravity. Int. J. Mol. Sci. 2020, 21, 3748. https://doi.org/10.3390/ijms21113748
Treffel L, Navasiolava N, Mkhitaryan K, Jouan E, Zuj K, Gauquelin-Koch G, Custaud M-A, Gharib C. DI-5-Cuffs: Lumbar Intervertebral Disc Proteoglycan and Water Content Changes in Humans after Five Days of Dry Immersion to Simulate Microgravity. International Journal of Molecular Sciences. 2020; 21(11):3748. https://doi.org/10.3390/ijms21113748
Chicago/Turabian StyleTreffel, Loïc, Nastassia Navasiolava, Karen Mkhitaryan, Emmanuelle Jouan, Kathryn Zuj, Guillemette Gauquelin-Koch, Marc-Antoine Custaud, and Claude Gharib. 2020. "DI-5-Cuffs: Lumbar Intervertebral Disc Proteoglycan and Water Content Changes in Humans after Five Days of Dry Immersion to Simulate Microgravity" International Journal of Molecular Sciences 21, no. 11: 3748. https://doi.org/10.3390/ijms21113748






