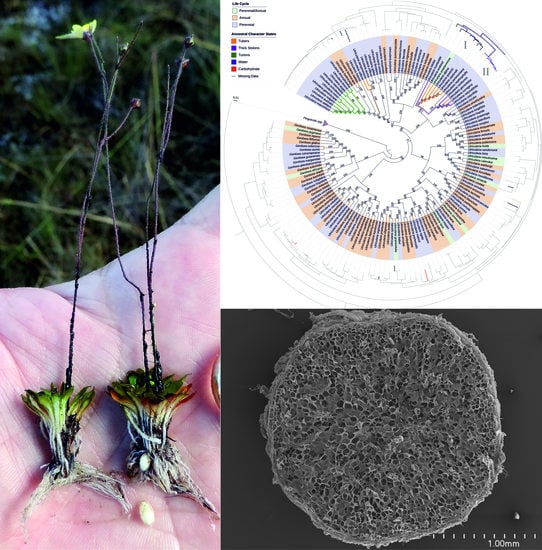
Structural Features of Carnivorous Plant (Genlisea, Utricularia) Tubers as Abiotic Stress Resistance Organs
Abstract
:1. Introduction
2. Results
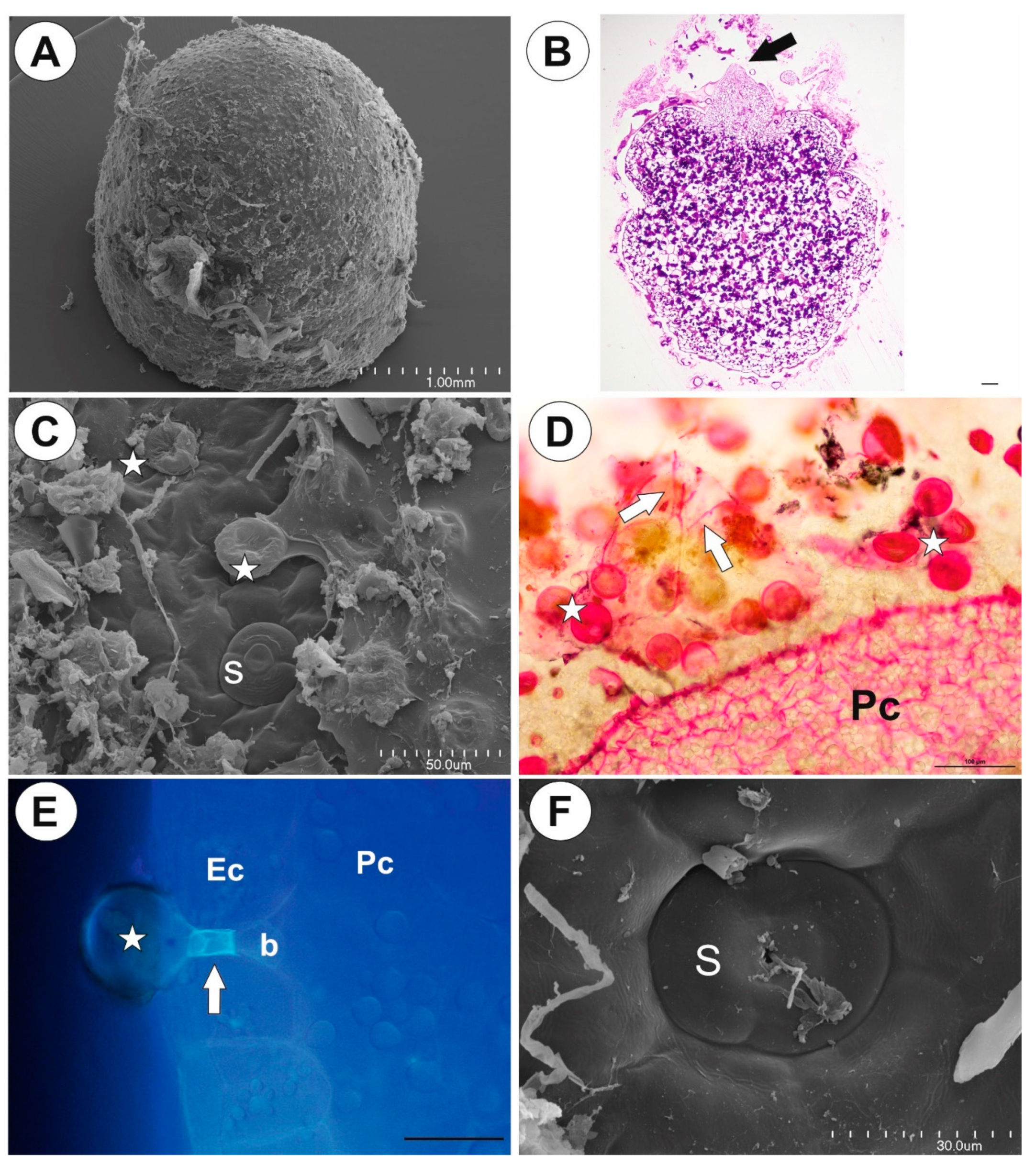
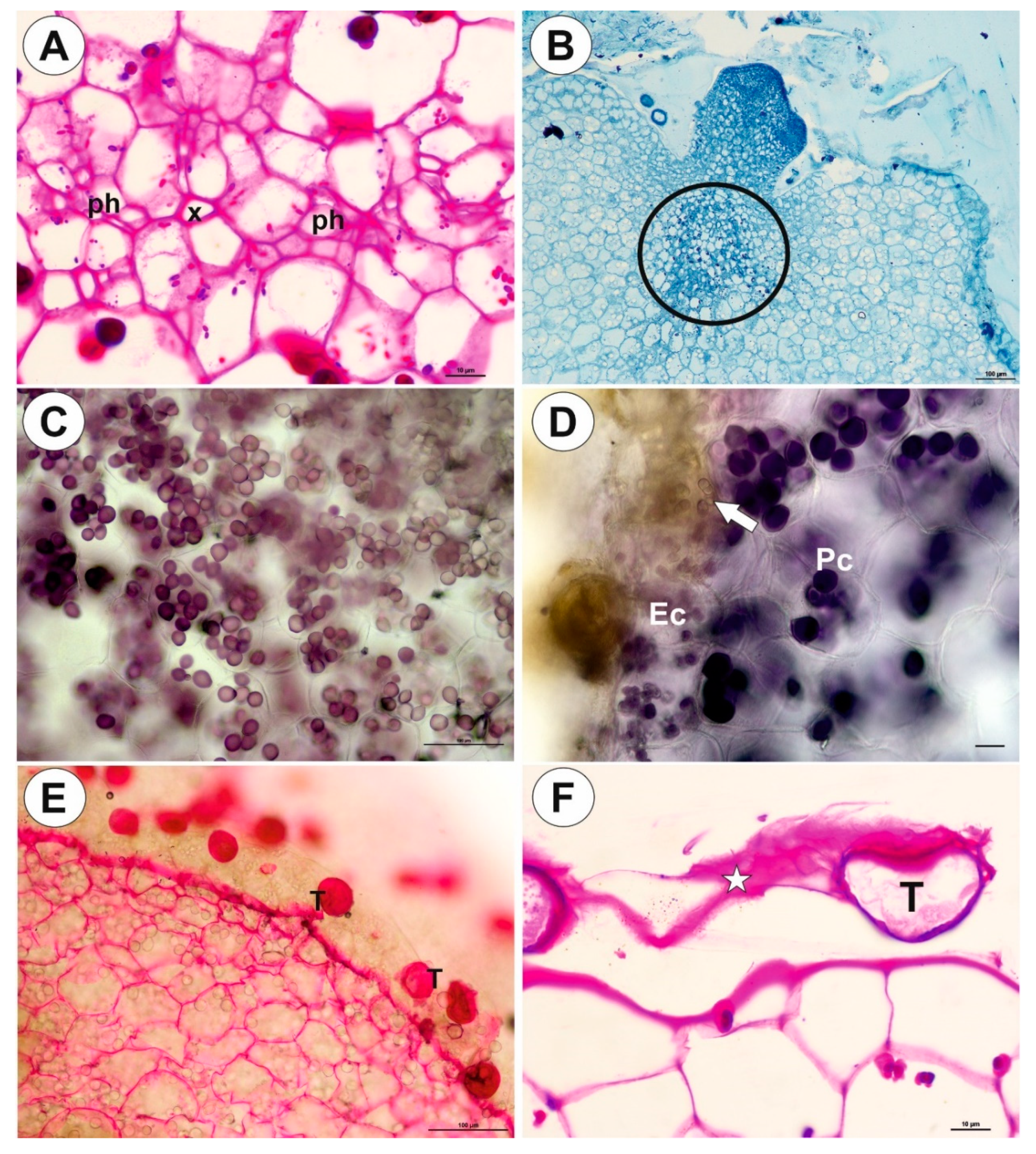
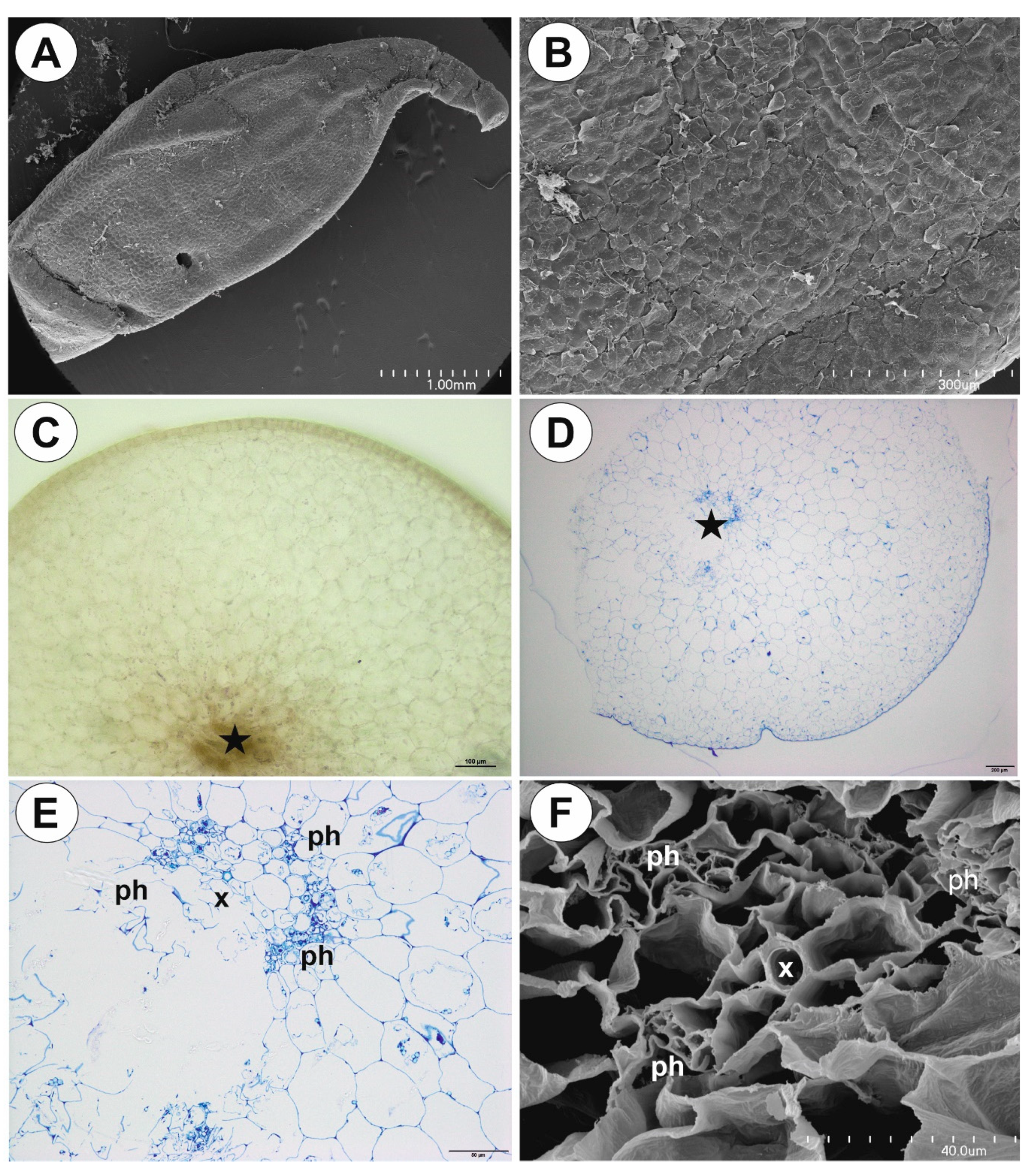
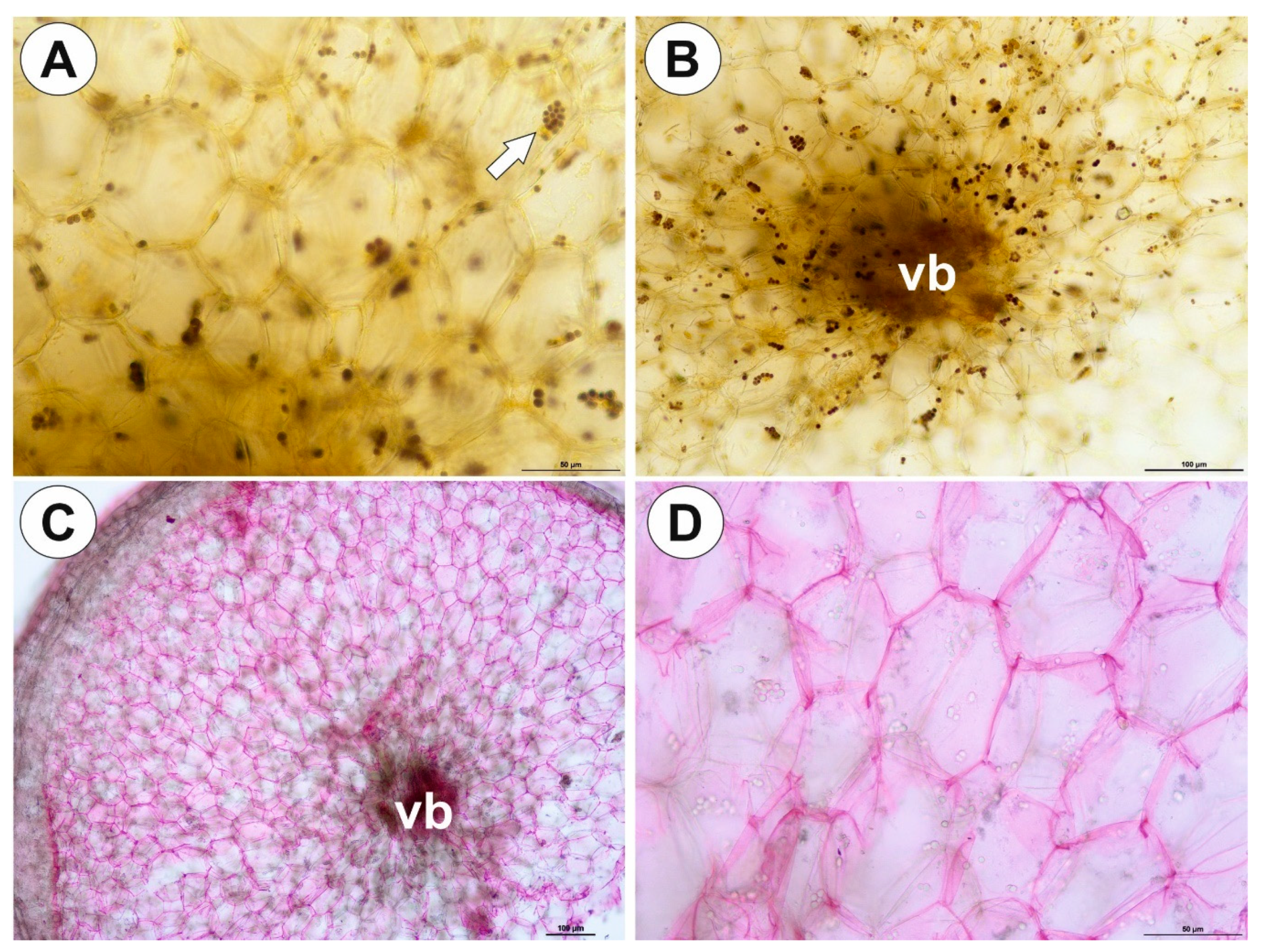
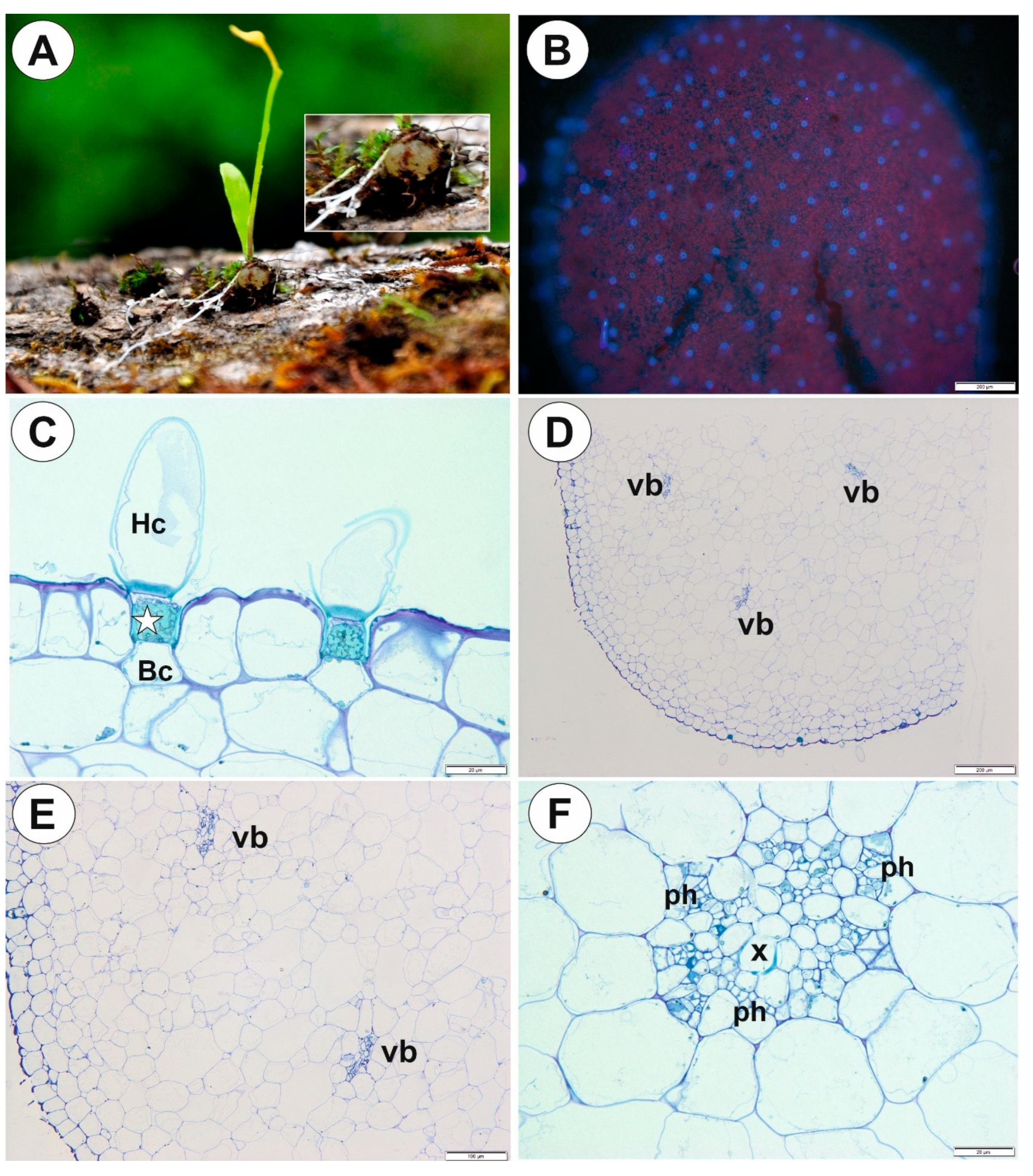
2.1. Genlisea tuberosa Rivadavia, Gonella & A. Fleischm
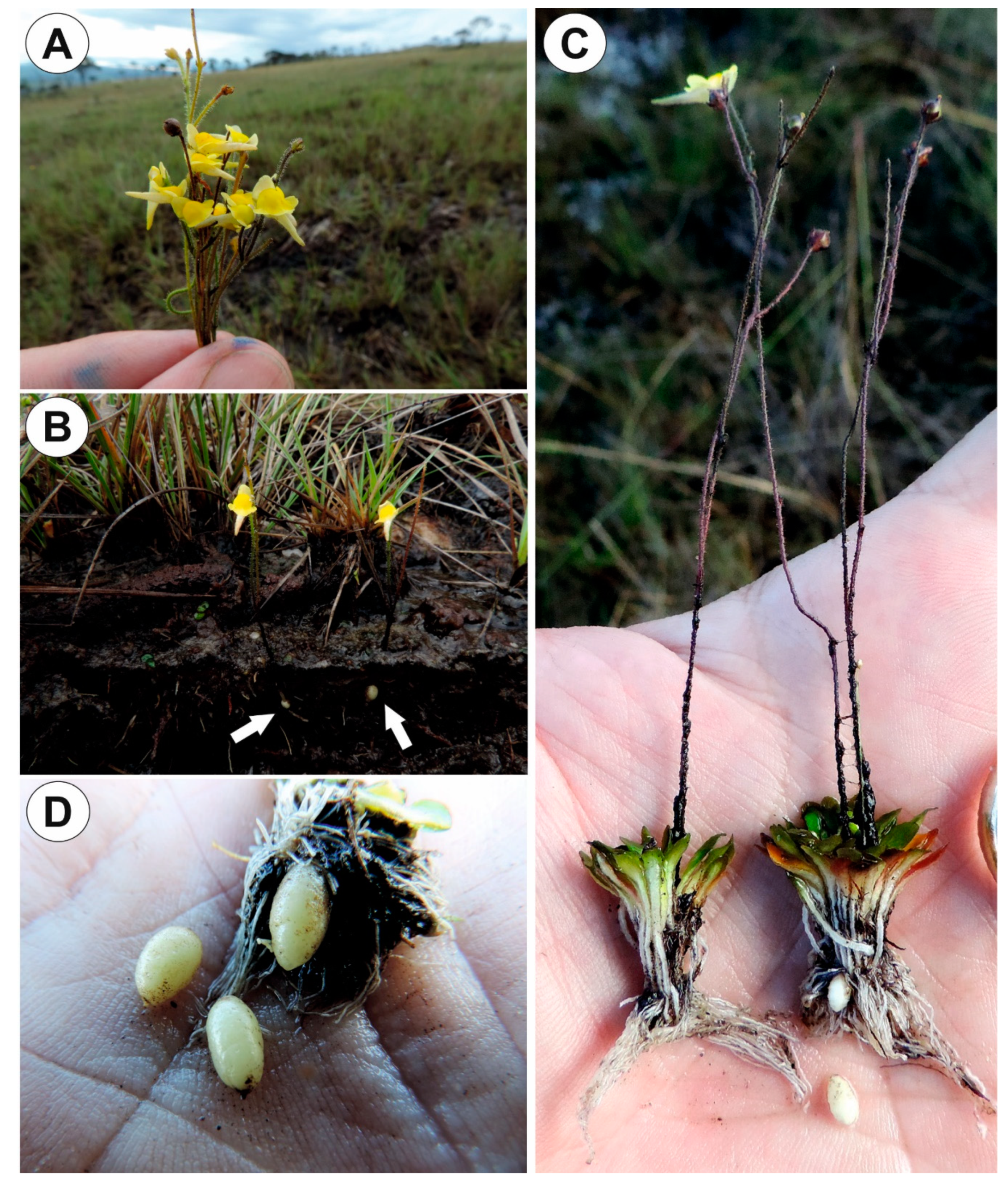
2.2. Utricularia menziesii R.Br.
2.3. Utricularia mannii Oliv.
2.4. Phylogenetic Analyses
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Król, E.; Płachno, B.J.; Adamec, L.; Stolarz, M.; Dziubińska, H.; Trebacz, K. Quite a few reasons for calling carnivores “the most wonderful plants in the world”. Ann. Bot. 2012, 109, 47–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poppinga, S.; Weisskopf, C.; Westermeier, A.S.; Masselter, T.; Speck, T. Fastest predators in plant kingdom: Functional morphology and biomechanics of suction traps found in the largest genus of carnivorous plants. AoB Plants 2015, 8, plv140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Płachno, B.J.; Muravnik, L.E. Functional anatomy of carnivorous traps. In Carnivorous Plants: Physiology, Ecology, and Evolution; Elisson, A.M., Adamec, L., Eds.; Oxford University Press: Oxford, UK, 2018; pp. 167–179. [Google Scholar]
- Płachno, B.J.; Świątek, P.; Miranda, V.F.O.; Stolarczyk, P. The structure and occurrence of a velum in Utricularia traps (Lentibulariaceae). Front. Plant Sci. 2019, 10, 302. [Google Scholar] [CrossRef]
- Rutishauser, R. Evolution of unusual morphologies in Lentibulariaceae (bladderworts and allies) and Podostemaceae (river-weeds): A pictorial report at the interface of developmental biology and morphological diversification. Ann. Bot. 2016, 117, 811–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reut, M.S.; Płachno, B.J. Unusual developmental morphology and anatomy of vegetative organs in Utricularia dichotoma—Leaf, shoot and root dynamics. Protoplasma 2020, 371–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitewoods, C.D.; Gonçalves, B.; Cheng, J.; Cui, M.; Kennaway, R.; Lee, K.; Bushell, C.; Yu, M.; Piao, C.; Coen, E. Evolution of carnivorous traps from planar leaves through simple shifts in gene expression. Science 2020, 367, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Adamec, L.; Świątek, P.; Kapusta, M.; Miranda, V.F.O. Life in the Current: Anatomy and Morphology of Utricularia neottioides. Int. J. Mol. Sci. 2020, 21, 4474. [Google Scholar] [CrossRef]
- Heslop-Harrison, Y. Pinguicula, L. J. Ecol. 2004, 92, 1071–1118. [Google Scholar] [CrossRef]
- Fleischmann, A.; Roccia, A. Systematics and evolution of Lentibulariaceae: I. Pinguicula, 1st ed.; Ellison, A.M., Adamec, L., Eds.; Oxford University Press: Oxford, UK, 2018; Volume 1, ISBN 9780198779841. [Google Scholar]
- Rivadavia, F. A Genlisea myth is confirmed. Carniv. Plant Newletter 2007, 36, 122–125. [Google Scholar]
- Fleischmann, A. Monograph of the Genus Genlisea; Redfern Natural History Productions: Dorset, UK, 2012; ISBN 9781908787002. [Google Scholar]
- Rivadavia, F.; Gonella, P.M.; Fleischmann, A. A New and Tuberous Species of Genlisea (Lentibulariaceae) from the Campos Rupestres of Brazil. Syst. Bot. 2013, 38, 464–470. [Google Scholar] [CrossRef]
- Taylor, P. The Genus Utricularia—A Taxonomic Monograph.; The Royal Botanic Gardens: London, UK, 1989; p. 724. [Google Scholar]
- Adamec, L. Ecophysiology of aquatic carnivorous plants. In Carnivorous Plants: Physiology, Ecology, and Evolution; Elisson, A.M., Adamec, L., Eds.; Oxford University Press: Oxford, UK, 2018; Volume 1. [Google Scholar]
- Adamec, L. Turion overwintering of aquatic carnivorous plants. Carniv. Plant Newletter 1999, 28, 19–24. [Google Scholar]
- Adamec, L. Respiration of turions and winter apices in aquatic carnivorous plants. Biologia (Bratisl). 2008, 63, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Adamec, L. Ecophysiological characteristics of turions of aquatic plants: A review. Aquat. Bot. 2018, 148, 64–77. [Google Scholar] [CrossRef]
- Płachno, B.J.; Adamec, L.; Kozieradzka-Kiszkurno, M.; Światek, P.; Kamińska, I. Cytochemical and ultrastructural aspects of aquatic carnivorous plant turions. Protoplasma 2014, 251, 1449–1454. [Google Scholar] [CrossRef] [Green Version]
- Rice, B. Tuberous organs in Utricularia, and new observations of sub-tuberous stolons on Utricularia radiata Small. Carniv. Plant Newsl. 2011, 40, 88–91. [Google Scholar]
- Rodrigues, F.G.; Marulanda, N.F.; Silva, S.R.; Płachno, B.J.; Adamec, L.; Miranda, V.F.O. Phylogeny of the “orchid-like” bladderworts (gen. Utricularia sect. Orchidioides and Iperua: Lentibulariaceae) with remarks on the stolon-tuber system. Ann. Bot. 2017, 120, 709–723. [Google Scholar]
- Pate, J.S.; Dixon, K.W. Tuberous, Cormous and Bulbous Plants: Biology of an Adaptive Strategy in Western Australia; University of Western Australia Press: Nedlands, Australia, 1982. [Google Scholar]
- Lowrie, A. Carnivorous Plants of Australia: Magnum Opus; Robinson, A., Ed.; Redfern Natural History Productions: Dorset, UK, 2013; Volume 1. [Google Scholar]
- Cross, A.T.; Davis, A.R.; Fleischmann, A.; Horner, J.D.; Jürgens, A.; Merritt, D.J.; Murza, G.L.; Turner, S.R. Reproductive biology and pollinator-prey conflicts. In Carnivorous Plants: Physiology, Ecology, and Evolution; Elisson, A.M., Adamec, L., Eds.; Oxford University Press: Oxford, UK, 2018; Volume 1. [Google Scholar]
- Menezes, C.G.; Gasparino, E.C.; Baleeiro, P.C.; Miranda, V.F.O. Seed morphology of bladderworts: A survey on Utricularia sect. Foliosa and sect. Psyllosperma (Lentibulariaceae) with taxonomic implications. Phytotaxa 2014, 167, 173. [Google Scholar] [CrossRef] [Green Version]
- Silveira, F.A.O.; Negreiros, D.; Barbosa, N.P.U.; Buisson, E.; Carmo, F.F.; Carstensen, D.W.; Conceição, A.A.; Cornelissen, T.G.; Echternacht, L.; Fernandes, G.W.; et al. Ecology and evolution of plant diversity in the endangered campo rupestre: A neglected conservation priority. Plant Soil 2016, 403, 129–152. [Google Scholar] [CrossRef] [Green Version]
- Jobson, R.W.; Baleeiro, P.C.; Reut, M.S. Molecular phylogeny of subgenus Polypompholyx (Utricularia; Lentibulariaceae) based on three plastid markers: Diversification and proposal for a new section. Aust. Syst. Bot. 2017, 30, 259–278. [Google Scholar] [CrossRef]
- Jobson, R.W.; Baleeiro, P.C.; Guisande, C. Systematics and evolution of Lentibulariaceae: III. Utricularia. In Carnivorous Plants: Physiology, Ecology, and Evolution; Elisson, A.M., Adamec, L., Eds.; Oxford University Press: Oxford, UK, 2018; pp. 89–104. [Google Scholar]
- Płachno, B.J.; Stpiczyńska, M.; Świątek, P.; Lambers, H.; Miranda, V.F.O.; Nge, F.J.; Stolarczyk, P.; Cawthray, G.R. Floral micromorphology of the bird-pollinated carnivorous plant species Utricularia menziesii R.Br. (Lentibulariaceae). Ann. Bot. 2018, 123, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Darwin, C. Insectivorous Plants; Murray, J., Ed.; London, 1875. Available online: http://darwin-online.org.uk/ (accessed on 5 May 2020).
- Adlassnig, W.; Peroutka, M.; Eder, G.; Pois, W.; Lichtscheidl, I.K. Ecophysiological observations on Drosophyllum lusitanicum. Ecol. Res. 2005, 21, 255–262. [Google Scholar] [CrossRef]
- Westermeier, A.S.; Fleischmann, A.; Müller, K.; Schäferhoff, B.; Rubach, C.; Speck, T.; Poppinga, S. Trap diversity and character evolution in carnivorous Lentibulariaceae). Sci. Rep. 2017, 7, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Borsch, T. Phylogenetics of Utricularia (Lentibulariaceae) and molecular evolution of the trnK intron in a lineage with high substitutional rates. Plant Syst. Evol. 2005, 250, 39–67. [Google Scholar] [CrossRef]
- Fleischmann, A.; Schäferhoff, B.; Heubl, G.; Rivadavia, F.; Barthlott, W.; Müller, K.F. Phylogenetics and character evolution in the carnivorous plant genus Genlisea A. St.-Hil. (Lentibulariaceae). Mol. Phylogenet. Evol. 2010, 56, 768–783. [Google Scholar] [CrossRef] [PubMed]
- Adlassnig, W.; Peroutka, M.; Lambers, H.; Lichtscheidl, I.K. The roots of carnivorous plants. Plant Soil 2005, 274, 127–140. [Google Scholar] [CrossRef]
- Compton, R.H. The morphology and anatomy of Utricularia brachiata Oliver. New Phytol. 1909, 8, 117–130. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Abrahão, A.; Pereira, C.; Teodoro, G.S.; Brum, M.; Alcantara, S.; Lambers, H. Ecophysiology of campos rupestres plants. In Ecology and Conservation of Mountaintop Grasslands in Brazil; Fernandes, G.W., Ed.; Springer International Publishing AG: Cham, Switzerland, 2016; pp. 227–272. [Google Scholar]
- de Joaquim, E.O.; de Figueiredo-Ribeiro, R.C.L.; Hayashi, A.H.; de Carvalho, M.A.M. Inulin contents and tissue distribution in underground storage organs of Asteraceae species from the Brazilian rocky fields. Botany 2014, 92, 827–836. [Google Scholar] [CrossRef]
- Carvalho, M.A.M.; Asega, A.F.; Figueiredo-Ribeiro, R.C.L. Fructans in Asteraceae from Brazilian cerrado. In Recent Advances In Fructooligosaccharides Research; Shiomi, N., Benkeblia, N., Onodera, S., Eds.; Research Signpost: Kerala, India, 2007; pp. 69–91. [Google Scholar]
- Auld, T.D.; Bradstock, R.A. Soil temperatures after the passage of a fire: Do they influence the germination of buried seeds? Aust. J. Ecol. 1996, 21, 106–109. [Google Scholar] [CrossRef]
- Meerts, P. Geoxylic suffrutices of African savannas: Short but remarkably similar to trees. J. Trop. Ecol. 2017, 33, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Simon, M.F.; Grether, R.; De Queiroz, L.P.; Skemae, C.; Pennington, R.T.; Hughes, C.E. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proc. Natl. Acad. Sci. USA 2009, 106, 20359–20364. [Google Scholar] [CrossRef] [Green Version]
- Pausas, J.G.; Lamont, B.B.; Paula, S.; Appezzato-da-Glória, B.; Fidelis, A. Unearthing belowground bud banks in fire-prone ecosystems. New Phytol. 2018, 217, 1435–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juniper, B.E.; Robins, R.J.; Joel, D.M. The Carnivorous Plants; Academic Press: London, UK, 1989; p. 353. [Google Scholar]
- Silva, S.R.; Gibson, R.; Adamec, L.; Domínguez, Y.; Miranda, V.F.O. Molecular phylogeny of bladderworts: A wide approach of Utricularia (Lentibulariaceae) species relationships based on six plastidial and nuclear DNA sequences. Mol. Phylogenet. Evol. 2018, 118, 244–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westermeier, A.S.; Fleischmann, A.; Müller, K.; Schäferhoff, B.; Rubach, C.; Speck, T.; Poppinga, S. Trap diversity and character evolution in carnivorous bladderworts (Utricularia, Lentibulariaceae). Sci. Rep. 2017, 7, 12052. [Google Scholar] [CrossRef]
- Reut, M.S.; Jobson, R.W. A phylogenetic study of subgenus Polypompholyx: A parallel radiation of Utricularia (Lentibulariaceae) throughout Australasia. Aust. Syst. Bot. 2010, 23, 152–161. [Google Scholar] [CrossRef]
- Jobson, R.W.; Playford, J.; Cameron, K.M.; Albert, V.A. Molecular Phylogenetics of Lentibulariaceae Inferred from Plastid rps16 Intron and trnL-F DNA Sequences: Implications for Character Evolution and Biogeography. Syst. Bot. 2003, 28, 157–171. [Google Scholar]
- Silva, S.R.; Pinheiro, D.G.; Penha, H.A.; Płachno, B.J.; Michael, T.P.; Meer, E.J.; Miranda, V.F.O.; Varani, A.M. Intraspecific Variation within the Utricularia amethystina Species Morphotypes Based on Chloroplast Genomes. Int. J. Mol. Sci. 2019, 20, 6130. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.R.; Michael, T.P.; Meer, E.J.; Pinheiro, D.G.; Varani, A.M.; Miranda, V.F.O. Comparative genomic analysis of Genlisea (corkscrew plants—Lentibulariaceae) chloroplast genomes reveals an increasing loss of the ndh genes. PLoS ONE 2018, 13, e0190321. [Google Scholar] [CrossRef] [Green Version]
- Bromham, L. Why do species vary in their rate of molecular evolution? Biol. Lett. 2009, 5, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Lehtonen, J.; Lanfear, R. Generation time, life history and the substitution rate of neutral mutations. Biol. Lett. 2014, 10, 20140801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.A.; Donoghue, M.J. Rates of molecular evolution are linked to life history in flowering plants. Science 2008, 322, 86–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Płachno, B.J.; Stpiczyńska, M.; Davies, K.L.; Świątek, P.; de Miranda, V.F.O. Floral ultrastructure of two Brazilian aquatic-epiphytic bladderworts: Utricularia cornigera Studnička and U. nelumbifolia Gardner (Lentibulariaceae). Protoplasma 2017, 254, 353–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphrey, C.D.; Pittman, F.E. A Simple Methylene Blue-Azure Ii-Basic Fuchsin Stain for Epoxy-Embedded Tissue Sections. Stain Technol. 1974, 49, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Johansen, D.A. Plant Microtechnique; McGraw-Hil Book Company Inc.: New York, NY, USA, 1940. [Google Scholar]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Kuzdowicz, A. Mikrotechnika roślinna; Panstw. Wyd. Roln. I Leśne: Warszawa, Polska, 1951. [Google Scholar]
- Kazutaka Katoh; John Rozewicki; Kazunori D Yamada MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [CrossRef] [PubMed] [Green Version]
- Kück, P.; Longo, G.C. FASconCAT-G: Extensive functions for multiple sequence alignment preparations concerning phylogenetic studies. Front. Zool. 2014, 11, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2014, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. Int. Symp. Inf. Theory 1973, 267–281. [Google Scholar]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [Green Version]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2017, 35, 518–522. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A modular system for evolutionary analysis. Version 3.61. Available online: http://mesquiteproject.org/ (accessed on 5 May 2020).
- Letunic, I.; Bork, P. Interactive Tree Of Life v2: Online annotation and display of phylogenetic trees made easy. Nucleic Acids Res. 2011, 39, W475–W478. [Google Scholar] [CrossRef]
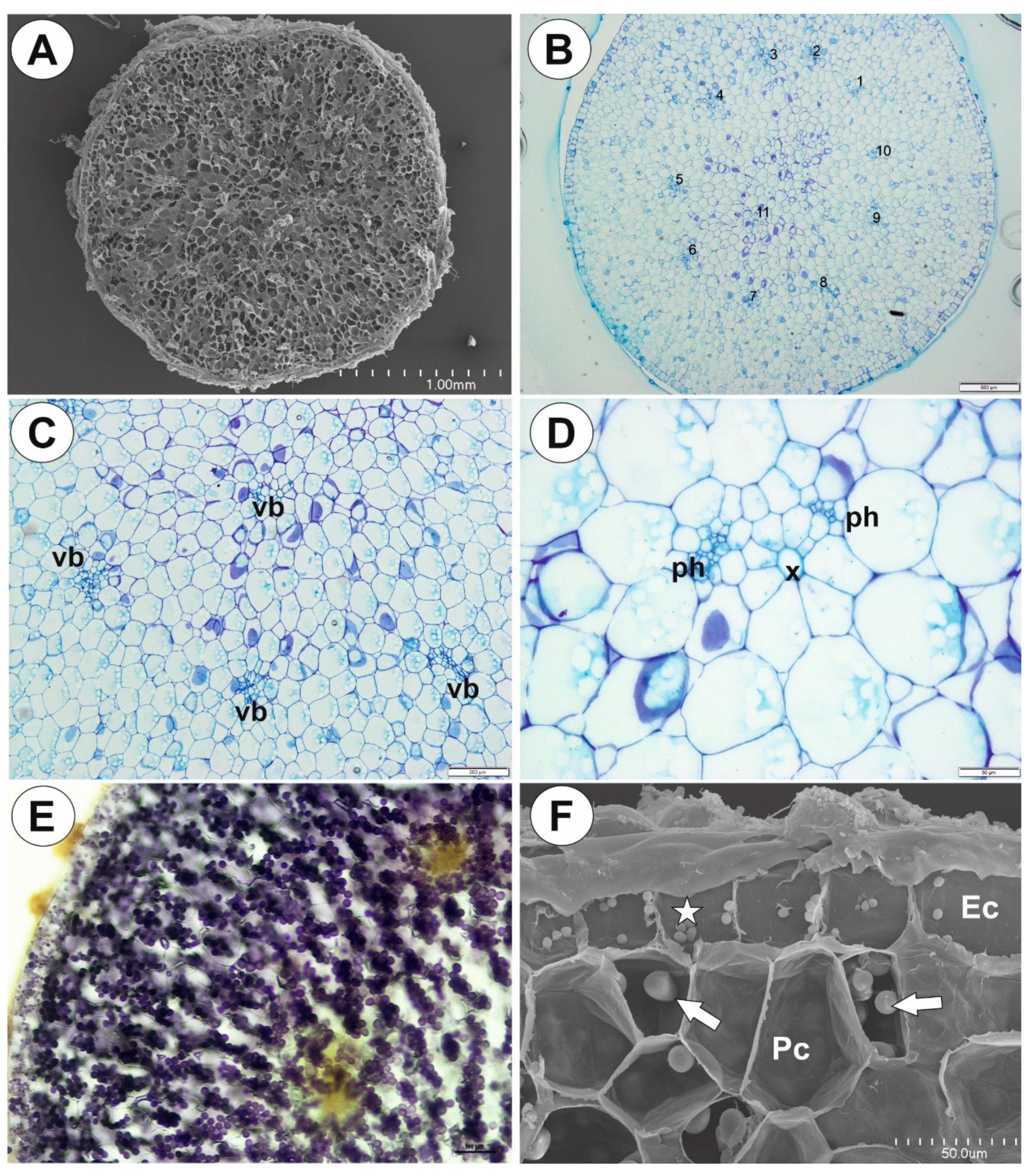



| Species | Cell Type | Diameter of Starch Grains (mean ± SD) |
|---|---|---|
| Genlisea tuberosa | Parenchyma | 17.34 ± 3.07 µm |
| Epidermis | 7.57 ± 1.53 µm | |
| Utricularia mannii | Parenchyma | 7.01 ± 1.46 µm |
| Utricularia menziesii | Parenchyma | 3.76 ± 0.70 µm |
| Species | Number of Starch Grains Per 100 µm2 (mean ± SD) |
|---|---|
| Genlisea tuberosa | 22.6 ± 3.59 |
| Utricularia mannii | 32.0 ± 5.83 |
| Utricularia menziesii | 36.4 ± 5.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płachno, B.J.; Silva, S.R.; Świątek, P.; Dixon, K.W.; Lustofin, K.; Seber, G.C.; Miranda, V.F.O. Structural Features of Carnivorous Plant (Genlisea, Utricularia) Tubers as Abiotic Stress Resistance Organs. Int. J. Mol. Sci. 2020, 21, 5143. https://doi.org/10.3390/ijms21145143
Płachno BJ, Silva SR, Świątek P, Dixon KW, Lustofin K, Seber GC, Miranda VFO. Structural Features of Carnivorous Plant (Genlisea, Utricularia) Tubers as Abiotic Stress Resistance Organs. International Journal of Molecular Sciences. 2020; 21(14):5143. https://doi.org/10.3390/ijms21145143
Chicago/Turabian StylePłachno, Bartosz J., Saura R. Silva, Piotr Świątek, Kingsley W. Dixon, Krzystof Lustofin, Guilherme C. Seber, and Vitor F. O. Miranda. 2020. "Structural Features of Carnivorous Plant (Genlisea, Utricularia) Tubers as Abiotic Stress Resistance Organs" International Journal of Molecular Sciences 21, no. 14: 5143. https://doi.org/10.3390/ijms21145143