Mesocellular Silica Foams (MCFs) with Tunable Pore Size as a Support for Lysozyme Immobilization: Adsorption Equilibrium and Kinetics, Biocomposite Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Characterization of MCF Supports
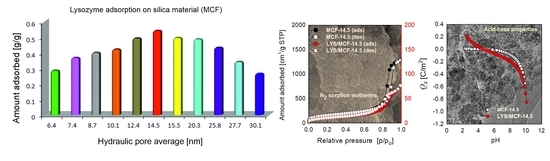
2.2. Adsorption Equilibrium and Kinetics
2.3. Physicochemical Properties of Protein/MCF Composites
2.3.1. Structural Analysis
2.3.2. Acid-Base Properties
2.4. Thermal Analysis (TG/DSC/FTIR/MS)
2.5. Microscopic Analysis (SEM, TEM)
3. Materials and Methods
3.1. Reagents
3.2. Synthesis of Mesocellular Foam
3.3. Adsorption Experiment
3.3.1. Adsorption Equilibrium
3.3.2. Adsorption Kinetics
3.4. Potentiometric Titration
3.5. Nitrogen Adsorption/Desorption Isotherms
3.6. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
3.7. Thermal Analysis Coupled with FTIR and MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kirkland, J.J.; Truszkowski, F.A.; Ricker, R.D. Atypical silica-based column packings for high-performance liquid chromatography. J. Chromatogr. A 2002, 965, 25. [Google Scholar] [CrossRef]
- Giacomelli, C.E.; Norde, W. The adsorption-desorption cycle. Reversibility of the BSA-Silica System. J. Colloid Interface Sci. 2001, 233, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Steri, D.; Monduzzi, M.; Salis, A. Ionic strength affects lysozyme adsorption and release from SBA-15 mesoporous silica. Microporous Mesoporous Mater. 2013, 170, 164–172. [Google Scholar] [CrossRef]
- Kubiak-Ossowska, K.; Cwieka, M.; Kaczynska, A.; Jachimska, B.; Mulheran, P.A. Lysozyme adsorption at a silica surface using simulation and experiment: Effects of pH on protein layer structure. Phys. Chem. Chem. Phys. 2015, 17, 24070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Heuvel, D.B.; Stawski, T.M.; Tobler, D.J.; Wirth, R.; Peacock, C.L.; Benning, L.G. Formation of Silica-Lysozyme Composites Through Co-Precipitation and Adsorption. Front. Mater. 2018, 5, 19. [Google Scholar] [CrossRef]
- Gustafsson, H.; Johansson, E.M.; Barrabino, A.; Odén, M.; Holmberg, K. Immobilization of lipase from Mucor miehei and Rhizopus oryzae into mesoporous silica-the effect of varied particle size and morphology. Colloids Surf. B 2012, 100, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Moerz, S.T.; Huber, P. Protein Adsorption into Mesopores: A Combination of Electrostatic Interaction, Counterion Release, and van der Waals Forces. Langmuir 2014, 30, 2729–2737. [Google Scholar] [CrossRef] [Green Version]
- Vinu, A.; Murugesan, V.; Tangermann, O.; Hartmann, M. Adsorption of Cytochrome c on Mesoporous Molecular Sieves: Influence of pH, Pore Diameter, and Aluminum Incorporation. Chem. Mater. 2004, 16, 3056–3065. [Google Scholar] [CrossRef]
- Diao, X.; Wang, Y.; Zhao, J.; Zhu, S. Effect of pore-size of mesoporous SBA-15 on adsorption of bovine serum albumin and lysozyme protein. Chin. J. Chem. Eng. 2010, 18, 493–499. [Google Scholar] [CrossRef]
- Lin, C.Y.; Yang, C.M.; Lindén, M. Influence of serum concentration and surface functionalization on the protein adsorption to mesoporous silica nanoparticles. RSC Adv. 2019, 9, 33912–33921. [Google Scholar] [CrossRef] [Green Version]
- Ran, R.; You, L.; Di, B.; Hao, W.; Su, M.; Yan, F.; Huang, L. A novel chiral mesoporous binaphthyl-silicas: Preparation, characterization, and application in HPLC. J. Sep. Sci. 2012, 35, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, A.; Yadav, S.; Smirniotis, P.G.; Pinto, N.G. Synthesis of ordered large pore SBA-15 spherical particles for adsorption of biomolecules. J. Chromatogr. A 2006, 1122, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.B.; Lee, J.W.; Shim, W.G.; Moon, H. Synthesis of functionalized SBA-15 with ordered large pore size and its adsorption properties of BSA. Microporous Mesoporous Mater. 2008, 110, 560–569. [Google Scholar] [CrossRef]
- Chen, S.; Shaoai, S.; Xiaoqiong, Z.; Qiang, H.; Liu, J.; Mingyu, D. Synthesis of large-pore mesostructured cellular foam silica spheres for the adsorption of biomolecules. J. Sep. Sci. 2014, 37, 2411–2417. [Google Scholar] [CrossRef] [PubMed]
- Zhifang, M.; Jing, B.; Yichen, W.; Xiue, J. Impact of Shape and Pore Size of Mesoporous Silica Nanoparticles on Serum Protein Adsorption and RBCs Hemolysis. Acs Appl. Mater. Interfaces 2014, 6, 2431–2438. [Google Scholar]
- Galarneau, A.; Mureseanu, M.; Atger, S.; Renard, G.; Fajula, F. Immobilization of lipase on silicas. Relevance of textural and interfacial properties on activity and selectivity. New J. Chem. 2006, 30, 562. [Google Scholar] [CrossRef]
- Cai, C.; Gao, Y.; Liu, Y.; Zhong, N.; Liu., N. Immobilization of Candida antarctica lipase B onto SBA-15 and their application in glycerolysis for diacylglycerols synthesis. Food Chem. 2016, 212, 205–212. [Google Scholar] [CrossRef]
- Zhong, N.; Chen, W.; Liu, L.; Chen, H. Immobilization of Rhizomucor miehei lipase onto the organic functionalized SBA-15: Their enzymatic properties and glycerolysis efficiencies for diacylglycerols production. Food Chem. 2019, 274, 739–746. [Google Scholar] [CrossRef]
- Miyahara, M.; Vinu, A.; Ariga, K. Adsorption myoglobin over mesoporous silica molecular sieves: Pore size effect and pore-filling model. Mater. Sci. Eng. C 2007, 27, 232–236. [Google Scholar] [CrossRef]
- Li, J.; Xiong, Z.; Zhou, L.; Han, X.; Liu, H. Effects of pore structure of mesoporous silicas on the electrochemical propertiesof hemoglobin. Microporous Mesoporous Mater. 2010, 130, 333–337. [Google Scholar] [CrossRef]
- Vinu, A.; Hartmann, M. Adsorption of cytochrome c on MCM-41 and SBA-15: Influence of pH. Stud. Surf. Sci. Catal. 2004, 154, 2987–2994. [Google Scholar]
- Meissner, J.; Prause, A.; diTommaso, C.; Bharti, B.; Findenegg, G.H. Protein Immobilization in Surface-Functionalized SBA-15: Predicting the Uptake Capacity from the Pore Structure. J. Phys. Chem. C 2015, 119, 2438–2446. [Google Scholar] [CrossRef]
- Pegah, S.N.Z.; Akerman, B. Immobilization of Enzymes in Mesoporous Silica Particles: Protein Concentration and Rotational Mobility in the Pores. J. Phys. Chem. B 2017, 121, 2575–2583. [Google Scholar]
- Katiyar, A.; Ji, L.; Smirniotis, P.G.; Pinto, N.G. Adsorption of bovine serum albumin and lysozyme on silicious MCM-41. Microporous Mesoporous Mater. 2005, 80, 311–320. [Google Scholar] [CrossRef]
- Atyaksheva, L.F.; Kasyanov, I.A.; Ivanova, I.I. Adsorptive Immobilization of Proteins on Mesoporous Molecular Sieves and Zeolites. Pet. Chem. 2019, 59, 327–337. [Google Scholar] [CrossRef]
- Hudson, S.; Cooney, J.; Magner, E. Proteins in Mesoporous Silicates. Angew. Chem. Int. Ed. 2008, 47, 8582–8594. [Google Scholar] [CrossRef]
- Russo, P.A.; Ribeiro Carrott, M.M.L.; Mourao, P.A.M.; Carrott, P.J.M. Tailoring the surface chemistry of mesocellular foams for protein adsorption. Colloids Surf. A 2011, 386, 25–35. [Google Scholar] [CrossRef]
- Essa, H.; Magner, E.; Cooney, J.; Hodnett, B. Influence of pH and ionic strength on the adsorption, leaching and activity of myoglobin immobilized onto ordered mesoporous silicates. J. Mol. Catal. B 2007, 49, 61. [Google Scholar] [CrossRef]
- Han, Y.; Watson, J.T.; Stucky, G.D.; Butler, A. Catalytic activity of mesoporous silicate-immobilized chloroperoxidase. J. Mol. Catal. B 2002, 17, 1. [Google Scholar] [CrossRef]
- Deryło-Marczewska, A.; Chrzanowska, A.; Marczewski, A.W. Morphological, structural and physicochemical characteristics of MCF surface with the adsorbed OVA and BSA proteins. Microporous Mesoporous Mater. 2020, 293, 109769. [Google Scholar] [CrossRef]
- Sing, K.S.W. Adsorption methods for the characterization of porous materials. Adv. Colloid Interface Sci. 1998, 76–77, 3–11. [Google Scholar] [CrossRef]
- Schmidt-Winkel, P.; Lukens Jr., W.W.; Yang, P.; Margolese, D.I.; Lettow, J.S.; Ying, J.Y. Microemulsion Templating of Siliceous Mesostructured Cellular Foams with Well-Defined Ultralarge Mesopores. Chem. Mater. 2000, 12, 686–696. [Google Scholar] [CrossRef]
- Santos, S.M.L.; Cecilia, J.A.; Vilarrasa-García, E.; Junior, I.J.S.; Rodríguez-Castell, E.; Azevedo, D.C.S. The effect of structure modifying agents in the SBA-15 for its application in the biomolecules adsorption. Microporous Mesoporous Mater. 2016, 232, 53–64. [Google Scholar] [CrossRef]
- Vinu, A.; Murugesan, V.; Hartmann, M. Adsorption of Lysozyme over Mesoporous Molecular Sieves MCM-41 and SBA-15: Influence of pH and Aluminum Incorporation. J. Phys. Chem. B 2004, 108, 7323–7330. [Google Scholar] [CrossRef]
- Moerz, S.T.; Huber, P. pH-Dependent Selective Protein Adsorption into Mesoporous Silica. J. Phys. Chem. C 2015, 119, 27072–27079. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Deryło Marczewska, A.; Marczewski, A.W.; Blachnio, M.; Tsyba, M.M.; Sapsay, V.I.; Klymchuk, D.O. Protein Adsorption by Nanostructured Carbons with Different Pore Sizes. Adsorption 2016, 22, 541–552. [Google Scholar] [CrossRef]
- David, A.E.; Wang, N.S.; Yang, V.C.; Yang, A.J. Chemically surface modified gel (CSMG): An excellent enzyme-immobilization matrix for industrial processes. J. Biotechnol. 2006, 125, 395–407. [Google Scholar] [CrossRef]
- Moelans, D.; Cool, P.; Baeyens, J.; Vansant, E.F. Using mesoporous silica materials to immobilise biocatalysis-enzymes. Catal. Commun. 2005, 6, 307–311. [Google Scholar] [CrossRef]
- Wisniewska, M.; Szewczuk-Karpisz, K.; Sternik, D. Adsorption and thermal properties of the bovine serum albumin–silicon dioxide system. J. Therm. Anal. Calorim. 2015, 120, 1355–1364. [Google Scholar] [CrossRef] [Green Version]
- Chrzanowska, A.; Derylo-Marczewska, A. Mesoporous silica/protein biocomposites: Surface, topography, thermal properties. Int. J. Biol. Macromol. 2019, 139, 531–542. [Google Scholar] [CrossRef]
- Chrzanowska, A.; Derylo-Marczewska, A.; Borowski, P. Comprehensive characterization of biocomposite surface based on the mesoporous silica and lysozyme molecules: Chemistry, morphology, topography, texture and micro-nanostructure. Appl. Surf. Sci. 2020, 525, 146512. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, J.; Huang, M.; Xu, Q.; Ma, M. The changes of secondary structures and properties of lysozyme along with the egg storage. Int. J. Biol. Macromol. 2016, 92, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.M.; Chaturvedi, S.K.; Rahman, S.K.; Ishtikhar, M.; Qadeer, A.; Ahmad, E.; Khan, R.H. Protonation favors aggregation of lysozyme with SDS. Soft Matter. 2014, 10, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Meissner, J.; Prause, A.; Bhuvnesh, B.; Findenegg, G.H. Characterization of protein adsorption onto silica nanoparticles: Influence of pH and ionic strength. Colloid Polym. Sci. 2015, 293, 3381–3391. [Google Scholar] [CrossRef] [Green Version]
- Perez, D.E.L.; Aradilla, D.; Del Valle, L.J.; Aleman, C. Capacitive composites made of conducting polymer and lysozyme: Toward the biocondenser. J. Phys. Chem. C 2013, 117, 6607–6619. [Google Scholar] [CrossRef]
- Fang, Y.; Yi, L.; Fang, Y. Unfolding of lysozyme induced by urea and guanidine hydrochloride studied by “Phase Diagram” method of fluorescence. Acta Chim. Sin. 2003, 61, 803–807. [Google Scholar]
- Buijs, J.; Hlady, V. Adsorption Kinetics, Conformation, and Mobility of the Growth Hormone and Lysozyme on Solid Surfaces, Studied with TIRF. J. Colloid Interface Sci. 1997, 190, 171–181. [Google Scholar] [CrossRef]
- Shanmugaraj, K.; Umadevi, P.; Senthilkumar, L.; Ilanchelian, M. Elucidation of Binding Mechanism of Photodynamic Therapeutic Agent Toluidine Blue O with Chicken Egg White Lysozyme by Spectroscopic and Molecular Dynamics Studies. Photochem. Photobiol. 2017, 93, 1043–1056. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Doadrio, A.L.; Sousa, E.M.B.; Doadrio, J.C.; Pérez-Pariente, J.; Izquierdo-Barba, I.; Vallet-Regǐi, M. Mesoporous SBA-15 HPLC evaluation for controlled gentamicin drug delivery. J. Control. Release 2004, 97, 125–132. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Marczewski, A.W.; Skrzypek, I.; Pikus, S.; Kozak, M. The effect of aging temperature on structure characteristics of ordered mesoporous silicas. Appl. Surf. Sci. 2005, 252, 625–632. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Marczewski, A.W.; Skrzypek, I.; Pikus, S. Effect of Block Copolymer Type on Formation of Mesoporous Silica Structure. Pol. J. Chem. 2008, 82, 205–212. [Google Scholar]
- Marczewski, A.W. Application of mixed order rate equations to adsorption of methylene blue on mesoporous carbons. Appl. Surf. Sci. 2010, 256, 5145–5152. [Google Scholar] [CrossRef]
- Jaroniec, M.; Madey, R. Physical Adsorption on Heterogeneous Solids; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Brandt, A.; Bülow, M.; Derylo-Marczewska, A.; Goworek, J.; Schmeißer, J.; Schöps, W.; Unger, B. Novel zeolite composites and consequences for rapid sorption processes. Adsorption 2007, 13, 267–279. [Google Scholar] [CrossRef]
- Marczewski, A.W. Analysis of kinetic Langmuir model. Part I: Integrated kinetic Langmuir equation (IKL): A new complete analytical solution of the Langmuir rate equation. Langmuir 2010, 26, 15229–15238. [Google Scholar] [CrossRef]
- Deryło-Marczewska, A.; Marczewski, A.W. Non-homogeneity Effects in Adsorption from Gas and Liquid Phases on Activated Carbons. Langmuir 1999, 15, 3981–3986. [Google Scholar] [CrossRef]
- De Boer, J.H.; Linsen, B.G.; Osinga, T.J. Studies on pore systems in catalysts: VI. The universal t curve. J. Catal. 1965, 4, 643–648. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyn, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Kao, C.K.; Lin, T.S.; Mou, C.Y. Enhanced Activity and Stability of Lysozyme by Immobilization in the Matching Nanochannels of Mesoporous Silica Nanoparticles. J. Phys. Chem. C 2014, 118, 6734–6743. [Google Scholar] [CrossRef]








| Material | Surface Area [m2/g] | Pore Volume [cm3/g] | Pore Diameter by BJH Adsorption | Pore Diameter by BJH Desorption | Pore Size (Average Hydraulic) [nm] | ||||
|---|---|---|---|---|---|---|---|---|---|
| PSD Average [nm] | Mode [nm] | PSD Average [nm] | Mode [nm] | ||||||
| aSBET | bSext | cVt | dVmes | eDav, ads. | fDmo, ads. | gDav,des. | hDmo,des. | iDh | |
| MCF-6.4 | 716 | 29 | 1.15 | 1.09 | 5.8 | 6.6 | 5.2 | 4.9 | 6.4 |
| MCF-7.4 | 685 | 38 | 1.26 | 1.18 | 7.2 | 7.9 | 6.5 | 6.4 | 7.4 |
| MCF-8.7 | 522 | 63 | 1.14 | 1.03 | 8.3 | 8.9 | 7.4 | 8.3 | 8.7 |
| MCF-10.1 | 533 | 72 | 1.32 | 1.19 | 9.9 | 10.1 | 8.8 | 9.3 | 10.1 |
| MCF-12.4 | 569 | 65 | 1.76 | 1.63 | 13.4 | 13.7 | 11.9 | 10.8 | 12.4 |
| MCF-14.5 | 547 | 135 | 1.98 | 1.74 | 16.5 | 16.6 | 13.6 | 12.6 | 14.5 |
| MCF-15.5 | 494 | 262 | 1.91 | 1.44 | 20.1 | 17.0 | 16.9 | 17.9 | 15.5 |
| MCF-20.3 | 435 | 41 | 2.21 | 2.00 | 25.2 | 27.7 | 20.6 | 22.2 | 20.3 |
| MCF-25.8 | 301 | 231 | 1.94 | 1.45 | 32.3 | 27.9 | 23.3 | 21.8 | 25.8 |
| MCF-27.7 | 267 | 67 | 1.85 | 1.73 | 34.5 | 38.0 | 26.8 | 29.6 | 27.7 |
| MCF-30.1 | 250 | 61 | 1.88 | 1.76 | 37.5 | 38.7 | 30.2 | 30.9 | 30.1 |
| Adsorption System | Fitted Isotherm | am | m | n | log K | R2 | SD(a) |
|---|---|---|---|---|---|---|---|
| LYS/MCF-14.5 | LF | 0.82 | 0.40 | 0.40 | 0.25 | 0.997 | 0.008 |
| LYS/MCF-6.4 | GL | 0.37 | 0.50 | 1 | −0.32 | 0.997 | 0.004 |
| LYS/MCF-30.1 | L | 0.29 | 1 | 1 | 0.71 | 0.985 | 0.006 |
| System | f1, logk1 | f2, logk2 | f3, logk3 | t1/2 [min] | SD(c)/co [%] | 1-R2 |
|---|---|---|---|---|---|---|
| LYS/MCF-6.4 | 0.054, −2.24 | 0.946, −3.91 | 0 | 358.7 | 0.337 | 0.0037 |
| LYS/MCF-14.5 | 0.231, 0.21 | 0.125, −2.48 | 0.644, −9.99 | 1.7 | 0.414 | 0.0027 |
| LYS/MCF-30.1 | 0.187, 0.55 | 0.813, −4.02 | 0 | 0.5 | 0.723 | 0.021 |
| Material | Surface Area [m2/g] | Pore Volume [cm3/g] | Pore Diameter by BJH Adsorption | Pore Diameter by BJH Desorption | Pore Size (Average Hydraulic) [nm] | ||||
|---|---|---|---|---|---|---|---|---|---|
| PSD Average [nm] | Mode [nm] | PSD Average [nm] | Mode [nm] | ||||||
| aSBET | bSext | cVt | dVmes | eDav, ads. | fDmo, ads. | gDav,des. | hDmo,des. | iDh | |
| MCF-6.4 | 716 | 29 | 1.15 | 1.09 | 5.8 | 6.6 | 5.2 | 4.9 | 6.4 |
| MCF-14.5 | 547 | 135 | 1.98 | 1.74 | 16.5 | 16.6 | 13.6 | 12.6 | 14.5 |
| MCF-30.1 | 250 | 61 | 1.88 | 1.76 | 37.5 | 38.7 | 30.2 | 30.9 | 30.1 |
| LYS/MCF-6.4 | 346 | 7 | 0.49 | 0.48 | 5.2 | 6.3 | 4.7 | 5.5 | 5.7 |
| LYS/MCF-14.5 | 38 | 22 | 0.12 | 0.08 | 12.5 | 12.2 | 10.9 | 10.7 | 12.6 |
| LYS/MCF-30.1 | 114 | 25 | 0.50 | 0.44 | 18.5 | 20.7 | 14.9 | 17.6 | 17.5 |
| Material | TG | DTG | ||||
|---|---|---|---|---|---|---|
| Mloss,IDT [%] | Mloss1 [%] | Mloss2 [%] | Mloss,TOTAL [%] | Tmax1 [°C] | Tmax2 [°C] | |
| 30–165 [°C] | 165–420 [°C] | 420–830 [°C] | ||||
| LYS/MCF-14.5 | 1.2 | 17.3 | 10.8 | 29.3 | 323 | 508 |
| LYS/MCF-6.4 | 1.3 | 13.4 | 8.9 | 23.6 | 327 | 498 |
| LYS/MCF-30.1 | 2.0 | 9.9 | 8.0 | 19.9 | 321 | 520 |
| Material | DSC | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tonset1 [°C] | Tpeak1 [°C] | Tend1 [°C] | ∆H [J/g] | Tonset2 [°C] | Tpeak2 [°C] | Tend2 [°C] | ∆H [J/g] | ||
| LYS/MCF-14.5 | 283 | 321 | 366 | −654 | 448 | 503 | 587 | −452 | |
| LYS/MCF-6.4 | 282 | 327 | 372 | −491 | 447 | 498 | 588 | −338 | |
| LYS/MCF-30.1 | 270 | 328 | 373 | −197 | 455 | 519 | 620 | −408 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrzanowska, A.; Derylo-Marczewska, A.; Wasilewska, M. Mesocellular Silica Foams (MCFs) with Tunable Pore Size as a Support for Lysozyme Immobilization: Adsorption Equilibrium and Kinetics, Biocomposite Properties. Int. J. Mol. Sci. 2020, 21, 5479. https://doi.org/10.3390/ijms21155479
Chrzanowska A, Derylo-Marczewska A, Wasilewska M. Mesocellular Silica Foams (MCFs) with Tunable Pore Size as a Support for Lysozyme Immobilization: Adsorption Equilibrium and Kinetics, Biocomposite Properties. International Journal of Molecular Sciences. 2020; 21(15):5479. https://doi.org/10.3390/ijms21155479
Chicago/Turabian StyleChrzanowska, Agnieszka, Anna Derylo-Marczewska, and Malgorzata Wasilewska. 2020. "Mesocellular Silica Foams (MCFs) with Tunable Pore Size as a Support for Lysozyme Immobilization: Adsorption Equilibrium and Kinetics, Biocomposite Properties" International Journal of Molecular Sciences 21, no. 15: 5479. https://doi.org/10.3390/ijms21155479