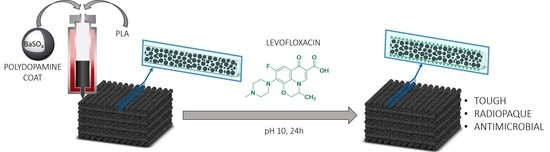
Benefits of Polydopamine as Particle/Matrix Interface in Polylactide/PD-BaSO4 Scaffolds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Improvements in the Mechanical Properties by Incorporation of Polydopamine Coated BaSO4 Particles into PLA
2.2. Biocompatibility Assessment
2.3. Adsorption/Release Test in 3D Printed Scaffolds
2.4. Antimicrobial Activity of 3D PLA/PD-BaSO4 Scaffolds with Levofloxacin
3. Materials and Methods
3.1. Materials
3.2. Coating with Polydopamine and Blending
3.3. 3D Printing of Radiopaque Scaffolds
3.4. Mechanical Properties
3.5. Adsorption/Release Test
3.6. Agar Disk Diffusion
3.7. Cell Culture
3.7.1. Human Dermal Fibroblasts
3.7.2. Cell Seeding
3.7.3. Cell Viability Studies
3.7.4. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMP-2 | Bone morphogenic protein 2 |
| PLA/BaSO4 | Composite of Polylactide and barium sulfate particles |
| PLA/PD-BaSO4 | Composite of polylactide and coated with polydopamine barium sulfate particles |
| CAD | Computer Aided Drawing |
| DNEM | Dulbecco’s modified Eagle’s medium |
| εr | Elongation at break |
| Fe3O4 | Ferrous Oxide |
| FBS | Fetal bovine serum |
| GPC | Gel Permeation Chromatography |
| HBSS | Hank’s balanced salt solution |
| HDFs | Human dermal fibroblasts |
| IGF-1 | Insulin growth factor 1 |
| MIC | Minimum inhibitory concentration |
| MWCNT | Multi-walled carbon nanotubes |
| PS | Penicillin-streptomycin |
| PBS | Phosphate buffer saline |
| PD-PLA | Polylactide cover by polydopamine |
| PD | Polydopamine |
| PU | Polyurethanes |
| RO | Radiopacity |
| RH | Relative Humidity |
| SEM | Scanning Electron Microscopy |
| S. aureus | Staphylococcus aureus |
| E | Tensile Modulus |
| σr | Tensile strength |
| TT | Tensile toughness |
| σy | Tensile Yield |
| TCP | Tissue culture plastic |
| TEM | Transmission Electron Microscopy |
| wt.% | Weight percent |
References
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Tajbakhsh, S.; Hajiali, F. A comprehensive study on the fabrication and properties of biocomposites of poly(lactic acid)/ceramics for bone tissue engineering. Mater. Sci. Eng. C 2017, 70, 897–912. [Google Scholar] [CrossRef]
- Martínez De Arenaza, I.; Sadaba, N.; Larrañaga, A.; Zuza, E.; Sarasua, J.R. High toughness biodegradable radiopaque composites based on polylactide and barium sulphate. Eur. Polym. J. 2015, 73, 88–93. [Google Scholar] [CrossRef]
- Sarasua, J.R.; Arraiza, A.L.; Balerdi, P.; Maiza, I. Crystallinity and mechanical properties of optically pure polylactides and their blends. Polym. Eng. Sci. 2005, 45, 745–753. [Google Scholar] [CrossRef]
- Sadaba, N.; Martini, R.; Barthelat, F.; Martínez de Arenaza, I.; Larrañaga, A.; Sarasua, J.R.; Zuza, E. Understanding the toughness mechanism prompted by submicron rigid particles in polylactide/barium sulfate composites. Polym. Test. 2018, 69, 340–349. [Google Scholar] [CrossRef]
- Grémare, A.; Guduric, V.; Bareille, R.; Heroguez, V.; Latour, S.; L’heureux, N.; Fricain, J.C.; Catros, S.; Le Nihouannen, D. Characterization of printed PLA scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2018, 106, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Nuutinen, J.-P.; Clerc, C.; Törmälä, P. Mechanical properties and in vitro degradation of self-reinforced radiopaque bioresorbable polylactide fibres. J. Biomater. Sci. Polym. Ed. 2003, 14, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-J.; Pan, Y.-H.; Tzeng, J.-J.; Wu, T.-L.; Fong, T.-H.; Feng, S.-W.; Huang, H.-M. Development and Testing of X-Ray Imaging-Enhanced Poly-L-Lactide Bone Screws. PLoS ONE 2015, 10, e0140354. [Google Scholar] [CrossRef] [Green Version]
- Noor Azman, N.Z.; Musa, N.F.L.; Nik Ab Razak, N.N.A.; Ramli, R.M.; Mustafa, I.S.; Abdul Rahman, A.; Yahaya, N.Z. Effect of Bi2O3 particle sizes and addition of starch into Bi2O3–PVA composites for X-ray shielding. Appl. Phys. A 2016, 122, 818. [Google Scholar] [CrossRef]
- Abunahel, B.M.; Mustafa, I.S.; Noor Azman, N.Z. Characteristics of X-ray attenuation in nano-sized bismuth oxide/epoxy-polyvinyl alcohol (PVA) matrix composites. Appl. Phys. A Mater. Sci. Process. 2018, 124, 1–7. [Google Scholar] [CrossRef]
- Zuza, E.; Meaurio, E.; Sarasua, J.-R. Biodegradable Polylactide-Based Composites. In Composites from Renewable and Sustainable Materials; InTech: Rijeka, Croatia, 2016. [Google Scholar]
- Xiu, H.; Qi, X.; Liu, Z.; Zhou, Y.; Bai, H.; Zhang, Q.; Fu, Q. Simultaneously reinforcing and toughening of polylactide/carbon fiber composites via adding small amount of soft poly(ether)urethane. Compos. Sci. Technol. 2016, 127, 54–61. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Parandoush, P.; Lin, D. A review on additive manufacturing of polymer-fiber composites. Compos. Struct. 2017, 182, 36–53. [Google Scholar] [CrossRef]
- Poh, P.S.P.; Chhaya, M.P.; Wunner, F.M.; De-Juan-Pardo, E.M.; Schilling, A.F.; Schantz, J.-T.; van Griensven, M.; Hutmacher, D.W. Polylactides in additive biomanufacturing. Adv. Drug Deliv. Rev. 2016, 107, 228–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Larrañaga, A.; Ramos, D.; Amestoy, H.; Zuza, E.; Sarasua, J.-R. Coating of bioactive glass particles with mussel-inspired polydopamine as a strategy to improve the thermal stability of poly(l -lactide)/bioactive glass composites. RSC Adv. 2015, 5, 65618–65626. [Google Scholar] [CrossRef] [Green Version]
- Ahn, B.K. Perspectives on Mussel-Inspired Wet Adhesion. J. Am. Chem. Soc. 2017, 139, 10166–10171. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, H.; Su, H.; Bi, X.; Bai, Y.; Chen, L.; Ge, D.; Shi, W.; Sun, Y. Polydopamine Nanocapsule: A Theranostic Agent for Photoacoustic Imaging and Chemo-Photothermal Synergistic Therapy. ACS Biomater. Sci. Eng. 2017, 3, 1799–1808. [Google Scholar] [CrossRef]
- Yu, X.; Tang, X.; He, J.; Yi, X.; Xu, G.; Tian, L.; Zhou, R.; Zhang, C.; Yang, K. Polydopamine Nanoparticle as a Multifunctional Nanocarrier for Combined Radiophotodynamic Therapy of Cancer. Part. Part. Syst. Charact. 2017, 34, 1600296. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Jia, G.; Jiang, Y.; Liu, Q.; Yang, X.; Pan, S. Improving osteogenesis of PLGA/HA porous scaffolds based on dual delivery of BMP-2 and IGF-1 via a polydopamine coating. RSC Adv. 2017, 7, 56732–56742. [Google Scholar] [CrossRef] [Green Version]
- Khoshnood, N.; Zamanian, A.; Massoudi, A. Mussel-inspired surface modification of titania nanotubes as a novel drug delivery system. Mater. Sci. Eng. C 2017, 77, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Yague, M.A.; Larrañaga, A.; Gladkovskaya, O.; Stanley, A.; Tadayyon, G.; Guo, Y.; Sarasua, J.R.; Tofail, S.A.M.; Zeugolis, D.I.; Pandit, A.; et al. Effects of Polydopamine Functionalization on Boron Nitride Nanotube Dispersion and Cytocompatibility. Bioconjug. Chem. 2015, 26, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Zhang, L.; Song, Y.; Chen, X.; Liu, M.; Wang, F.; Hu, X. Modification of carbon nanotubes by a novel biomimetic approach towards the enhancement of the mechanical properties of polyurethane. Polymer 2016, 92, 231–238. [Google Scholar] [CrossRef]
- Pekkan, G.; Aktas, A.; Pekkan, K. Comparative radiopacity of bone graft materials. J. Cranio-Maxillofac. Surg. 2012, 40, e1–e4. [Google Scholar] [CrossRef]
- Clauss, M.; Trampuz, A.; Borens, O.; Bohner, M.; Ilchmann, T. Biofilm formation on bone grafts and bone graft substitutes: Comparison of different materials by a standard in vitro test and microcalorimetry. Acta Biomater. 2010, 6, 3791–3797. [Google Scholar] [CrossRef]
- Zuiderduin, W.C.J.; Westzaan, C.; Huétink, J.; Gaymans, R.J. Toughening of polypropylene with calcium carbonate particles. Polymer 2002, 44, 261–275. [Google Scholar] [CrossRef]
- Kim, G.M.; Michler, G.H. Micromechanical deformation processes in toughened and particle filled semicrystalline polymers: Part 2. model representation for micromechanical deformation processes. Polymer 1998, 39, 5699–5703. [Google Scholar] [CrossRef]
- Subramanian, A.S.; Tey, J.N.; Zhang, L.; Ng, B.H.; Roy, S.; Wei, J.; Hu, X. ‘Matthew’ Synergistic bond strengthening in epoxy adhesives using polydopamine/MWCNT hybrids. Polymer 2016, 82, 285–294. [Google Scholar] [CrossRef]
- Rodrigues, N.; Benning, M.; Ferreira, A.M.; Dixon, L.; Dalgarno, K. Manufacture and Characterisation of Porous PLA Scaffolds. Procedia CIRP 2016, 49, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Ghassemieh, E. Morphology and compression behaviour of biodegradable scaffolds produced by the sintering process. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2008, 222, 1247–1262. [Google Scholar] [CrossRef]
- Deplaine, H.; Acosta-Santamaría, V.A.; Vidaurre, A.; Gómez Ribelles, J.L.; Doblaré, M.; Ochoa, I.; Gallego Ferrer, G. Evolution of the properties of a poly(L-lactic acid) scaffold with double porosity during in vitro degradation in a phosphate-buffered saline solution. J. Appl. Polym. Sci. 2014, 131, 1–10. [Google Scholar] [CrossRef]
- Khodaei, M.; Amini, K.; Valanezhad, A. Fabrication and Characterization of Poly Lactic Acid Scaffolds by Fused Deposition Modeling for Bone Tissue Engineering. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2020, 35, 248–251. [Google Scholar] [CrossRef]
- Chung, T.-W.; Wang, Y.-Z.; Huang, Y.-Y.; Pan, C.-I.; Wang, S.-S. Poly (e-caprolactone) Grafted With Nano-structured Chitosan Enhances Growth of Human Dermal Fibroblasts. Artif. Organs 2006, 30, 35–41. [Google Scholar] [CrossRef]
- Navarro, M.; Engel, E.; Planell, J.A.; Amaral, I.; Barbosa, M.; Ginebra, M.P. Surface characterization and cell response of a PLA/CaP glass biodegradable composite material. J. Biomed. Mater. Res. Part A 2008, 85, 477–486. [Google Scholar] [CrossRef]
- Meaurio, E.; Sanchez-Rexach, E.; Butron, A.; Sarasua, J.R. The conformation of chloramphenicol in the ordered and disordered phases. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 211, 383–392. [Google Scholar] [CrossRef]
- Ferreira, M.; Rzhepishevska, O.; Grenho, L.; Malheiros, D.; Gonçalves, L.; Almeida, A.J.; Jordão, L.; Ribeiro, I.A.; Ramstedt, M.; Gomes, P.; et al. Levofloxacin-loaded bone cement delivery system: Highly effective against intracellular bacteria and Staphylococcus aureus biofilms. Int. J. Pharm. 2017, 532, 241–248. [Google Scholar] [CrossRef]
- Cicuéndez, M.; Doadrio, J.C.; Hernández, A.; Portolés, M.T.; Izquierdo-Barba, I.; Vallet-Regí, M. Multifunctional pH sensitive 3D scaffolds for treatment and prevention of bone infection. Acta Biomater. 2018, 65, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Martin, V.; Ribeiro, I.A.C.; Alves, M.M.; Gonçalves, L.; Almeida, A.J.; Grenho, L.; Fernandes, M.H.; Santos, C.F.; Gomes, P.S.; Bettencourt, A.F. Understanding intracellular trafficking and anti-inflammatory effects of minocycline chitosan-nanoparticles in human gingival fibroblasts for periodontal disease treatment. Int. J. Pharm. 2019, 572, 118821. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. M02-A12 Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard-Twelfth Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]







| PD-BaSO4 (wt.%) | E (MPa) | σy (MPa) | σr (MPa) | εb (%) | TT (J/m3) |
|---|---|---|---|---|---|
| 0 | 1243 ± 96 | - | 67.1 ± 0.5 | 9.2 ± 0.5 | 3.8 ± 0.6 |
| 0.5 | 1309 ± 51 | 78.7 ± 0.4 | 56.3 ± 5.4 | 132.2 ± 13.1 | 67.5 ± 6.1 |
| 1 | 1396 ± 109 | 77.7 ± 0.8 | 56.0 ± 4.8 | 146.3 ± 9.8 | 73.6 ± 4.6 |
| 2 | 1417 ± 86 | 78.4 ± 1.7 | 62.5 ± 5.2 | 182.2 ± 5.9 | 95.1 ± 1.7 |
| 5 | 1415 ± 49 | 75.7 ± 0.6 | 57.6± 5.8 | 171.9 ± 2.9 | 85.9 ± 0.8 |
| 10 | 1350 ± 124 | 74.1 ± 0.8 | 55.3 ± 4.3 | 154.6 ± 8.6 | 76.2 ± 2.8 |
| Material | Temperature (°C) | Pressure (Bar) | Speed(mm/s) | Post-Flow(s) | Pre-Flow(s) |
|---|---|---|---|---|---|
| PLA | 25 | 5.0 | 3.5 | 0.11 | 0.04 |
| PLA/PD-BaSO4 | 25 | 4.4 | 4.1 | 0.11 | 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadaba, N.; Larrañaga, A.; Orpella-Aceret, G.; Bettencourt, A.F.; Martin, V.; Biggs, M.; Ribeiro, I.A.C.; Ugartemendia, J.M.; Sarasua, J.-R.; Zuza, E. Benefits of Polydopamine as Particle/Matrix Interface in Polylactide/PD-BaSO4 Scaffolds. Int. J. Mol. Sci. 2020, 21, 5480. https://doi.org/10.3390/ijms21155480
Sadaba N, Larrañaga A, Orpella-Aceret G, Bettencourt AF, Martin V, Biggs M, Ribeiro IAC, Ugartemendia JM, Sarasua J-R, Zuza E. Benefits of Polydopamine as Particle/Matrix Interface in Polylactide/PD-BaSO4 Scaffolds. International Journal of Molecular Sciences. 2020; 21(15):5480. https://doi.org/10.3390/ijms21155480
Chicago/Turabian StyleSadaba, Naroa, Aitor Larrañaga, Gemma Orpella-Aceret, Ana F. Bettencourt, Victor Martin, Manus Biggs, Isabel A. C. Ribeiro, Jone M. Ugartemendia, Jose-Ramon Sarasua, and Ester Zuza. 2020. "Benefits of Polydopamine as Particle/Matrix Interface in Polylactide/PD-BaSO4 Scaffolds" International Journal of Molecular Sciences 21, no. 15: 5480. https://doi.org/10.3390/ijms21155480