Synthesis, CYP24A1-Dependent Metabolism and Antiproliferative Potential against Colorectal Cancer Cells of 1,25-Dihydroxyvitamin D2 Derivatives Modified at the Side Chain and the A-Ring
Abstract
:1. Introduction
2. Results and Discussion
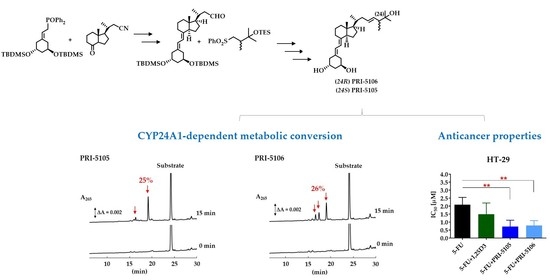
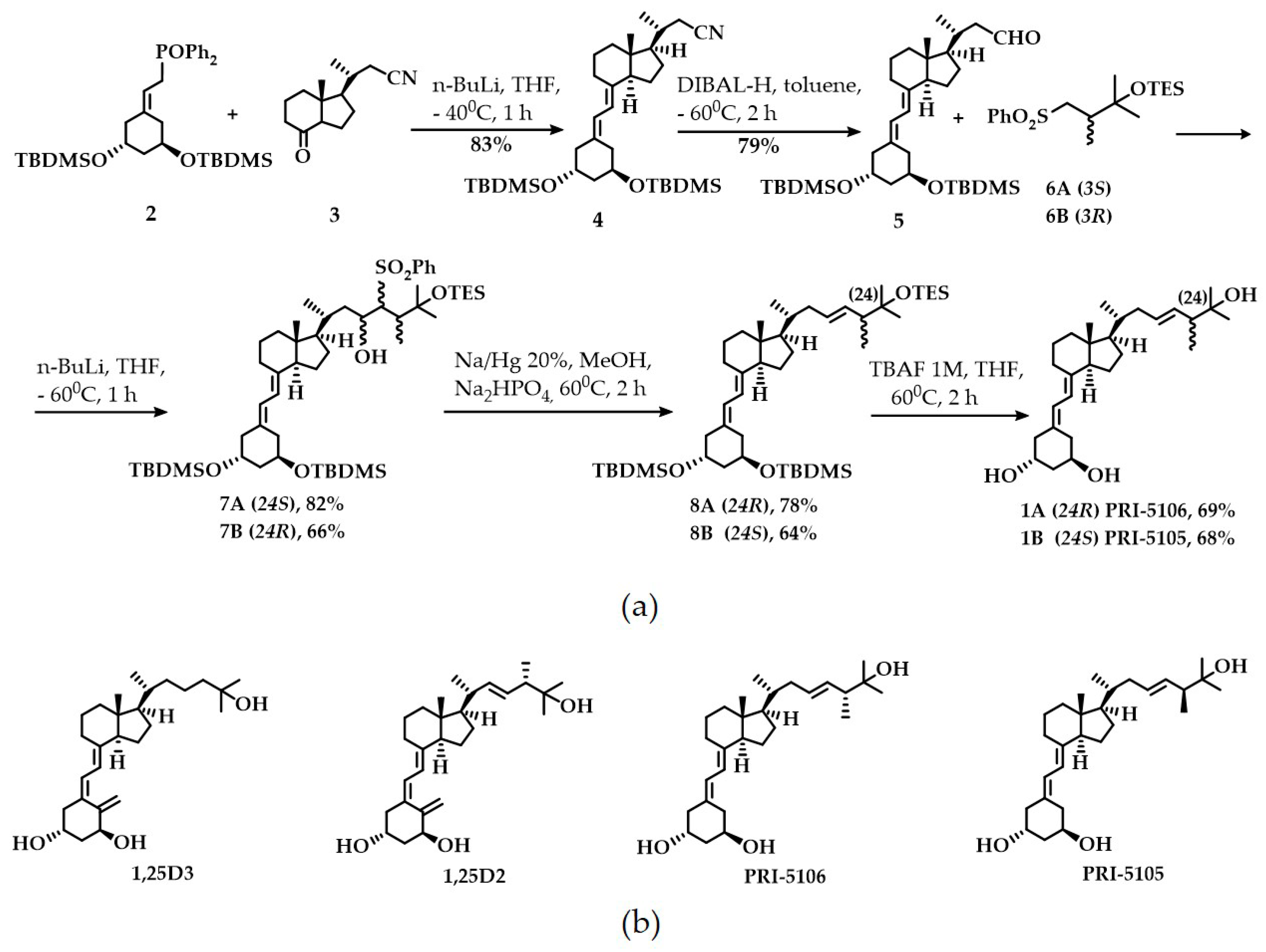
2.1. Synthesis and CYP24A1-Dependent Metabolism
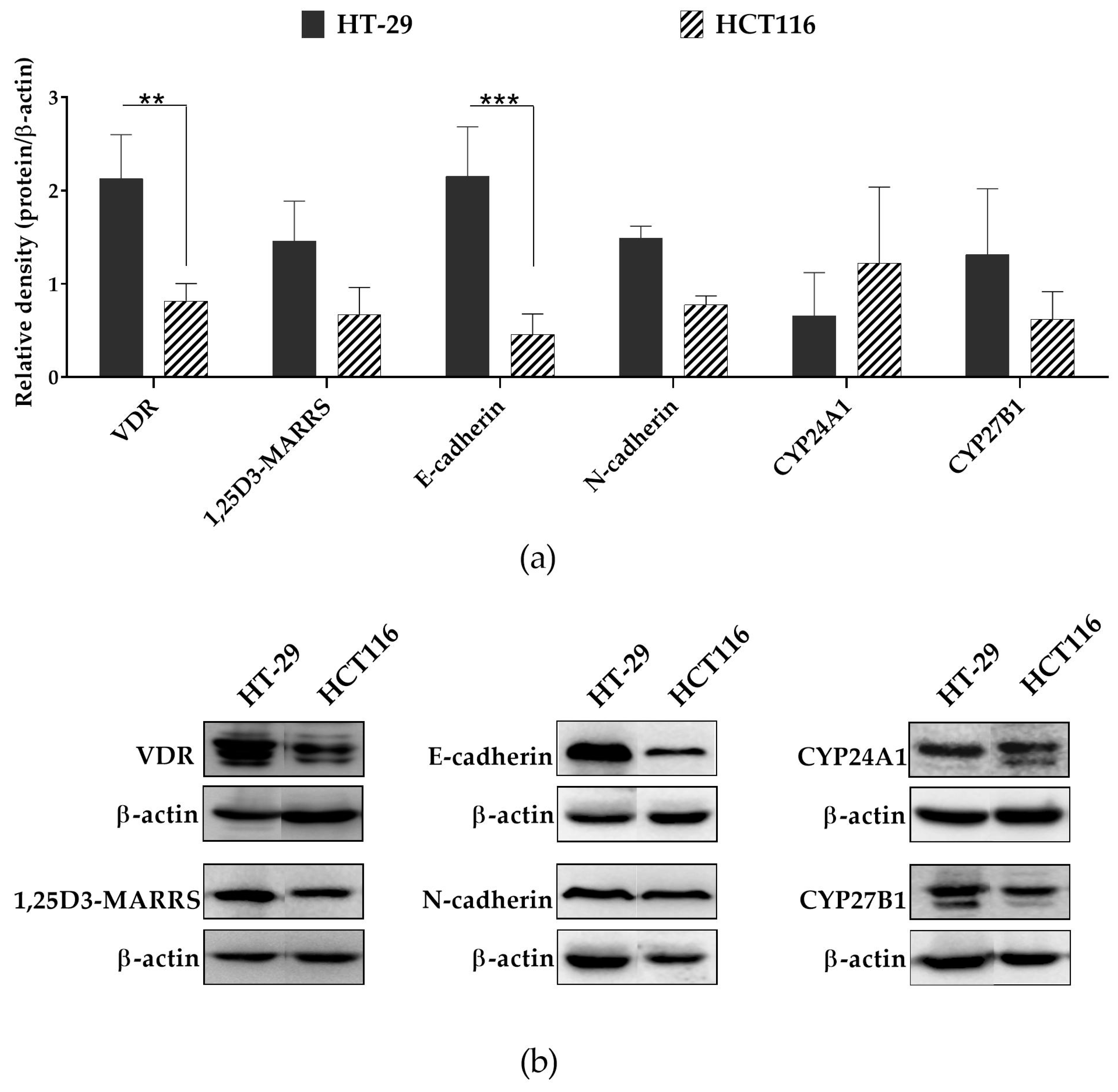
2.2. Anticancer Potential
3. Materials and Methods
3.1. Synthetic and Analytical Chemistry
3.2. Synthesis
3.2.1. (1R,3R,7E,20R)-1,3-Bis(tert-butyldimethylsilyloxy)-20-methyl-19-nor-9,10-secopregna-5,7-diene-21-carbonitrile 4
3.2.2. (1R,3R,7E,20R)-1,3-Bis(tert-butyldimethylsilyloxy)-20-methyl-19-nor-9,10-secopregna-5,7-diene-21-carboaldehyde 5
3.2.3. (1R,3R,7E,22E,24R)-24-Methyl-19-nor-20a-homo-9,10-secocholesta-5,7,22-triene-1,3,25-triol 1A
3.2.4. (1R,3R,7E,22E,24S)-24-Methyl-19-nor-20a-homo-9,10-secocholesta-5,7,22-triene-1,3,25-triol 1B
3.3. Metabolic Resistance of VDDs to CYP24A1-Dependent Degradation
3.4. Antiproliferative Activity of VDDs
3.5. Protein Expression
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 1,25D2 | 1,25-dihydroxyvitamin D2 |
| 1,25D3 | 1,25-dihydroxyvitamin D3 |
| 5-FU | 5-fluorouracil |
| CMS | consensus molecular subtypes |
| CRC | colorectal cancer |
| CYP24A1 | 25-hydroxyvitamin D3-24-hydroxylase |
| CYP27B1 | 25-hydroxyvitamin D3-1α-hydroxylase |
| EMT | epithelial-mesenchymal transition |
| MARRS | membrane associated rapid response steroid-binding protein |
| VDD | vitamin D derivative |
| VDR | vitamin D receptor |
References
- Leyssens, C.; Verlinden, L.; Verstuyf, A. The future of vitamin D analogs. Front. Physiol. 2014, 5, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, M.J.; Murray, A.; Synnott, N.C.; O’Donovan, N.; Crown, J. Vitamin D analogues: Potential use in cancer treatment. Crit. Rev. Oncol. Hemat. 2017, 112, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Studzinski, G.P.; Gocek, E.; Coffman, F.; Danilenko, M. Effects of Vitamin D Derivatives on Differentiation, Cell Cycle, and Apoptosis in Hematological Malignancies. In Vitamin D, 4th ed.; Feldman, D., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 2, pp. 761–799. [Google Scholar]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J. Vitamin D and breast cancer: Past and present. J. Steroid Biochem. Mol. Biol. 2018, 177, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, E.; Wallace, G.R.; Browng, G. The use of 1α,25-dihydroxyvitamin D3 as an anticancer agent. Int. J. Mol. Sci. 2016, 17, 729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trynda, J.; Turlej, E.; Milczarek, M.; Pietraszek, A.; Chodyński, M.; Kutner, A.; Wietrzyk, J. Antiproliferative Activity and in Vivo Toxicity of Double-Point Modified Analogs of 1,25-Dihydroxyergocalciferol. Int. J. Mol. Sci. 2015, 16, 24873–24894. [Google Scholar] [CrossRef] [Green Version]
- Berkowska, K.; Corcoran, A.; Grudzień, M.; Jakuszak, A.; Chodyński, M.; Kutner, A.; Marcinkowska, E. Investigating the Role of VDR and Megalin in Semi-Selectivity of Side-Chain Modified 19-nor Analogs of Vitamin D. Int. J. Mol. Sci. 2019, 20, 4183. [Google Scholar] [CrossRef] [Green Version]
- Milczarek, M.; Psurski, M.; Kutner, A.; Wietrzyk, J. Vitamin D analogs enhance the anticancer activity of 5-fluorouracil in an in vivo mouse colon cancer model. BMC Cancer 2013, 13, 294. [Google Scholar] [CrossRef] [Green Version]
- Milczarek, M.; Filip-Psurska, B.; Swietnicki, W.; Kutner, A.; Wietrzyk, J. Vitamin D analogs combined with 5-fluorouracil in human HT-29 colon cancer treatment. Oncol. Rep. 2014, 32, 491–504. [Google Scholar] [CrossRef] [Green Version]
- Berg, K.C.G.; Eide, P.W.; Eilertsen, I.A.; Johannessen, B.; Bruun, J.; Danielsen, S.A.; Bjørnslett, M.; Meza-Zepeda, L.A.; Eknæs, M.; Lind, G.E.; et al. Multi-omics of 34 colorectal cancer cell lines—A resource for biomedical studies. Mol. Cancer 2017, 16, 116. [Google Scholar] [CrossRef]
- Schroll, M.M.; Ludwig, K.R.; Bauer, K.M.; Hummon, A.B. Calcitriol supplementation causes decreases in tumorigenic proteins and different proteomic and metabolomic signatures in right versus left-sided colon cancer. Metabolites 2018, 8, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimoto, N.; Inaba, Y.; Yamada, S.; Makishima, M.; Shimizu, M.; Yamamoto, K. 2-Methylene 19-nor-25-dehydro-1α-hydroxyvitamin D3 26,23-lactones: Synthesis, biological activities and molecular basis of passive antagonism. Bioorg. Med. Chem. 2008, 16, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Nachliely, M.; Trachtenberg, A.; Khalfin, B.; Nalbandyan, K.; Cohen-Lahav, M.; Yasuda, K.; Sakaki, T.; Kutner, A.; Danilenko, M. Dimethyl fumarate and vitamin D derivatives cooperatively enhance VDR and Nrf2 signaling in differentiating AML cells in vitro and inhibit leukemia progression in a xenograft mouse model. J. Steroid Biochem. Mol. Biol. 2019, 188, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Bolla, N.R.; Corcoran, A.; Yasuda, K.; Chodyński, M.; Krajewski, K.; Cmoch, P.; Marcinkowska, E.; Brown, G.; Sakaki, T.; Kutner, A. Synthesis and evaluation of geometric analogs of 1α,25-dihydroxyvitamin D2 as potential therapeutics. J. Steroid Biochem. Mol. Biol. 2016, 164, 50–55. [Google Scholar] [CrossRef]
- Danilenko, M.; Studzinski, G.P. Enhancement by other compounds of the anti-cancer activity of vitamin D3 and its analogs. Exp. Cell Res. 2004, 298, 339–358. [Google Scholar] [CrossRef]
- Milczarek, M.; Rossowska, J.; Klopotowska, D.; Stachowicz, M.; Kutner, A.; Wietrzyk, J. Tacalcitol increases the sensitivity of colorectal cancer cells to 5-fluorouracil by downregulating the thymidylate synthase. J. Steroid Biochem. Mol. Biol. 2019, 190, 139–151. [Google Scholar] [CrossRef]
- Wheelock, M.J.; Shintani, Y.; Maeda, M.; Fukumoto, Y.; Johnson, K.R. Cadherin switching. J. Cell Sci. 2008, 121, 727–735. [Google Scholar] [CrossRef] [Green Version]
- Sakaki, T.; Sawada, N.; Komai, K.; Shiozawa, S.; Yamada, S.; Yamamoto, K.; Ohyama, Y.; Inouye, K. Dual metabolic pathway of 25-hydroxyvitamin D3 catalyzed by human CYP24. Eur. J. Biochem. 2000, 267, 6158–6165. [Google Scholar] [CrossRef] [Green Version]
- Urushino, N.; Yasuda, K.; Ikushiro, S.; Kamakura, M.; Ohta, M.; Sakaki, T. Metabolism of 1α,25-dihydroxyvitamin D2 by human CYP24A1. Biochem. Biophys. Res. Commun. 2009, 384, 144–148. [Google Scholar] [CrossRef]
- Nevozhay, D. Cheburator software for automatically calculating drug inhibitory concentrations from in vitro screening assays. PLoS ONE 2014, 9, e106186. [Google Scholar] [CrossRef] [Green Version]
- Wietrzyk, J.; Milczarek, M.; Kutner, A. The Effect of Combined Treatment on Head and Neck Human Cancer Cell Lines with Novel Analogs of Calcitriol and Cytostatics. Oncol. Res. 2007, 16, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Generalized Equations for the Analysis of Inhibitions of Michaelis-Menten and Higher-Order Kinetic Systems with Two or More Mutually Exclusive and Nonexclusive Inhibitors. Eur. J. Biochem. 1981, 115, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]

| Compound | 1,25D2 | PRI-5105 | PRI-5106 |
|---|---|---|---|
| Metabolic conversion (%) | 33 ± 4.2 | 25 ± 1.9 | 26 ± 3.3 |
| Compound/CI | IC50 [µM] | |
|---|---|---|
| HT-29 | HCT116 | |
| 1,25D3 | 0.34 ± 0.4 | nd |
| PRI-5105 | 0.15 ± 0.1 | nd |
| PRI-5106 | 0.09 ± 0.01 | nd |
| 5-FU | 2.09 ± 0.5 | 2.60 ± 0.1 |
| 5-FU + 1,25D3 | 1.49 ± 0.7 | 3.07 ± 0.5 |
| CI | 0.8 ± 0.3 | - |
| 5-FU + PRI-5105 | 0.71 ± 0.4 ** | 2.66 ± 0.6 |
| CI | 0.4 ± 0.2 | - |
| 5-FU + PRI-5106 | 0.78 ± 0.3 ** | 2.69 ± 0.4 |
| CI | 0.5 ± 0.04 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milczarek, M.; Chodyński, M.; Pietraszek, A.; Stachowicz-Suhs, M.; Yasuda, K.; Sakaki, T.; Wietrzyk, J.; Kutner, A. Synthesis, CYP24A1-Dependent Metabolism and Antiproliferative Potential against Colorectal Cancer Cells of 1,25-Dihydroxyvitamin D2 Derivatives Modified at the Side Chain and the A-Ring. Int. J. Mol. Sci. 2020, 21, 642. https://doi.org/10.3390/ijms21020642
Milczarek M, Chodyński M, Pietraszek A, Stachowicz-Suhs M, Yasuda K, Sakaki T, Wietrzyk J, Kutner A. Synthesis, CYP24A1-Dependent Metabolism and Antiproliferative Potential against Colorectal Cancer Cells of 1,25-Dihydroxyvitamin D2 Derivatives Modified at the Side Chain and the A-Ring. International Journal of Molecular Sciences. 2020; 21(2):642. https://doi.org/10.3390/ijms21020642
Chicago/Turabian StyleMilczarek, Magdalena, Michał Chodyński, Anita Pietraszek, Martyna Stachowicz-Suhs, Kaori Yasuda, Toshiyuki Sakaki, Joanna Wietrzyk, and Andrzej Kutner. 2020. "Synthesis, CYP24A1-Dependent Metabolism and Antiproliferative Potential against Colorectal Cancer Cells of 1,25-Dihydroxyvitamin D2 Derivatives Modified at the Side Chain and the A-Ring" International Journal of Molecular Sciences 21, no. 2: 642. https://doi.org/10.3390/ijms21020642