Light Energy Dose and Photosensitizer Concentration Are Determinants of Effective Photo-Killing against Caries-Related Biofilms
Abstract
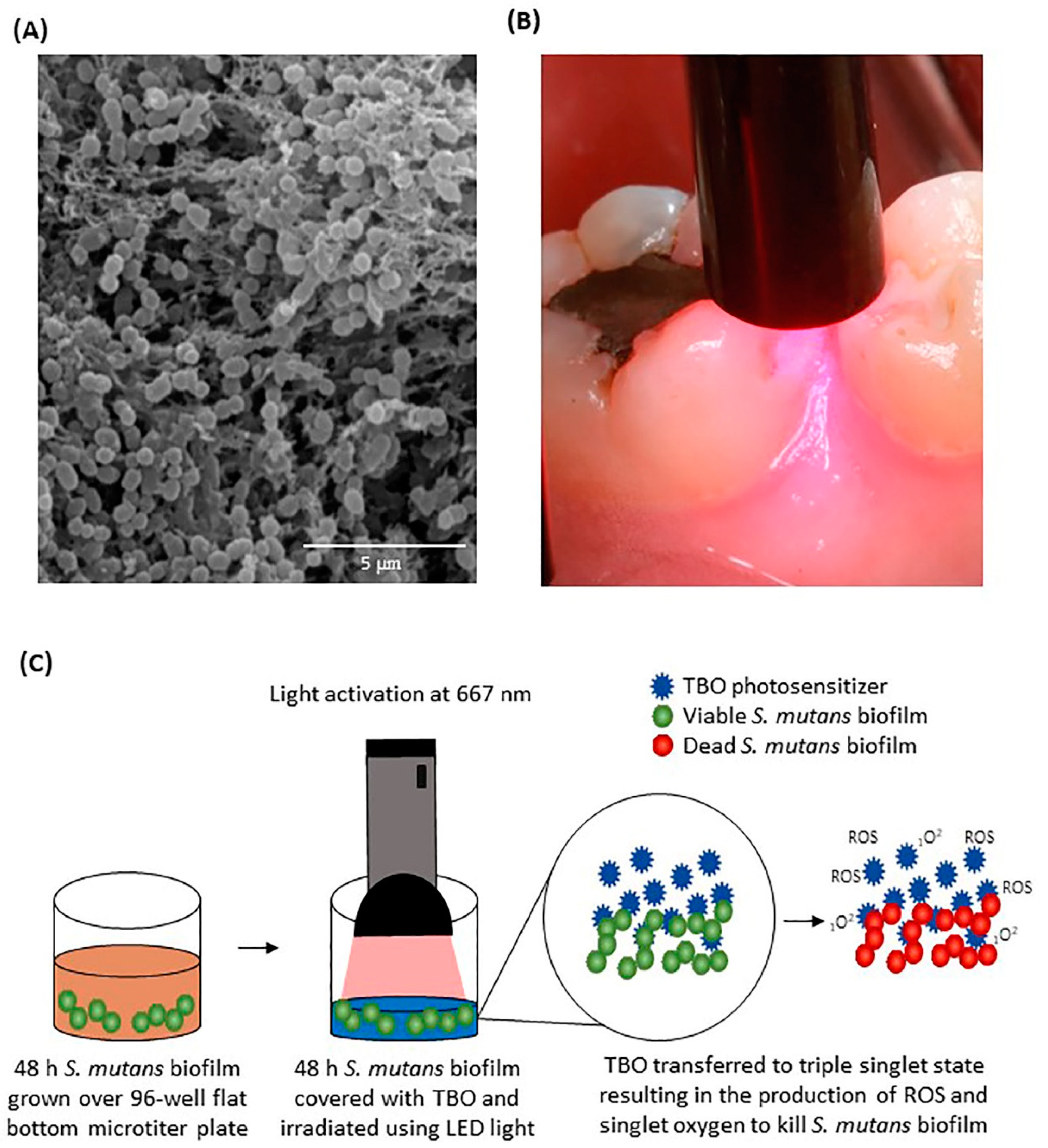
:1. Introduction
2. Results
2.1. Spectra of TBO and LED
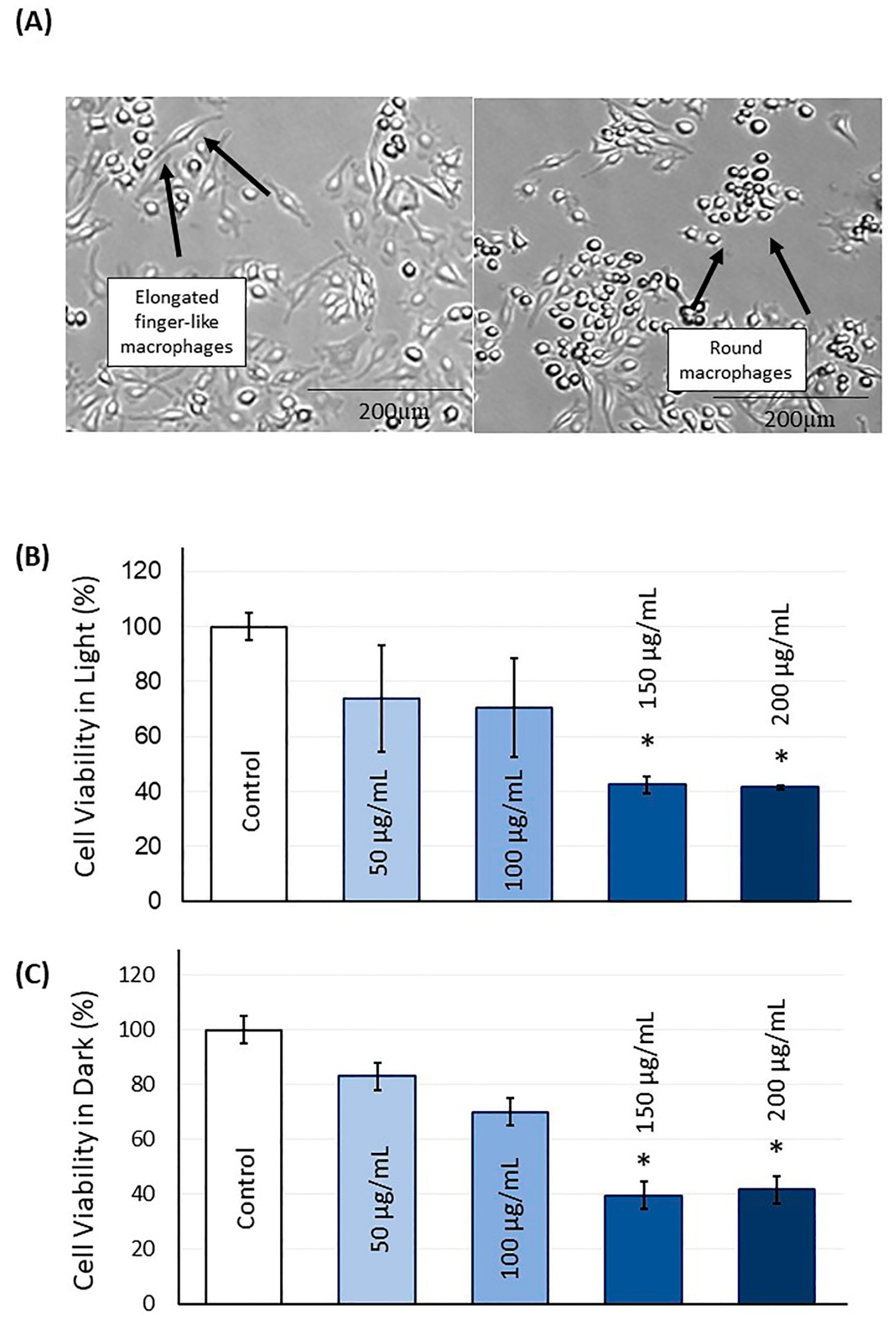
2.2. TBO Cytotoxicity
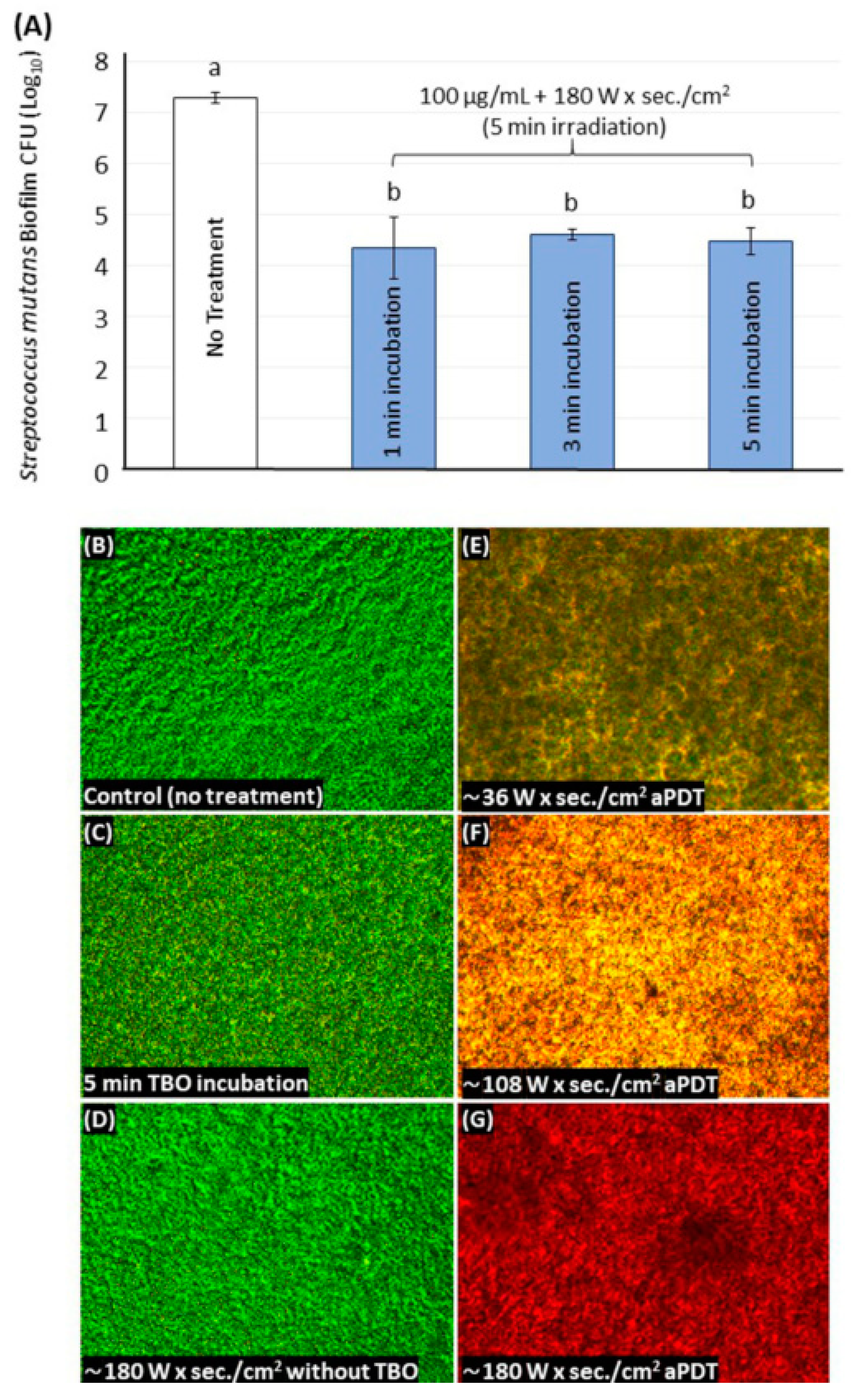
2.3. TBO Determining Parameters and S. mutans Photoinactivation
2.4. Live/Dead Assays for the S. mutans Biofilm
3. Discussion
4. Material and Methods
4.1. Photosensitizer TBO and Light-Emitting Diode (LED)
4.2. Light and Dark Cell Cytotoxicity Assay
4.3. S. mutans Biofilm Model
4.4. In Vitro Photosensitization of S. mutans Biofilms for Optimization of the Dosimetry
4.5. CFU Counting Assay
4.6. Live/Dead Staining of Biofilms
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nedeljkovic, I.; De Munck, J.; Vanloy, A.; Declerck, D.; Lambrechts, P.; Peumans, M.; Teughels, W.; Van Meerbeek, B.; Van Landuyt, K.L. Secondary caries: Prevalence, characteristics, and approach. Clin. Oral Investig. 2020, 24, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Wrasse, E.O.; Garcia, I.M.; Baierle, R.J.; de Souza, V.S.; Scholten, J.D.; Collares, F.M. Quantum chemistry study of the interaction between ionic liquid-functionalized TiO2 quantum dots and methacrylate resin: Implications for dental materials. Biophys. Chem. 2020, 265, 106435. [Google Scholar] [CrossRef]
- Garcia, I.M.; Rodrigues, S.B.; Balbinot, G.d.S.; Visioli, F.; Leitune, V.C.B.; Collares, F.M. Quaternary ammonium compound as antimicrobial agent in resin-based sealants. Clin. Oral Investig. 2020, 24, 777–784. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Hwang, G.; Liu, Y.; Gao, L.; Kilpatrick-Liverman, L.; Santarpia, P.; Zhou, X.; Koo, H. l-Arginine Modifies the Exopolysaccharide Matrix and Thwarts Streptococcus mutans Outgrowth within Mixed-Species Oral Biofilms. J. Bacteriol. 2016, 198, 2651–2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, J.-N.; Jung, J.-E.; Lee, M.-H.; Choi, H.-M.; Jeon, J.-G. Sucrose challenges to Streptococcus mutans biofilms and the curve fitting for the biofilm changes. FEMS Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial interactions in dental biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Misaki, T.; Naka, S.; Wato, K.; Nagasawa, Y.; Nomura, R.; Otsugu, M.; Matsumoto-Nakano, M.; Nakano, K.; Kumagai, H.; et al. Specific strains of Streptococcus mutans, a pathogen of dental caries, in the tonsils, are associated with IgA nephropathy. Sci. Rep. 2019, 9, 20130. [Google Scholar] [CrossRef]
- Lemos, J.A.; Burne, R.A. A model of efficiency: Stress tolerance by Streptococcus mutans. Microbiol. Read. Engl. 2008, 154, 3247–3255. [Google Scholar] [CrossRef] [Green Version]
- Du, Q.; Fu, M.; Zhou, Y.; Cao, Y.; Guo, T.; Zhou, Z.; Li, M.; Peng, X.; Zheng, X.; Li, Y.; et al. Sucrose promotes caries progression by disrupting the microecological balance in oral biofilms: An in vitro study. Sci. Rep. 2020, 10, 2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitwalli, H.; Alsahafi, R.; Balhaddad, A.A.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Emerging Contact-Killing Antibacterial Strategies for Developing Anti-Biofilm Dental Polymeric Restorative Materials. Bioengineering 2020, 7, 83. [Google Scholar] [CrossRef]
- Di Nisio, C.; De Colli, M.; di Giacomo, V.; Rapino, M.; Di Valerio, V.; Marconi, G.D.; Gallorini, M.; Di Giulio, M.; Cataldi, A.; Zara, S. A dual role for β1 integrin in an in vitro Streptococcus mitis/human gingival fibroblasts co-culture model in response to TEGDMA. Int. Endod. J. 2015, 48, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Øilo, M.; Bakken, V. Biofilm and Dental Biomaterials. Materials 2015, 8, 2887–2900. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [Green Version]
- Misba, L.; Kulshrestha, S.; Khan, A.U. Antibiofilm action of a toluidine blue O-silver nanoparticle conjugate on Streptococcus mutans: A mechanism of type I photodynamic therapy. Biofouling 2016, 32, 313–328. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial photodynamic therapy: Overview of a promising approach to fight antibiotic-resistant bacterial infections. J. Clin. Transl. Res. 2015, 1, 140–167. [Google Scholar]
- Steiner-Oliveira, C.; Longo, P.L.; Aranha, A.C.C.; Ramalho, K.M.; Mayer, M.P.A.; Eduardo, C.d.P. Randomized in vivo evaluation of photodynamic antimicrobial chemotherapy on deciduous carious dentin. J. Biomed. Opt. 2015, 20, 108003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, M.A.S.; Rolim, J.P.M.L.; Passos, V.F.; Lima, R.A.; Zanin, I.C.J.; Codes, B.M.; Rocha, S.S.; Rodrigues, L.K.A. Photodynamic antimicrobial chemotherapy and ultraconservative caries removal linked for management of deep caries lesions. Photodiagnosis Photodyn. 2015, 12, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, A.; Grinholc, M. Combined Antimicrobial Activity of Photodynamic Inactivation and Antimicrobials–State of the Art. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- de Melo, M.A.S.; Rolim, J.P.M.L.; Zanin, I.C.J.; Barros, E.B.; da-Costa, E.F.; Rodrigues, L.K.A. Characterization of antimicrobial photodynamic therapy-treated Streptococci mutans: An atomic force microscopy study. Photomed. Laser Surg. 2013, 31, 105–109. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; Garcia, I.M.; Ibrahim, M.S.; Rolim, J.P.M.L.; Gomes, E.A.B.; Martinho, F.C.; Collares, F.M.; Xu, H.; Melo, M.A.S. Prospects on Nano-Based Platforms for Antimicrobial Photodynamic Therapy against Oral Biofilms. Photobiomodul Photomed. Laser Surg. 2020. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.B.; Ferrisse, T.M.; Marques, R.S.; de Annunzio, S.R.; Brighenti, F.L.; Fontana, C.R. Effect of Photodynamic Therapy on Microorganisms Responsible for Dental Caries: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 3585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, T.; Mogi, M.; Okabe, I.; Okada, K.; Goto, H.; Sasaki, Y.; Fujimura, T.; Fukuda, M.; Mitani, A. Adjunctive Application of Antimicrobial Photodynamic Therapy in Nonsurgical Periodontal Treatment: A Review of Literature. Int. J. Mol. Sci. 2015, 16, 24111–24126. [Google Scholar] [CrossRef] [Green Version]
- Rolim, J.P.M.L.; de-Melo, M.A.S.; Guedes, S.F.; Albuquerque-Filho, F.B.; de Souza, J.R.; Nogueira, N.A.P.; Zanin, I.C.J.; Rodrigues, L.K.A. The antimicrobial activity of photodynamic therapy against Streptococcus mutans using different photosensitizers. J. Photochem. Photobiol. Biol. 2012, 106, 40–46. [Google Scholar] [CrossRef]
- Lima, J.P.M.; Melo, M.A.S.D.; Borges, F.M.C.; Teixeira, A.H.; Steiner-Oliveira, C.; Santos, M.N.D.; Rodrigues, L.K.A.; Zanin, I.C.J. Evaluation of the antimicrobial effect of photodynamic antimicrobial therapy in an in situ model of dentine caries. Eur. J. Oral Sci. 2009, 117, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, R.; Seraj, B.; Chiniforush, N.; Rokouei, M.; Mousavi, N.; Ghadimi, S. Effect of antimicrobial photodynamic therapy on the counts of salivary Streptococcus mutans in children with severe early childhood caries. Photodiagnosis Photodyn. 2017, 18, 319–322. [Google Scholar] [CrossRef]
- Darmani, H.; Tawalbeh, K.H.; Al-Hiyasat, A.S.; Al-Akhras, M.-A. Comparison of the Photosensitivity of Biofilms of Different Genera of Cariogenic Bacteria in Tooth Slices. Pol. J. Microbiol. 2018, 67, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Reis, A.C.M.; Regis, W.F.M.; Rodrigues, L.K.A. Scientific evidence in antimicrobial photodynamic therapy: An alternative approach for reducing cariogenic bacteria. Photodiagnosis Photodyn. 2019, 26, 179–189. [Google Scholar] [CrossRef]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef]
- Zanin, I.C.J.; Lobo, M.M.; Rodrigues, L.K.A.; Pimenta, L.A.F.; Höfling, J.F.; Gonçalves, R.B. Photosensitization of in vitro biofilms by toluidine blue O combined with a light-emitting diode. Eur. J. Oral Sci. 2006, 114, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, J.-F.; Dussault, S.; Viau, G.; Gad, F.; Boushira, M.; Bissonnette, R. Photodynamic therapy with toluidine blue in Jurkat cells: Cytotoxicity, subcellular localization and apoptosis induction. Photochem. Photobiol. Sci. 2002, 1, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Chepurna, O.; Grebinyk, A.; Petrushko, Y.; Prylutska, S.; Grebinyk, S.; Yashchuk, V.M.; Matyshevska, O.; Ritter, U.; Dandekar, T.; Frohme, M.; et al. LED-based portable light source for photodynamic therapy. In Proceedings of the Optics in Health Care and Biomedical Optics IX., International Society for Optics and Photonics, Hangzhou, China, 21–23 October 2019; Volume 11190, p. 111901A. [Google Scholar]
- Breskey, J.D.; Lacey, S.E.; Vesper, B.J.; Paradise, W.A.; Radosevich, J.A.; Colvard, M.D. Photodynamic Therapy: Occupational Hazards and Preventative Recommendations for Clinical Administration by Healthcare Providers. Photomed. Laser Surg. 2013, 31, 398–407. [Google Scholar] [CrossRef] [Green Version]
- de-Paula, D.M.; Melo, M.A.S.; Lima, J.P.M.; Nobre-dos-Santos, M.; Zanin, I.C.J.; Rodrigues, L.K.A. In vitro assessment of thermal changes in human teeth during photodynamic antimicrobial chemotherapy performed with red light sources. Laser Phys. 2010, 20, 1475–1480. [Google Scholar] [CrossRef]
- Fumes, A.C.; da Silva Telles, P.D.; Corona, S.A.M.; Borsatto, M.C. Effect of aPDT on Streptococcus mutans and Candida albicans present in the dental biofilm: Systematic review. Photodiagnosis Photodyn. 2018, 21, 363–366. [Google Scholar] [CrossRef]
- Nagata, J.Y.; Hioka, N.; Kimura, E.; Batistela, V.R.; Terada, R.S.S.; Graciano, A.X.; Baesso, M.L.; Hayacibara, M.F. Antibacterial photodynamic therapy for dental caries: Evaluation of the photosensitizers used and light source properties. Photodiagnosis Photodyn. 2012, 9, 122–131. [Google Scholar] [CrossRef]
- Soria-Lozano, P.; Gilaberte, Y.; Paz-Cristobal, M.P.; Pérez-Artiaga, L.; Lampaya-Pérez, V.; Aporta, J.; Pérez-Laguna, V.; García-Luque, I.; Revillo, M.J.; Rezusta, A. In vitro effect photodynamic therapy with differents photosensitizers on cariogenic microorganisms. BMC Microbiol. 2015, 15, 187. [Google Scholar] [CrossRef] [Green Version]
- Burmølle, M.; Ren, D.; Bjarnsholt, T.; Sørensen, S.J. Interactions in multispecies biofilms: Do they actually matter? Trends Microbiol. 2014, 22, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Elias, S.; Banin, E. Multi-species biofilms: Living with friendly neighbors. FEMS Microbiol. Rev. 2012, 36, 990–1004. [Google Scholar] [CrossRef]
- AlQranei, M.S.; Aljohani, H.; Majumdar, S.; Senbanjo, L.T.; Chellaiah, M.A. C-phycocyanin attenuates RANKL-induced osteoclastogenesis and bone resorption in vitro through inhibiting ROS levels, NFATc1 and NF-κB activation. Sci. Rep. 2020, 10, 2513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.-J.; Rice, K.C. Understanding the Streptococcus mutans Cid/Lrg System through CidB Function. Appl. Environ. Microbiol 2016, 82, 6189–6203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felix Gomez, G.G.; Lippert, F.; Ando, M.; Zandona, A.F.; Eckert, G.J.; Gregory, R.L. Photoinhibition of Streptococcus mutans Biofilm-Induced Lesions in Human Dentin by Violet-Blue Light. Dent. J. 2019, 7, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balhaddad, A.A.; Ibrahim, M.S.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Concentration dependence of quaternary ammonium monomer on the design of high-performance bioactive composite for root caries restorations. Dent. Mater. 2020, 36, e266–e278. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balhaddad, A.A.; AlQranei, M.S.; Ibrahim, M.S.; Weir, M.D.; Martinho, F.C.; Xu, H.H.K.; Melo, M.A.S. Light Energy Dose and Photosensitizer Concentration Are Determinants of Effective Photo-Killing against Caries-Related Biofilms. Int. J. Mol. Sci. 2020, 21, 7612. https://doi.org/10.3390/ijms21207612
Balhaddad AA, AlQranei MS, Ibrahim MS, Weir MD, Martinho FC, Xu HHK, Melo MAS. Light Energy Dose and Photosensitizer Concentration Are Determinants of Effective Photo-Killing against Caries-Related Biofilms. International Journal of Molecular Sciences. 2020; 21(20):7612. https://doi.org/10.3390/ijms21207612
Chicago/Turabian StyleBalhaddad, Abdulrahman A., Mohammed S. AlQranei, Maria S. Ibrahim, Michael D. Weir, Frederico C. Martinho, Hockin H. K. Xu, and Mary Anne S. Melo. 2020. "Light Energy Dose and Photosensitizer Concentration Are Determinants of Effective Photo-Killing against Caries-Related Biofilms" International Journal of Molecular Sciences 21, no. 20: 7612. https://doi.org/10.3390/ijms21207612
APA StyleBalhaddad, A. A., AlQranei, M. S., Ibrahim, M. S., Weir, M. D., Martinho, F. C., Xu, H. H. K., & Melo, M. A. S. (2020). Light Energy Dose and Photosensitizer Concentration Are Determinants of Effective Photo-Killing against Caries-Related Biofilms. International Journal of Molecular Sciences, 21(20), 7612. https://doi.org/10.3390/ijms21207612







