Platinum-Based Drugs Cause Mitochondrial Dysfunction in Cultured Dorsal Root Ganglion Neurons
Abstract
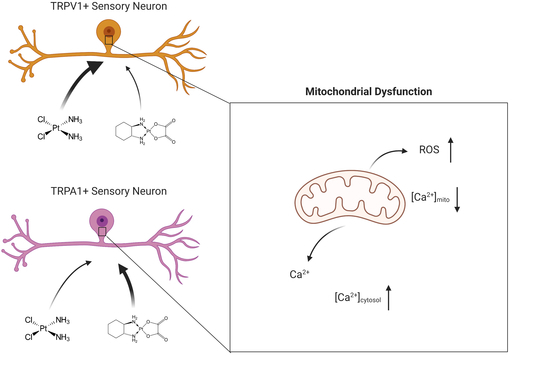
:1. Introduction
2. Results
2.1. Viability of DRG Neurons Is Not Changed by Small Concentrations of Cis- or Oxaliplatin after 24 h
2.2. ROS Production Is Increased in DRG Neurons after Cis- or Oxaliplatin Treatment for 24 h
2.3. Cis- and Oxaliplatin Induce Mitochondrial Stress in DRG Neurons
2.4. Change in Cytosolic and Mitochondrial Calcium Concentrations of TRPA1- or TRPV1-Positive DRG Neurons Is Caused by Exposure to Cisplatin
2.5. Exposure to Oxaliplatin Changes Cytosolic and Mitochondrial Calcium Concentrations of TRPA1- or TRPV1-Positive DRG Neurons
2.6. Exposure to Cis- or Oxaliplatin Results in Differences in Cytosolic and Mitochondrial Calcium in TRPA1- or TRPV1-Positive DRG Neurons
2.7. Modulation of ROS Production in TRPA1- or TRPV1-Positive DRG Neurons after Cisplatin Exposure
2.8. Modulation of ROS Production in TRPA1- or TRPV1-Positive DRG Neurons after Oxaliplatin Exposure
2.9. ROS Production Is Different in TRPA1- and TRPV1-Positive DRG Neurons
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Culture of DRG Neurons
4.3. Plating Density of DRG Neurons
4.4. Exposure of Sensory Neurons with Cisplatin or Oxaliplatin
4.5. Viability Assay
4.6. Immunocytochemical Staining of TRPA1- or TRPV1-Positive DRG Neurons
4.7. ROS Staining
4.8. Cytosolic Calcium Concentration
4.9. Intramitochondrial Calcium Concentration
4.10. Mitochondrial Stress Assay
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cersosimo, R.J. Cisplatin neurotoxicity. Cancer Treat. Rev. 1989, 16, 195–211. [Google Scholar] [CrossRef]
- Taillade, L.; Soria, J.C.; Andre, F.; Grunenwald, D.; Dunant, A.; Pignon, J.P.; Le Chevalier, T. Adjuvant chemotherapy in non-small cell lung cancer. Bull. Cancer 2004, 91, 63–67. [Google Scholar] [PubMed]
- Barabas, K.; Milner, R.; Lurie, D.; Adin, C. Cisplatin: A review of toxicities and therapeutic applications. Vet. Comp. Oncol. 2008, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Misset, J.L.; Bleiberg, H.; Sutherland, W.; Bekradda, M.; Cvitkovic, E. Oxaliplatin clinical activity: A review. Crit. Rev. Oncol. Hematol. 2000, 35, 75–93. [Google Scholar] [CrossRef]
- Joseph, E.K.; Levine, J.D. Comparison of oxaliplatin- and cisplatin-induced painful peripheral neuropathy in the rat. J. Pain Off. J. Am. Pain Soc. 2009, 10, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Thompson, S.W.; Davis, L.E.; Kornfeld, M.; Hilgers, R.D.; Standefer, J.C. Cisplatin neuropathy. Clinical, electrophysiologic, morphologic, and toxicologic studies. Cancer 1984, 54, 1269–1275. [Google Scholar] [CrossRef]
- Windebank, A.J.; Grisold, W. Chemotherapy-induced neuropathy. J. Peripher. Nerv. Syst. 2008, 13, 27–46. [Google Scholar] [CrossRef]
- Cavaletti, G. Peripheral neurotoxicity of platinum-based chemotherapy. Nat. Rev. Cancer 2008, 8. [Google Scholar] [CrossRef] [Green Version]
- Weickhardt, A.; Wells, K.; Messersmith, W. Oxaliplatin-induced neuropathy in colorectal cancer. J. Oncol. 2011, 2011, 201593. [Google Scholar] [CrossRef] [Green Version]
- Miltenburg, N.C.; Boogerd, W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat. Rev. 2014, 40, 872–882. [Google Scholar] [CrossRef]
- Canta, A.; Pozzi, E.; Carozzi, V.A. Mitochondrial Dysfunction in Chemotherapy-Induced Peripheral Neuropathy (CIPN). Toxics 2015, 3, 198–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, W.H.; Zheng, H.; Bennett, G.J. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience 2012, 203, 194–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansson, P.; Backonja, M.; Bouhassira, D. Usefulness and limitations of quantitative sensory testing: Clinical and research application in neuropathic pain states. Pain 2007, 129, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Mahn, F. Types of topical treatment for peripheral neuropathic pain: Mechanism of action and indications. Schmerz 2010, 24, 317–325. [Google Scholar] [CrossRef]
- Nickel, F.T.; DeCol, R.; Jud, S.; Fasching, P.A.; Seifert, F.; Maihofner, C. Inhibition of hyperalgesia by conditioning electrical stimulation in a human pain model. Pain 2011, 152, 1298–1303. [Google Scholar] [CrossRef]
- Leo, M.; Schmitt, L.I.; Erkel, M.; Melnikova, M.; Thomale, J.; Hagenacker, T. Cisplatin-induced neuropathic pain is mediated by upregulation of N-type voltage-gated calcium channels in dorsal root ganglion neurons. Exp. Neurol. 2017, 288, 62–74. [Google Scholar] [CrossRef]
- Leo, M.; Schmitt, L.I.; Jastrow, H.; Thomale, J.; Kleinschnitz, C.; Hagenacker, T. Cisplatin alters the function and expression of N-type voltage-gated calcium channels in the absence of morphological damage of sensory neurons. Mol. Pain 2017, 13, 1744806917746565. [Google Scholar] [CrossRef]
- Schmitt, L.I.; Leo, M.; Kleinschnitz, C.; Hagenacker, T. Oxaliplatin Modulates the Characteristics of Voltage-Gated Calcium Channels and Action Potentials in Small Dorsal Root Ganglion Neurons of Rats. Mol. Neurobiol. 2018, 55, 8842–8855. [Google Scholar] [CrossRef]
- Kawashiri, T.; Egashira, N.; Kurobe, K.; Tsutsumi, K.; Yamashita, Y.; Ushio, S.; Yano, T.; Oishi, R. L type Ca(2)+ channel blockers prevent oxaliplatin-induced cold hyperalgesia and TRPM8 overexpression in rats. Mol. Pain 2012, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Gil, I.; Zian, D.; Vazquez-Villa, H.; Hernandez-Torres, G.; Martinez, R.F.; Khiar-Fernandez, N.; Rivera, R.; Kihara, Y.; Devesa, I.; Mathivanan, S.; et al. A Novel Agonist of the Type 1 Lysophosphatidic Acid Receptor (LPA1), UCM-05194, Shows Efficacy in Neuropathic Pain Amelioration. J. Med. Chem. 2020, 63, 2372–2390. [Google Scholar] [CrossRef]
- Macpherson, L.J.; Dubin, A.E.; Evans, M.J.; Marr, F.; Schultz, P.G.; Cravatt, B.F.; Patapoutian, A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007, 445, 541–545. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassini, R.; Gees, M.; Harrison, S.; De Siena, G.; Materazzi, S.; Moretto, N.; Failli, P.; Preti, D.; Marchetti, N.; Cavazzini, A.; et al. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain 2011, 152, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Tominaga, T. Structure and function of TRPV1. Pflug. Arch. 2005, 451, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Honore, P.; Zhong, C.; Gauvin, D.; Mikusa, J.; Hernandez, G.; Chandran, P.; Gomtsyan, A.; Brown, B.; Bayburt, E.K.; et al. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J. Neurosci. 2006, 26, 9385–9393. [Google Scholar] [CrossRef] [Green Version]
- Eastman, A.; Barry, M.A. Interaction of trans-diamminedichloroplatinum(II) with DNA: Formation of monofunctional adducts and their reaction with glutathione. Biochemistry 1987, 26, 3303–3307. [Google Scholar] [CrossRef] [PubMed]
- Siau, C.; Bennett, G.J. Dysregulation of cellular calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anesth. Analg. 2006, 102, 1485–1490. [Google Scholar] [CrossRef]
- Podratz, J.L.; Knight, A.M.; Ta, L.E.; Staff, N.P.; Gass, J.M.; Genelin, K.; Schlattau, A.; Lathroum, L.; Windebank, A.J. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol. Dis. 2011, 41, 661–668. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.H.; Bennett, G.J. Effects of mitochondrial poisons on the neuropathic pain produced by the chemotherapeutic agents, paclitaxel and oxaliplatin. Pain 2012, 153, 704–709. [Google Scholar] [CrossRef] [Green Version]
- Kelley, M.R.; Jiang, Y.; Guo, C.; Reed, A.; Meng, H.; Vasko, M.R. Role of the DNA base excision repair protein, APE1 in cisplatin, oxaliplatin, or carboplatin induced sensory neuropathy. PLoS ONE 2014, 9, e106485. [Google Scholar] [CrossRef]
- Tomaszewski, A.; Busselberg, D. Cisplatin modulates voltage gated channel currents of dorsal root ganglion neurons of rats. Neurotoxicology 2007, 28, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Gorgun, M.F.; Zhuo, M.; Englander, E.W. Cisplatin Toxicity in Dorsal Root Ganglion Neurons Is Relieved by Meclizine via Diminution of Mitochondrial Compromise and Improved Clearance of DNA Damage. Mol. Neurobiol. 2017, 54, 7883–7895. [Google Scholar] [CrossRef] [PubMed]
- Ta, L.E.; Espeset, L.; Podratz, J.; Windebank, A.J. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. Neurotoxicology 2006, 27, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.W.; Liu, S.H.; Young, Y.H.; Lin-Shiau, S.Y. D-Methionine attenuated cisplatin-induced vestibulotoxicity through altering ATPase activities and oxidative stress in guinea pigs. Toxicol. Appl. Pharmacol. 2006, 215, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xiao, W.H.; Bennett, G.J. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exp. Neurol. 2011, 232, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marullo, R.; Werner, E.; Degtyareva, N.; Moore, B.; Altavilla, G.; Ramalingam, S.S.; Doetsch, P.W. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS ONE 2013, 8, e81162. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Tang, B.; Yu, P.W.; Tang, B.; Hao, Y.X.; Lei, X.; Luo, H.X.; Zeng, D.Z. Autophagy protects against oxaliplatin-induced cell death via ER stress and ROS in Caco-2 cells. PLoS ONE 2012, 7, e51076. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, C.; Vasko, M.R.; Kelley, M.R. Implications of apurinic/apyrimidinic endonuclease in reactive oxygen signaling response after cisplatin treatment of dorsal root ganglion neurons. Cancer Res. 2008, 68, 6425–6434. [Google Scholar] [CrossRef] [Green Version]
- Massicot, F.; Hache, G.; David, L.; Chen, D.; Leuxe, C.; Garnier-Legrand, L.; Rat, P.; Laprevote, O.; Coudore, F. P2X7 Cell Death Receptor Activation and Mitochondrial Impairment in Oxaliplatin-Induced Apoptosis and Neuronal Injury: Cellular Mechanisms and In Vivo Approach. PLoS ONE 2013, 8, e66830. [Google Scholar] [CrossRef]
- Vincent, A.M.; Kato, K.; McLean, L.L.; Soules, M.E.; Feldman, E.L. Sensory neurons and schwann cells respond to oxidative stress by increasing antioxidant defense mechanisms. Antioxid. Redox Signal. 2009, 11, 425–438. [Google Scholar] [CrossRef]
- Jiao, L.; Li, D.D.; Yang, C.L.; Peng, R.Q.; Guo, Y.Q.; Zhang, X.S.; Zhu, X.F. Reactive oxygen species mediate oxaliplatin-induced epithelial-mesenchymal transition and invasive potential in colon cancer. Tumour Biol. 2016, 37, 8413–8423. [Google Scholar] [CrossRef] [PubMed]
- Splettstoesser, F.; Florea, A.M.; Busselberg, D. IP(3) receptor antagonist, 2-APB, attenuates cisplatin induced Ca2+-influx in HeLa-S3 cells and prevents activation of calpain and induction of apoptosis. Br. J. Pharmacol. 2007, 151, 1176–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, C.; Chen, J.; Xu, H. Changes in [Ca2+]i and IP3 levels in the process of cisplatin-induced apoptosis of gastric carcinoma. Zhonghua zhong liu za zhi [Chin. J. Oncol.] 1999, 21, 256–258. [Google Scholar] [PubMed]
- Boehmerle, W.; Splittgerber, U.; Lazarus, M.B.; McKenzie, K.M.; Johnston, D.G.; Austin, D.J.; Ehrlich, B.E. Paclitaxel induces calcium oscillations via an inositol 1,4,5-trisphosphate receptor and neuronal calcium sensor 1-dependent mechanism. Proc. Natl. Acad. Sci. USA 2006, 103, 18356–18361. [Google Scholar] [CrossRef] [Green Version]
- Sittl, R.; Lampert, A.; Huth, T.; Schuy, E.T.; Link, A.S.; Fleckenstein, J.; Alzheimer, C.; Grafe, P.; Carr, R.W. Anticancer drug oxaliplatin induces acute cooling-aggravated neuropathy via sodium channel subtype Na(V)1.6-resurgent and persistent current. Proc. Natl. Acad. Sci. USA 2012, 109, 6704–6709. [Google Scholar] [CrossRef] [Green Version]
- Miyake, T.; Nakamura, S.; Meng, Z.; Hamano, S.; Inoue, K.; Numata, T.; Takahashi, N.; Nagayasu, K.; Shirakawa, H.; Mori, Y.; et al. Distinct Mechanism of Cysteine Oxidation-Dependent Activation and Cold Sensitization of Human Transient Receptor Potential Ankyrin 1 Channel by High and Low Oxaliplatin. Front. Physiol. 2017, 8, 878. [Google Scholar] [CrossRef] [Green Version]
- Materazzi, S.; Fusi, C.; Benemei, S.; Pedretti, P.; Patacchini, R.; Nilius, B.; Prenen, J.; Creminon, C.; Geppetti, P.; Nassini, R. TRPA1 and TRPV4 mediate paclitaxel-induced peripheral neuropathy in mice via a glutathione-sensitive mechanism. Pflug. Arch. Eur. J. Physiol. 2012, 463, 561–569. [Google Scholar] [CrossRef]
- Naziroglu, M.; Braidy, N. Thermo-Sensitive TRP Channels: Novel Targets for Treating Chemotherapy-Induced Peripheral Pain. Front. Physiol. 2017, 8, 1040. [Google Scholar] [CrossRef]
- Hagenacker, T.; Splettstoesser, F.; Greffrath, W.; Treede, R.D.; Busselberg, D. Capsaicin differentially modulates voltage-activated calcium channel curren. Brain Res. 2005, 1062, 74–85. [Google Scholar] [CrossRef]








| Primary/Secondary Antibody | Dilution, Species | Maker |
|---|---|---|
| Anti-TRPA1-ATTO-594 | 1:200, rabbit | Alomone Labs, Israel |
| Anti-TRPV1 Alexa Fluo 488 | 1:200, rabbit | Santa Cruz Biotechnology, USA |
| Anti-TRPA1 (extracellular) | 1:200, rabbit | Alomone Labs, Israel |
| Anti-TRPV1 (extracellular) | 1:200, rabbit | Alomone Labs, Israel |
| Alexa-Fluo 488 | 1:200, goat anti-rabbit | DIANOVA, Germany |
| Cy3 | 1:200, goat anti-rabbit | DIANOVA, Germany |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leo, M.; Schmitt, L.-I.; Küsterarent, P.; Kutritz, A.; Rassaf, T.; Kleinschnitz, C.; Hendgen-Cotta, U.B.; Hagenacker, T. Platinum-Based Drugs Cause Mitochondrial Dysfunction in Cultured Dorsal Root Ganglion Neurons. Int. J. Mol. Sci. 2020, 21, 8636. https://doi.org/10.3390/ijms21228636
Leo M, Schmitt L-I, Küsterarent P, Kutritz A, Rassaf T, Kleinschnitz C, Hendgen-Cotta UB, Hagenacker T. Platinum-Based Drugs Cause Mitochondrial Dysfunction in Cultured Dorsal Root Ganglion Neurons. International Journal of Molecular Sciences. 2020; 21(22):8636. https://doi.org/10.3390/ijms21228636
Chicago/Turabian StyleLeo, Markus, Linda-Isabell Schmitt, Patricia Küsterarent, Andrea Kutritz, Tienush Rassaf, Christoph Kleinschnitz, Ulrike B. Hendgen-Cotta, and Tim Hagenacker. 2020. "Platinum-Based Drugs Cause Mitochondrial Dysfunction in Cultured Dorsal Root Ganglion Neurons" International Journal of Molecular Sciences 21, no. 22: 8636. https://doi.org/10.3390/ijms21228636