Biochemical and Behavioral Consequences of Ethanol Intake in a Mouse Model of Metabolic Syndrome
Abstract
:1. Introduction
2. Results
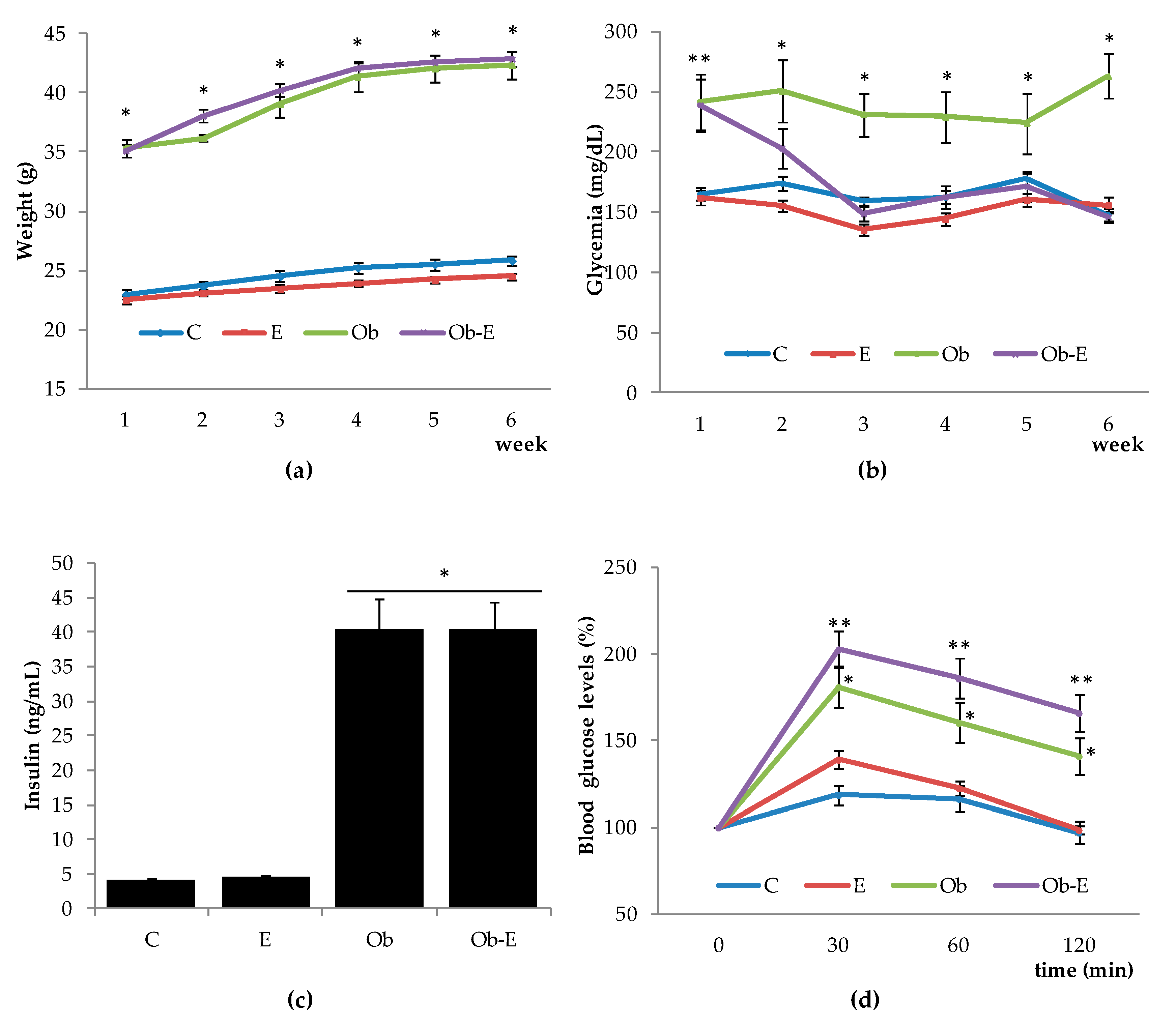
2.1. Effects of Ethanol Intake with Regard to Weight, Glucose Overdose, Insulin, and Basal Glycemia
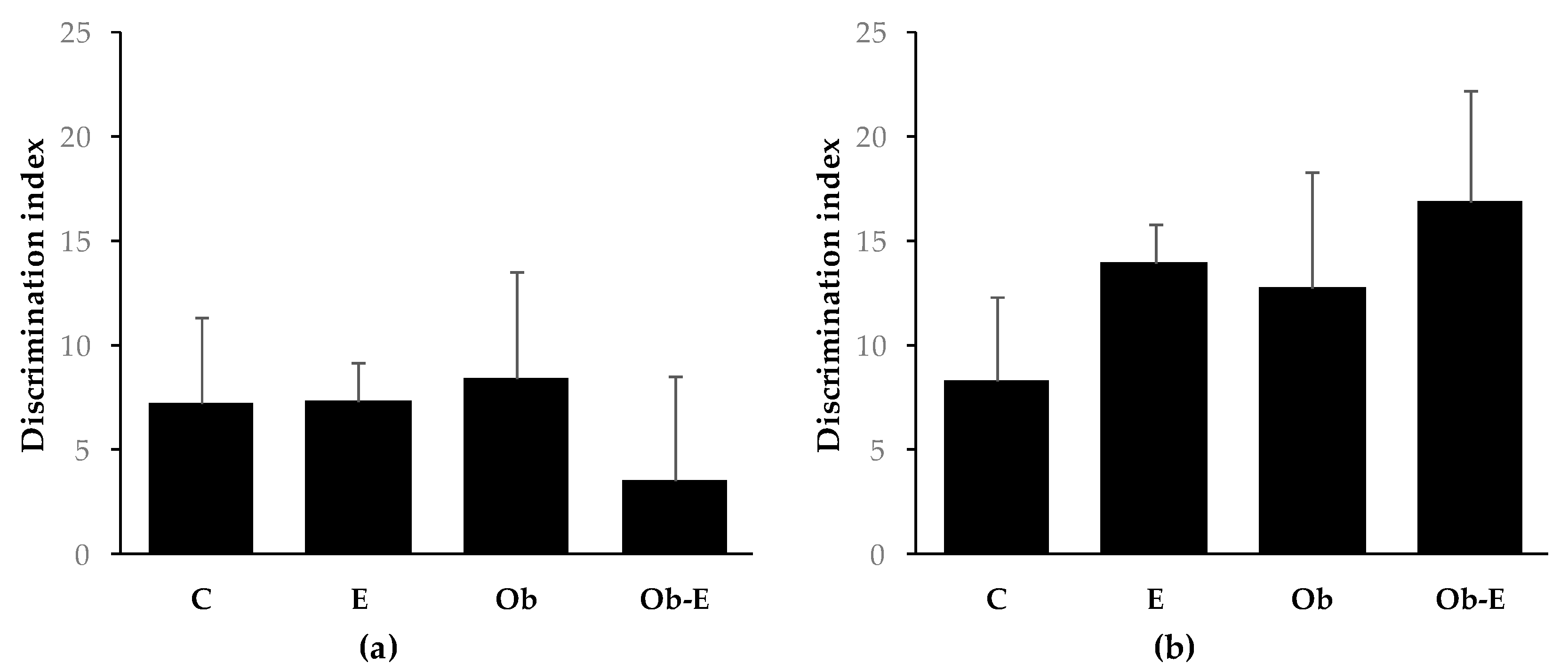
2.2. Cognitive Effects of Ethanol Consumption
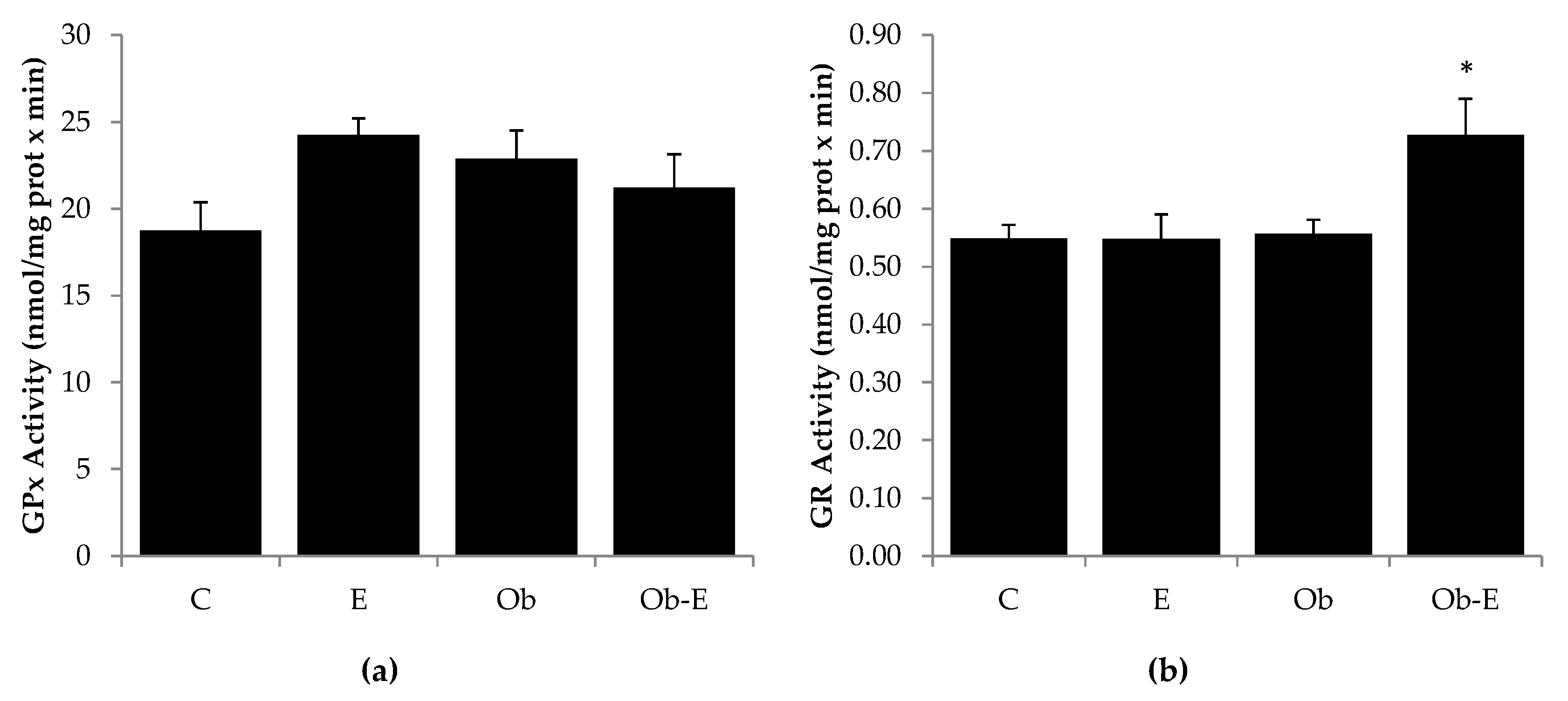
2.3. Effects of Ethanol Intake on GR and GPx Activity
2.4. Effects of Ethanol Consumption on GSH, GSSG, GSH/GSSG Ratio, and L-Cysteine
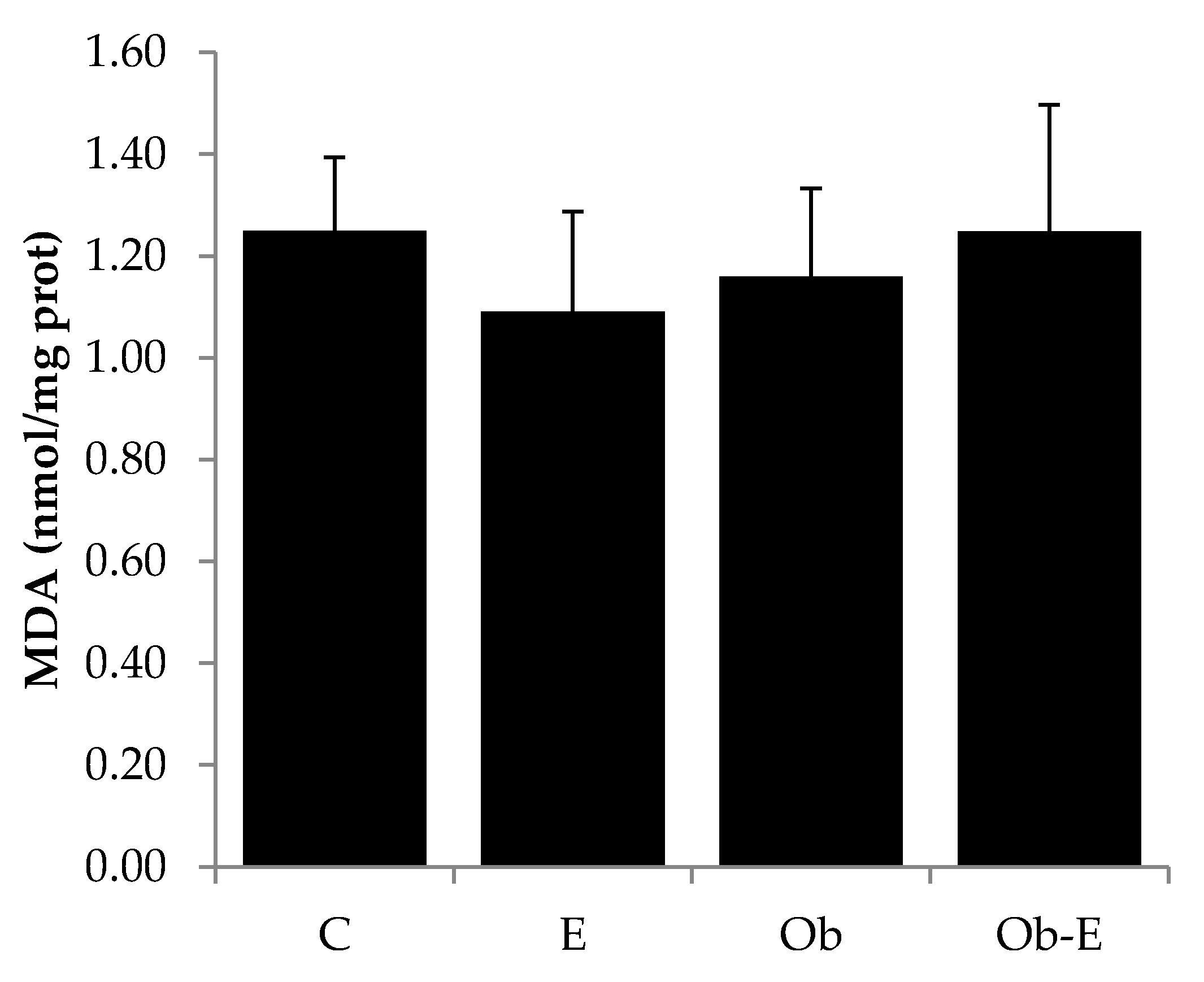
2.5. Effects of Ethanol Administration on MDA Levels
3. Discussion
3.1. MS Mice Model and Chronic Ethanol Consumption
3.2. Chronic Ethanol Consumption and MS Modulate Oxidative Status in the Brain of Mice
3.3. Behavioral Effects of Chronic Ethanol Consumption in MS Mice
4. Materials and Methods
4.1. Animals
4.2. Drugs and Chemicals
4.3. Experimental Procedure
4.3.1. Glycemia, Insulinemia, and Glucose Overload Tests
4.3.2. Memory and Learning Test
4.3.3. Antioxidant Defenses
GSH, GSSG, and L-Cysteine
Glutathione Peroxidase Activity (GPx)
Glutathione Reductase Activity (GR)
4.3.4. Lipid Peroxidation
4.3.5. Protein Content
4.3.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| cAMP | Cyclic adenosine monophosphate |
| DI | Discrimination index |
| DTNB | 5,5′-dithiobis(2-nitrobenzoic acid) |
| EDTA | Ethylenediaminetetraacetic acid |
| GABA | Γ-aminobutyric acid |
| GPx | Glutathione peroxidase activity |
| GR | Glutathione reductase activity |
| GSH | Glutathione |
| GSSG | Glutathione disulfide |
| HPLC | High-performance liquid chromatography |
| L-Cys | L-cysteine |
| MDA | Malondialdehyde |
| MS | Metabolic syndrome |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| T2D | Type 2 diabetes |
| WHO | World Health Organization |
References
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Lorenzo, C.; Okoloise, M.; Williams, K.; Stern, M.P.; Haffner, S.M.; San Antonio Heart Study. The Metabolic Syndrome as Predictor of Type 2 Diabetes: The San Antonio Heart Study. Diabetes Care 2003, 26, 3153–3159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome; International Diabetes Federation: Brussels, Belgium, 2006. [Google Scholar]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marott, S.C.W.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Benn, M. Components of the Metabolic Syndrome and Risk of Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 3212–3221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginsberg, H.N.; Maccallum, P.R. The Obesity, Metabolic Syndrome, and Type 2 Diabetes Mellitus Pandemic: Part I. Increased Cardiovascular Disease Risk and the Importance of Atherogenic Dyslipidemia in Persons With the Metabolic Syndrome and Type 2 Diabetes Mellitus. J. CardioMetab. Syndr. 2009, 4, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hsieh, M.-C.; Chang, S.-J. Metabolic syndrome, diabetes, and hyperuricemia. Curr. Opin. Rheumatol. 2013, 25, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Shinkov, A.; Borissova, A.-M.; Kovatcheva, R.; Vlahov, J.; Dakovska, L.; Atanassova, I.; Petkova, P. Increased prevalence of depression and anxiety among subjects with metabolic syndrome and known type 2 diabetes mellitus—A population-based study. Postgrad. Med. 2018, 130, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.-P.; Willett, W.C.; Hu, F.B. Sugar-Sweetened Beverages and Risk of Metabolic Syndrome and Type 2 Diabetes: A meta-analysis. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef] [Green Version]
- Earnest, C.P.; Johannsen, N.M.; Swift, D.L.; Gillison, F.B.; Mikus, C.R.; Lucía, A.; Kramer, K.; Lavie, C.J.; Church, T.S. Aerobic and Strength Training in Concomitant Metabolic Syndrome and Type 2 Diabetes. Med. Sci. Sports Exerc. 2014, 46, 1293–1301. [Google Scholar] [CrossRef] [Green Version]
- Wannamethee, S.G.; Shaper, A.G.; Perry, I.J.; Alberti, K.G.M.M. Alcohol consumption and the incidence of type II diabetes. J. Epidemiol. Community Health 2002, 56, 542–548. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.-A.; Lee, J.-H.; Lim, S.-Y.; Ha, H.-S.; Kwon, H.-S.; Park, Y.-M.; Lee, W.-C.; Kang, M.-I.; Yim, H.-W.; Yoon, K.-H.; et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J. Diabetes Investig. 2013, 4, 334–343. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Hayden, M.R.; Tyagi, S.C. Islet Redox Stress: The Manifold Toxicities of Insulin Resistance, Metabolic Syndrome and Amylin Derived Islet Amyloid in Type 2 Diabetes Mellitus. JOP 2002, 3, 86–108. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12110767 (accessed on 8 April 2019). [PubMed]
- Robertson, R.; Harmon, J.; Tran, P.O.T.; Poitout, V. Cell Glucose Toxicity, Lipotoxicity, and Chronic Oxidative Stress in Type 2 Diabetes. Diabetes 2004, 53 (Suppl. 1), S119–S124. Available online: http://www.ncbi.nlm.nih.gov/pubmed/14749276 (accessed on 8 April 2019). [CrossRef] [PubMed] [Green Version]
- Bi, Y.; Wang, T.; Xu, M.; Xu, Y.; Li, M.; Lu, J.; Zhu, X.; Ning, G. Advanced research on risk factors of type 2 diabetes. Diabetes Metab. Res. Rev. 2012, 28, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Fromenty, B.; Vadrot, N.; Massart, J.; Turlin, B.; Barri-Ova, N.; Letteron, P.; Fautrel, A.; Robin, M.-A. Chronic Ethanol Consumption Lessens the Gain of Body Weight, Liver Triglycerides, and Diabetes in Obese ob/ob Mice. J. Pharmacol. Exp. Ther. 2009, 331, 23–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaller, G.; Kretschmer, S.; Gouya, G.; Haider, D.G.; Mittermayer, F.; Riedl, M.; Wagner, O.; Pacini, G.; Wolzt, M.; Ludvik, B. Alcohol Acutely Increases Vascular Reactivity together with Insulin Sensitivity in Type 2 Diabetic Men. Exp. Clin. Endocrinol. Diabetes 2009, 118, 57–60. [Google Scholar] [CrossRef]
- Koppes, L.L.J.; Dekker, J.M.; Hendriks, H.F.; Bouter, L.M.; Heine, R.J. Moderate Alcohol Consumption Lowers the Risk of Type 2 Diabetes: A Meta-Analysis of Prospective Observational Studies. Diabetes Care 2005, 28, 719–725. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15735217 (accessed on 8 April 2019). [CrossRef] [Green Version]
- Howard, A.A.; Arnsten, J.H.; Gourevitch, M.N. Effect of Alcohol Consumption on Diabetes Mellitus: A Systematic Review. Ann. Intern. Med. 2004, 140, 211–219. Available online: http://www.ncbi.nlm.nih.gov/pubmed/14757619 (accessed on 7 April 2019). [CrossRef]
- Knott, C.S.; Bell, S.; Britton, A. Alcohol Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-analysis of More Than 1.9 Million Individuals from 38 Observational Studies. Diabetes Care 2015, 38, 1804–1812. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wang, Y.; Lam, K.S.L.; Xu, A. Moderate Wine Consumption in the Prevention of Metabolic Syndrome and Its Related Medical Complications. Endocr. Metab. Immune Disord. Drug Targets 2008, 8, 89–98. Available online: http://www.ncbi.nlm.nih.gov/pubmed/18537695 (accessed on 8 April 2019). [CrossRef]
- Li, X.-H.; Yu, F.-F.; Zhou, Y.-H.; He, J. Association between alcohol consumption and the risk of incident type 2 diabetes: A systematic review and dose-response meta-analysis. Am. J. Clin. Nutr. 2016, 103, 818–829. [Google Scholar] [CrossRef] [Green Version]
- Pietraszek, A.; Gregersen, S.; Hermansen, K. Alcohol and type 2 diabetes. A review. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 366–375. [Google Scholar] [CrossRef]
- Baliunas, D.; Taylor, B.J.; Irving, H.; Roerecke, M.; Patra, J.; Mohapatra, S.; Rehm, J. Alcohol as a Risk Factor for Type 2 Diabetes: A systematic review and meta-analysis. Diabetes Care 2009, 32, 2123–2132. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Wang, X.; Zhang, Y. Specific types of alcoholic beverage consumption and risk of type 2 diabetes: A systematic review and meta-analysis. J. Diabetes Investig. 2017, 8, 56–68. [Google Scholar] [CrossRef] [Green Version]
- De Gaetano, G.; Costanzo, S.; Di Castelnuovo, A.; Badimon, L.; Bejko, D.; Alkerwi, A.; Chiva-Blanch, G.; Estruch, R.; La Vecchia, C.; Panico, S.; et al. Effects of moderate beer consumption on health and disease: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 443–467. [Google Scholar] [CrossRef] [Green Version]
- Cofán, M.; Nicolas, J.M.; Sola-Fernandez, J.; Robert, J.; Tobias, E.; Sacanella, E.; Estruch, R.; Urbano-Marquez, A. Acute Ethanol Treatment Decreases Intracellular Calcium-Ion Transients in Mouse Single Skeletal Muscle Fibres In Vitro. Alcohol Alcohol. 2000, 35, 134–138. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10787387 (accessed on 8 April 2019). [CrossRef] [Green Version]
- De Beun, R.; Schneider, R.; Klein, A.; Lohmann, A.; Schreiber, R.; De Vry, J. The calcium channel agonist BAY k 8644 reduces ethanol intake and preference in alcohol-preferring AA rats. Psychopharmacology 1996, 127, 302–310. [Google Scholar] [CrossRef]
- Masalkar, P.D.; Abhang, S.A. Oxidative stress and antioxidant status in patients with alcoholic liver disease. Clin. Chim. Acta 2005, 355, 61–65. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H. Mechanism of Generation of Oxidative Stress and Pathophysiology of Type 2 Diabetes Mellitus: How Are They Interlinked? J. Cell. Biochem. 2017, 118, 3577–3585. [Google Scholar] [CrossRef]
- Sakuraba, H.; Mizukami, H.; Yagihashi, N.; Wada, R.; Hanyu, C.; Yagihashi, S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia 2002, 45, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Ford, E.S.; Mokdad, A.H.; Giles, W.H.; Brown, D.W. The Metabolic Syndrome and Antioxidant Concentrations: Findings From the Third National Health and Nutrition Examination Survey. Diabetes 2003, 52, 2346–2352. [Google Scholar] [CrossRef] [Green Version]
- Demircan, N.; Gurel, A.; Armutcu, F.; Ünalacak, M.; Aktunc, E.; Atmaca, H.M. The evaluation of serum cystatin C, malondialdehyde, and total antioxidant status in patients with metabolic syndrome. Med. Sci. Monit. 2008, 14, CR97–CR101. [Google Scholar]
- Kennedy, A.J.; Ellacott, K.L.; King, V.L.; Hasty, A.H. Mouse models of the metabolic syndrome. Dis. Model. Mech. 2010, 3, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Fairus, A.; Soelaiman, I.N. Animal models of metabolic syndrome: A review. Nutr. Metab. 2016, 13, 65. [Google Scholar] [CrossRef] [Green Version]
- Pascual, M.; Baliño, P.; Alfonso-Loeches, S.; Aragón, C.M.G.; Guerri, C. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav. Immun. 2011, 25 (Suppl. 1), S80–S91. [Google Scholar] [CrossRef]
- Pascual, M.; Baliño, P.; Aragón, C.M.G.; Guerri, C. Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: Role of TLR4 and TLR2. Neuropharmacology 2015, 89, 352–359. [Google Scholar] [CrossRef]
- Baliño, P.; Ledesma, J.C.; Aragón, C.M.G. In Vivo Study of Ethanol-Activated Brain Protein Kinase A: Manipulations of Ca2+ Distribution and Flux. Alcohol. Clin. Exp. Res. 2014, 38, 629–640. [Google Scholar] [CrossRef]
- Kahn, S.E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 2003, 46, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Yagihashi, S. Advances in pathology of diabetes from pancreatic islets to neuropathy-a tribute to Paul Langerhans. Pathol. Int. 2015, 65, 157–169. [Google Scholar] [CrossRef]
- Yagihashi, S.; Inaba, W.; Mizukami, H. Dynamic pathology of islet endocrine cells in type 2 diabetes: β-Cell growth, death, regeneration and their clinical implications. J. Diabetes Investig. 2016, 7, 155–165. [Google Scholar] [CrossRef]
- Steiner, J.L.; Crowell, K.T.; Lang, C.H. Impact of Alcohol on Glycemic Control and Insulin Action. Biomolecules 2015, 5, 2223–2246. [Google Scholar] [CrossRef] [Green Version]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pr. 2014, 105, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.; Singh, S.P. Effect of ethanol and its metabolites on glucose mediated insulin release from isolated islets of rats. Metabolism 1979, 28, 85–89. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hwang, J.-Y.; Lee, D.Y.; Song, E.H.; Park, K.J.; Kim, G.H.; Jeong, E.A.; Lee, Y.J.; Go, M.J.; Kim, D.J.; et al. Chronic Ethanol Consumption Inhibits Glucokinase Transcriptional Activity byAtf3and Triggers Metabolic Syndromein Vivo. J. Biol. Chem. 2014, 289, 27065–27079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Luo, Y.; Feng, A.; Li, T.; Yang, X.; Nofech-Mozes, R.; Yu, M.; Wang, C.; Li, Z.; Yi, F.; et al. Ethanol induced impairment of glucose metabolism involves alterations of GABAergic signaling in pancreatic β-cells. Toxicology 2014, 326, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Van De Wiel, A. Diabetes mellitus and alcohol. Diabetes Metab. Res. Rev. 2004, 20, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Tetzschner, R.; Nørgaard, K.; Ranjan, A.G. Effects of alcohol on plasma glucose and prevention of alcohol-induced hypoglycemia in type 1 diabetes-A systematic review with GRADE. Diabetes Metab. Res. Rev. 2018, 34, e2965. [Google Scholar] [CrossRef]
- Monnier, L.; Colette, C.; Dunseath, G.J.; Owens, D.R. The Loss of Postprandial Glycemic Control Precedes Stepwise Deterioration of Fasting With Worsening Diabetes. Diabetes Care 2007, 30, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Pratley, R.E.; Weyer, C. The role of impaired early insulin secretion in the pathogenesis of Type II diabetes mellitus. Diabetologia 2001, 44, 929–945. [Google Scholar] [CrossRef] [Green Version]
- Ceriello, A. The possible role of postprandial hyperglycaemia in the pathogenesis of diabetic complications. Diabetologia 2003, 46, M9–M16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baliño, P.; Romero-Cano, R.; Sánchez-Andrés, J.V.; Valls, V.; Aragón, C.G.; Muriach, M.; Romero, R. Effects of Acute Ethanol Administration on Brain Oxidative Status: The Role of Acetaldehyde. Alcohol. Clin. Exp. Res. 2019, 43, 1672–1681. [Google Scholar] [CrossRef] [PubMed]
- Quertemont, E.; Grant, K.; Correa, M.; Arizzi, M.N.; Salamone, J.D.; Tambour, S.; Aragon, C.M.G.; McBride, W.J.; Rodd, Z.; Goldstein, A.; et al. The Role of Acetaldehyde in the Central Effects of Ethanol. Alcohol. Clin. Exp. Res. 2005, 29, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Aragon, C.; Amit, Z. Differences in ethanol-induced behaviors in normal and acatalasemic mice: Systematic examination using a biobehavioral approach. Pharmacol. Biochem. Behav. 1993, 44, 547–554. [Google Scholar] [CrossRef]
- Arizzi-LaFrance, M.N.; Correa, M.; Aragon, C.M.G.; Salamone, J.D. Motor Stimulant Effects of Ethanol Injected into the Substantia Nigra Pars Reticulata: Importance of Catalase-Mediated Metabolism and the Role of Acetaldehyde. Neuropsycho-Pharmacology 2006, 31, 997–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarragon, E.; Baliño, P.; Aragón, C.M.G.; Tarragon, E. Centrally formed acetaldehyde mediates ethanol-induced brain PKA activation. Neurosci. Lett. 2014, 580, 68–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuru-Aoyagi, K.; Potts, M.B.; Trivedi, A.; Bs, T.P.; Raber, J.; Wendland, M.; Bs, C.P.C.; Koh, S.-E.; Ferriero, D.; Noble-Haeusslein, L.J.; et al. Glutathione peroxidase activity modulates recovery in the injured immature brain. Ann. Neurol. 2009, 65, 540–549. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Vallejo, V.; Benlloch-Navarro, S.; Trachsel-Moncho, L.; López-Pedrajas, R.; Almansa, I.; Romero, F.J.; Miranda, M. Alterations in glutamate cysteine ligase content in the retina of two retinitis pigmentosa animal models. Free Radic. Biol. Med. 2016, 96, 245–254. [Google Scholar] [CrossRef]
- Kane, C.J.M.; Chang, J.Y.; Roberson, P.K.; Garg, T.K.; Han, L. Ethanol exposure of neonatal rats does not increase biomarkers of oxidative stress in isolated cerebellar granule neurons. Alcohol 2008, 42, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Sommavilla, M.; Sánchez-Villarejo, M.V.; Almansa, I.; Sánchez-Vallejo, V.; Barcia, J.M.; Romero, F.J.; Miranda, M. The effects of acute ethanol exposure and ageing on rat brain glutathione metabolism. Free Radic. Res. 2012, 46, 1076–1081. [Google Scholar] [CrossRef]
- Wlodek, L.; Rommelspacher, H. Ethanol-induced changes in the content of thiol compounds and of lipid peroxidation in livers and brains from mice: Protection by thiazolidine derivatives. Alcohol Alcohol. 1994, 29, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Argüelles, S.; Machado, A.; Ayala, A. “In vitro” effect of lipid peroxidation metabolites on elongation factor-2. Biochim. Biophys. Acta 2006, 1760, 445–452. [Google Scholar] [CrossRef]
- Łuczaj, W.; Skrzydlewska, E. DNA damage caused by lipid peroxidation products. Cell. Mol. Biol. Lett. 2003, 8, 391–413. [Google Scholar] [PubMed]
- Johnsen-Soriano, S.; Francisco, B.-M.; Miranda, M.; Asensio, S.; Barcia, J.M.; Romá, J.; Monfort, P.; Felipo, V.; Romero, F.J. Ebselen Prevents Chronic Alcohol-Induced Rat Hippocampal Stress and Functional Impairment. Alcohol. Clin. Exp. Res. 2007, 31, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Almansa, I.; Barcia, J.M.; López-Pedrajas, R.; Muriach, M.; Miranda, M.; Romero, F.J. Naltrexone Reverses Ethanol-Induced Rat Hippocampal and Serum Oxidative Damage. Oxidative Med. Cell. Longev. 2013, 2013, 296898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, G.; Anderson, G.; Dean, O.; Berk, M.; Galecki, P.; Martin-Subero, M.; Maes, M. The Glutathione System: A New Drug Target in Neuroimmune Disorders. Mol. Neurobiol. 2014, 50, 1059–1084. [Google Scholar] [CrossRef]
- Reed, D.; Babson, J.; Beatty, P.; Brodie, A.; Ellis, W.; Potter, D. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal. Biochem. 1980, 106, 55–62. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Parkhill, L.K.; Burk, R.F. Hepatic cytosolic non selenium-dependent glutathione peroxidase activity: Its nature and the effect of selenium deficiency. J. Nutr. 1978, 108, 981–987. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef]
- Esterbauer, H.; Eckl, P.; Ortner, A. Possible mutagens derived from lipids and lipid precursors. Mutat. Res. Genet. Toxicol. 1990, 238, 223–233. [Google Scholar] [CrossRef]
- Richard, M.J.; Guiraud, P.; Meo, J.; Favier, A. High-performance liquid chromatographic separation of malondialde-hyde-thiobarbituric acid adduct in biological materials (plasma and human cells) using a commercially available reagent. J. Chromatogr. 1992, 577, 9–18. [Google Scholar] [CrossRef]
- Romero, F.J.; Bosch-Morell, F.; Jareño, E.J.; Romero, B.; Marín, N.; Romá, J. Lipid peroxidation products and antioxidants in human disease. Environ. Health Perspect. 1998, 106 (Suppl. 5), 1229–1234. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baliño, P.; Romero-Cano, R.; Muriach, M. Biochemical and Behavioral Consequences of Ethanol Intake in a Mouse Model of Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 807. https://doi.org/10.3390/ijms22020807
Baliño P, Romero-Cano R, Muriach M. Biochemical and Behavioral Consequences of Ethanol Intake in a Mouse Model of Metabolic Syndrome. International Journal of Molecular Sciences. 2021; 22(2):807. https://doi.org/10.3390/ijms22020807
Chicago/Turabian StyleBaliño, Pablo, Ricard Romero-Cano, and María Muriach. 2021. "Biochemical and Behavioral Consequences of Ethanol Intake in a Mouse Model of Metabolic Syndrome" International Journal of Molecular Sciences 22, no. 2: 807. https://doi.org/10.3390/ijms22020807




