Increased Purinergic Responses Dependent on P2Y2 Receptors in Hepatocytes from CCl4-Treated Fibrotic Mice
Abstract
1. Introduction
2. Results
2.1. Hepatic Fibrosis Induced by CCl4
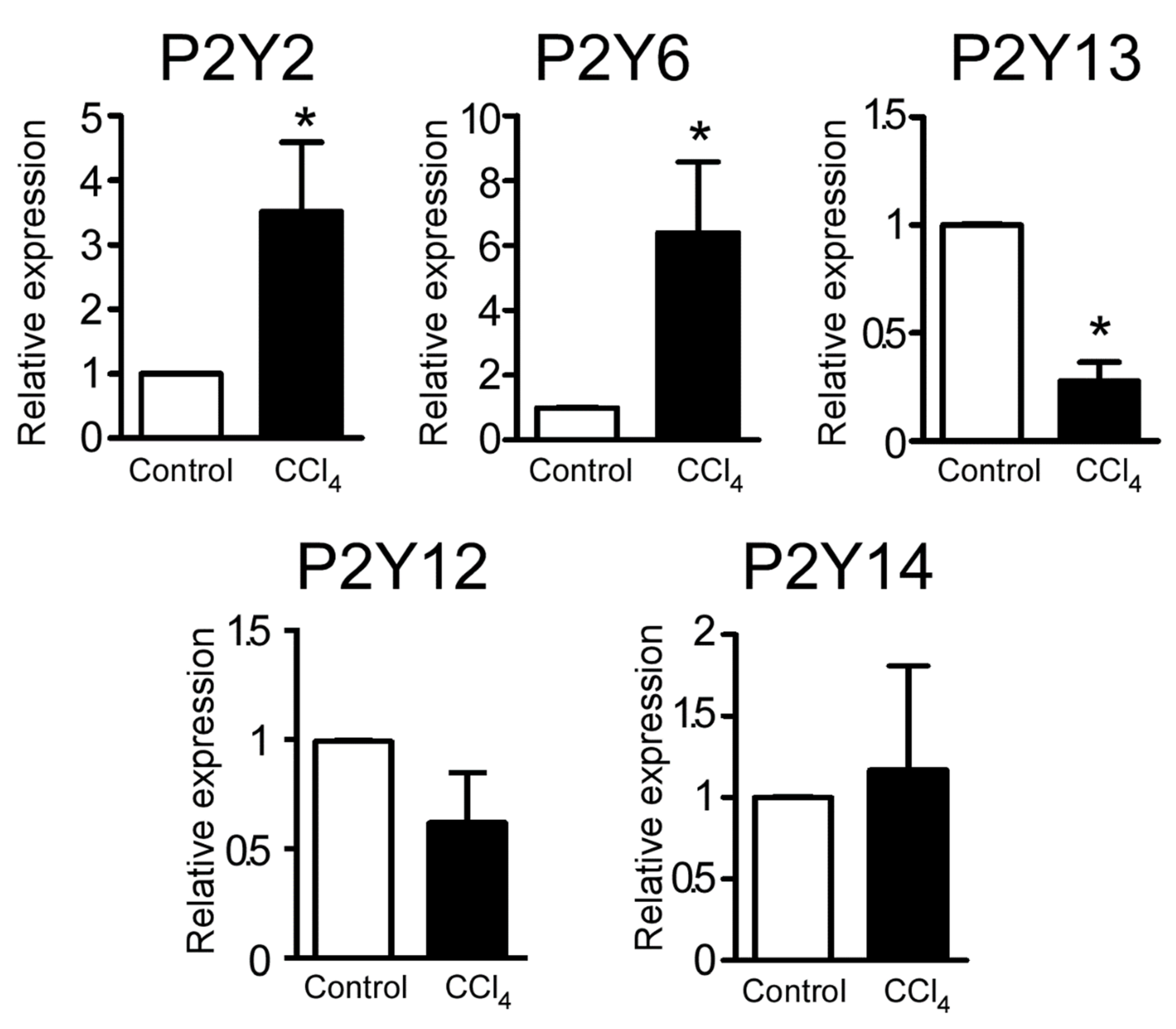
2.2. CCl4-Induced Changes in the Expression Level of P2Y Receptor Transcripts
2.3. The Fibrotic State Induced by CCl4 Alters the Distribution and Abundance of P2Y2R
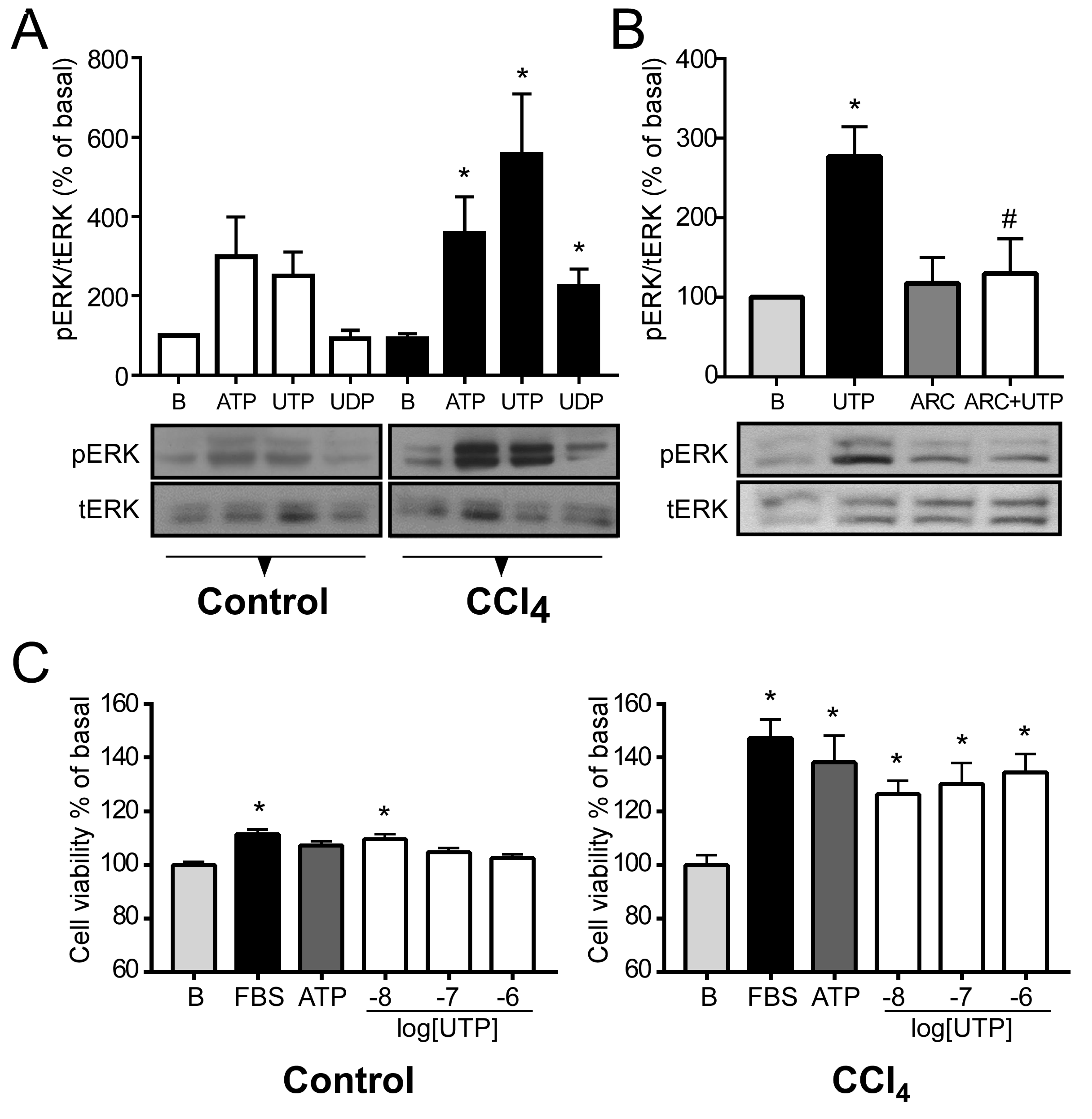
2.4. P2Y2R Function is Exacerbated in Hepatocytes from CCl4-Treated Mice
2.5. Analysis of Gene Expression in Response to UTP in Hepatocytes from CCl4-Induced Fibrotic Mice
2.5.1. Differences in UTP-Dependent Transcriptional Response between Healthy and Fibrotic Hepatocytes
2.5.2. Enhanced Proliferative Response in CCl4-Treated Hepatocytes
2.5.3. Detection of Potential Cellular Mechanisms Regulated by P2Y2R in Fibrotic Hepatocytes
3. Discussion
4. Materials and Methods
4.1. Animals and Induction of Fibrosis with CCl4
4.2. Histological Analysis
4.3. Mitotic Index
4.4. Reverse Transcription and Quantitative Polymerase Chain Reaction (qPCR)
4.5. Immunofluorescence
4.6. Hepatocyte Isolation
4.7. Analysis of the Induction of ERK Phosphorylation and P2Y2R by Western blot
4.8. Cell Viability Assay
4.9. cDNA Microarray Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ralevic, V.; Burnstock, G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar] [PubMed]
- Dixon, C.J.; White, P.J.; Hall, J.F.; Kingston, S.; Boarder, M.R. Regulation of human hepatocytes by P2Y receptors: Control of glycogen phosphorylase, Ca2+, and mitogen-activated protein kinases. J. Pharmacol. Exp. Ther. 2005, 313, 1305–1313. [Google Scholar] [CrossRef]
- Thevananther, S.; Sun, H.; Li, D.; Arjunan, V.; Awad, S.S.; Wyllie, S.; Zimmerman, T.L.; Goss, J.A.; Karpen, S.J. Extracellular ATP activates c-jun N-terminal kinase signaling and cell cycle progression in hepatocytes. Hepatology 2004, 39, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Tackett, B.C.; Sun, H.; Mei, Y.; Maynard, J.P.; Cheruvu, S.; Mani, A.; Hernandez-Garcia, A.; Vigneswaran, N.; Karpen, S.J.; Thevananther, S. P2Y2 purinergic receptor activation is essential for efficient hepatocyte proliferation in response to partial hepatectomy. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G1073–G1087. [Google Scholar] [CrossRef]
- Szabo, G.; Petrasek, J. Inflammasome activation and function in liver disease. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 387–400. [Google Scholar] [CrossRef]
- Adinolfi, E.; Giuliani, A.L.; De Marchi, E.; Pegoraro, A.; Orioli, E.; Di Virgilio, F. The P2X7 receptor: A main player in inflammation. Biochem. Pharmacol. 2018, 151, 234–244. [Google Scholar] [CrossRef]
- Franceschini, A.; Capece, M.; Chiozzi, P.; Falzoni, S.; Sanz, J.M.; Sarti, A.C.; Bonora, M.; Pinton, P.; Di Virgilio, F. The P2X7 receptor directly interacts with the NLRP3 inflammasome scaffold protein. FASEB J. 2015, 29, 2450–2461. [Google Scholar] [CrossRef]
- Schulien, I.; Hockenjos, B.; van Marck, V.; Ayata, C.K.; Follo, M.; Thimme, R.; Hasselblatt, P. Extracellular ATP and purinergic P2Y2 receptor signaling promote liver tumorigenesis in mice by exacerbating DNA damage. Cancer Res. 2019, 80, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Iracheta-Vellve, A.; Petrasek, J.; Satishchandran, A.; Gyongyosi, B.; Saha, B.; Kodys, K.; Fitzgerald, K.A.; Kurt-Jones, E.A.; Szabo, G. Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice. J. Hepatol. 2015, 63, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.; Sabbaghziarani, F.; Omidi, A.; Dehpour, A.R.; Omidi, N.; Ghasemi, S.; Pasbakhsh, P.; Ragerdi Kashani, I. Therapeutic value of melatonin post-treatment on CCl4-induced fibrotic rat liver. Can. J. Physiol. Pharmacol. 2016, 94, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Muñoz, R.; Díaz-Muñoz, M.; Suárez-Cuenca, J.A.; Trejo-Solís, C.; López, V.; Sánchez-Sevilla, L.; Yáñez, L.; De Sánchez, V.C. Adenosine reverses a preestablished CCl4-induced micronodular cirrhosis through enhancing collagenolytic activity and stimulating hepatocyte cell proliferation in rats. Hepatology 2001, 34, 677–687. [Google Scholar] [CrossRef]
- Dreisig, K.; Kornum, B.R. A critical look at the function of the P2Y11 receptor. Purinergic Signal. 2016, 12, 427–437. [Google Scholar] [PubMed]
- Rafehi, M.; Burbiel, J.C.; Attah, I.Y.; Abdelrahman, A.; Müller, C.E. Synthesis, characterization, and in vitro evaluation of the selective P2Y2 receptor antagonist AR-C118925. Purinergic Signal. 2017, 13, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Kraus, A.; Grampp, S.; Goppelt-Struebe, M.; Schreiber, R.; Kunzelmann, K.; Peters, D.J.M.; Leipziger, J.; Schley, G.; Schödel, J.; Eckardt, K.-U.; et al. P2Y2R is a direct target of HIF-1α and mediates secretion-dependent cyst growth of renal cyst-forming epithelial cells. Purinergic Signal. 2016, 12, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Hoque, R.; Sohail, M.A.; Salhanick, S.; Malik, A.F.; Ghani, A.; Robson, S.C.; Mehal, W.Z. P2X7 receptor-mediated purinergic signaling promotes liver injury in acetaminophen hepatotoxicity in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1171–G1179. [Google Scholar] [CrossRef] [PubMed]
- Ayata, C.K.; Ganal, S.C.; Hockenjos, B.; Willim, K.; Vieira, R.P.; Grimm, M.; Robaye, B.; Boeynaems, J.M.; Di Virgilio, F.; Pellegatti, P.; et al. Purinergic P2Y₂ receptors promote neutrophil infiltration and hepatocyte death in mice with acute liver injury. Gastroenterology 2012, 143, 1620–1629. [Google Scholar] [CrossRef]
- Chatterjee, S.; Rana, R.; Corbett, J.; Kadiiska, M.B.; Goldstein, J.; Mason, R.P. P2X7 receptor-NADPH oxidase axis mediates protein radical formation and Kupffer cell activation in carbon tetrachloride-mediated steatohepatitis in obese mice. Free Radic. Biol. Med. 2012, 52, 1666–1679. [Google Scholar] [CrossRef]
- Von Kügelgen, I.; Harden, T.K. Molecular pharmacology, physiology, and structure of the P2Y receptors. Adv. Pharmacol. 2011, 61, 373–415. [Google Scholar]
- Warny, M.; Aboudola, S.; Robson, S.C.; Sévigny, J.; Communi, D.; Soltoff, S.P.; Kelly, C.P. P2Y(6) nucleotide receptor mediates monocyte interleukin-8 production in response to UDP or lipopolysaccharide. J. Biol. Chem. 2001, 276, 26051–26056. [Google Scholar] [CrossRef]
- Vieira, R.P.; Müller, T.; Grimm, M.; von Gernler, V.; Vetter, B.; Dürk, T.; Cicko, S.; Ayata, C.K.; Sorichter, S.; Robaye, B.; et al. Purinergic receptor type 6 contributes to airway inflammation and remodeling in experimental allergic airway inflammation. Am. J. Respir. Crit. Care Med. 2011, 184, 215–223. [Google Scholar] [CrossRef]
- Hou, M.; Harden, T.K.; Kuhn, C.M.; Baldetorp, B.; Lazarowski, E.; Pendergast, W.; Möller, S.; Edvinsson, L.; Erlinge, D. UDP acts as a growth factor for vascular smooth muscle cells by activation of P2Y _6 receptors. Am. J. Physiol. Circ. Physiol. 2002, 282, H784–H792. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Cuevas, F.G.; Zárate-Díaz, E.P.; Garay, E.; Arellano, R.O. Functional expression and intracellular signaling of UTP-sensitive P2Y receptors in theca-interstitial cells. Reprod. Biol. Endocrinol. 2010, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H.; Mishra, S.K.; Shukla, V.; Langer, D.; Gampe, K.; Grimm, I.; Delic, J.; Braun, N. Ecto-nucleotidases, molecular properties and functional impact. An. R. Acad. Nac. Farm. 2007, 73, 537–566. [Google Scholar]
- Lichtenstein, L.; Serhan, N.; Espinosa-Delgado, S.; Fabre, A.; Annema, W.; Tietge, U.J.F.; Robaye, B.; Boeynaems, J.-M.; Laffargue, M.; Perret, B.; et al. Increased atherosclerosis in P2Y13/apolipoprotein E double-knockout mice: Contribution of P2Y13 to reverse cholesterol transport. Cardiovasc. Res. 2015, 106, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Spann, N.K.; Glass, C.K. Sterols and oxysterols in immune cell function. Nat. Immunol. 2013, 14, 893. [Google Scholar] [CrossRef] [PubMed]
- Kietzmann, T. Metabolic zonation of the liver: The oxygen gradient revisited. Redox Biol. 2017, 11, 622–630. [Google Scholar] [CrossRef]
- Jhandier, M.N.; Kruglov, E.A.; Lavoie, E.G.; Sévigny, J.; Dranoff, J.A. Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of NTPDase2. J. Biol. Chem. 2005, 280, 22986–22992. [Google Scholar] [CrossRef]
- Beldi, G.; Wu, Y.; Sun, X.; Imai, M.; Enjyoji, K.; Csizmadia, E.; Candinas, D.; Erb, L.; Robson, S.C. Regulated catalysis of extracellular nucleotides by vascular CD39/ENTPD1 is required for liver regeneration. Gastroenterology 2008, 135, 1751–1760. [Google Scholar] [CrossRef][Green Version]
- Graubardt, N.; Fahrner, R.; Trochsler, M.; Keogh, A.; Breu, K.; Furer, C.; Stroka, D.; Robson, S.C.; Slack, E.; Candinas, D.; et al. Promotion of liver regeneration by natural killer cells in a murine model is dependent on extracellular adenosine triphosphate phosphohydrolysis. Hepatology 2013, 57, 1969–1979. [Google Scholar] [CrossRef]
- Tak, E.; Jun, D.Y.; Kim, S.-H.; Park, G.-C.; Lee, J.; Hwang, S.; Song, G.-W.; Lee, S.-G. Upregulation of P2Y2 nucleotide receptor in human hepatocellular carcinoma cells. J. Int. Med. Res. 2016, 44, 1234–1247. [Google Scholar] [CrossRef]
- Carini, R.; Alchera, E.; De Cesaris, M.G.; Splendore, R.; Piranda, D.; Baldanzi, G.; Albano, E. Purinergic P2Y2 receptors promote hepatocyte resistance to hypoxia. J. Hepatol. 2006, 45, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.W.D.; Boll, M.; Stampfl, A. Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Crit. Rev. Toxicol. 2003, 33, 105–136. [Google Scholar] [CrossRef] [PubMed]
- Aho, J.; Helenius, M.; Vattulainen-Collanus, S.; Alastalo, T.P.; Koskenvuo, J. Extracellular ATP protects endothelial cells against DNA damage. Purinergic Signal. 2016, 12, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Caja, L.; Bertran, E.; Campbell, J.; Fausto, N.; Fabregat, I. The transforming growth factor-beta (TGF-β) mediates acquisition of a mesenchymal stem cell-like phenotype in human liver cells. J. Cell. Physiol. 2011, 226, 1214–1223. [Google Scholar] [CrossRef]
- Cicchini, C.; Amicone, L.; Alonzi, T.; Marchetti, A.; Mancone, C.; Tripodi, M. Molecular mechanisms controlling the phenotype and the EMT/MET dynamics of hepatocyte. Liver Int. 2015, 35, 302–310. [Google Scholar] [CrossRef]
- Zeisberg, M.; Yang, C.; Martino, M.; Duncan, M.B.; Rieder, F.; Tanjore, H.; Kalluri, R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J. Biol. Chem. 2007, 282, 23337–23347. [Google Scholar] [CrossRef]
- Aragona, M.; Dekoninck, S.; Rulands, S.; Lenglez, S.; Mascré, G.; Simons, B.D.; Blanpain, C. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat. Commun. 2017, 8, 14684. [Google Scholar] [CrossRef]
- Ke, Q.; Costa, M. Hypoxia-inducible factor-1 (HIF-1). Mol. Pharmacol. 2006, 70, 1469–1480. [Google Scholar] [CrossRef]
- Lee, L.B.H. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. In American Registry of Pathology; McGraw-Hill Book Company: New York, NY, USA, 1968; Volume 3, p. 258. [Google Scholar]
- McGavin, M.D.; Carlton, W.W.; Zachary, J.F. Thomson’s Special Veterinary Pathology, 3rd ed.; Mosby Publication: Maryland Heights, MO, USA, 2001. [Google Scholar]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Rivera-Zavala, J.B.; Molina-Aguilar, C.; Pérez-Mendoza, M.; Olguín-Martínez, M.; Hernández-Muñoz, R.; Báez-Ruiz, G.A.; Díaz-Muñoz, M. Daytime restricted feeding modifies the daily regulation of fatty acid β-oxidation and the lipoprotein profile in rats. Br. J. Nutr. 2017, 117, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Mederacke, I.; Dapito, D.H.; Affò, S.; Uchinami, H.; Schwabe, R.F. High-yield and high-purity isolation of hepatic stellate cells from normal and fibrotic mouse livers. Nat. Protoc. 2015, 10, 305–315. [Google Scholar] [CrossRef]
- Vázquez-Victorio, G.; Caligaris, C.; Del Valle-Espinosa, E.; Sosa-Garrocho, M.; González-Arena, N.R.; Reyes-Cruz, G.; Briones-Orta, M.A.; Macías-Silva, M. Novel regulation of Ski protein stability and endosomal sorting by actin cytoskeleton dynamics in hepatocytes. J. Biol. Chem. 2015, 290, 4487–4499. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ramírez, A.S.; Díaz-Muñoz, M.; Battastini, A.M.; Campos-Contreras, A.; Olvera, A.; Bergamin, L.; Glaser, T.; Jacintho Moritz, C.E.; Ulrich, H.; Vázquez-Cuevas, F.G. Cellular Migration Ability Is Modulated by Extracellular Purines in Ovarian Carcinoma SKOV-3 Cells. J. Cell. Biochem. 2017, 118, 4468–4478. [Google Scholar] [CrossRef] [PubMed]
- Tabas-Madrid, D.; Nogales-Cadenas, R.; Pascual-Montano, A. GeneCodis3: A non-redundant and modular enrichment analysis tool for functional genomics. Nucleic Acids Res. 2012, 40, W478–W483. [Google Scholar] [CrossRef] [PubMed]
- Nogales-Cadenas, R.; Carmona-Saez, P.; Vazquez, M.; Vicente, C.; Yang, X.; Tirado, F.; Carazo, J.M.; Pascual-Montano, A. GeneCodis: Interpreting gene lists through enrichment analysis and integration of diverse biological information. Nucleic Acids Res. 2009, 37, W317–W322. [Google Scholar] [CrossRef]
- Carmona-Saez, P.; Chagoyen, M.; Tirado, F.; Carazo, J.M.; Pascual-Montano, A. GENECODIS: A web-based tool for finding significant concurrent annotations in gene lists. Genome Biol. 2007, 8, R3. [Google Scholar] [CrossRef]



| Up-Regulated Transcripts | Down-Regulated Transcripts | ||||
|---|---|---|---|---|---|
| GO:0006281 DNA Repair | GO:0045944 Negative Regulation of Transcription by RNA Polymerase II | ||||
| Symbol | Z-score | Name | Symbol | Z-score | Name |
| Fbxo18 | 2.74 | F-Box DNA Helicase 1 | Traf7 | −2.86 | TNF Receptor Associated Factor 7 |
| Nthl1 | 3.06 | Nth Like DNA Glycosylase 1 | Rbl1 | −2.71 | RB Transcriptional Corepressor Like 1 |
| Pold4 | 3.81 | DNA Polymerase Delta 4, Accessory Subunit | Cdx4 | −2.65 | Caudal Type Homeobox 4 |
| Rpa3 | 5.51 | Replication protein A | Nfib | −2.37 | Nuclear Factor I B |
| Eid3 | 2.87 | EP300 Interacting Inhibitor Of Differentiation 3 | Setdb1 | −2.21 | SET Domain Bifurcated Histone Lysine |
| Smc3 | 3.05 | Structural Maintenance Of Chromosomes 3 | Pkig | −2.16 | CAMP-Dependent Protein Kinase Inhibitor Gamma |
| GO:0006974 Cellular response to DNA damage stimulus | GO:0030336: Negative regulation of cell migration | ||||
| Symbol | Z-score | Name | Symbol | Z-score | Name |
| Foxo1 | 1.72 | Forkhead Box O1 | Nkx2-1 | −2.21 | NK2 Homeobox 1 |
| Aatf | 3.58 | Apoptosis Antagonizing Transcription Factor | Pdgfb | −2.11 | Platelet Derived Growth Factor Subunit B |
| Zfp238 | 2.27 | Zinc Finger And BTB Domain Containing 18 | Dach1 | −2.06 | Dachshund Family Transcription Factor 1 |
| Baz1b | 2.52 | Bromodomain Adjacent To Zinc Finger Domain 1B | Erdr1 | −1.94 | Erythroid differentiation regulator |
| Uba6 | 2.11 | Ubiquitin Like Modifier Activating Enzyme 6 | Arid2 | −1.89 | AT-Rich Interaction Domain 2 |
| Rbbp5 | 2.27 | RB Binding Protein 5 | Rhob | −1.9 | Ras Homolog Family Member B |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velázquez-Miranda, E.; Molina-Aguilar, C.; González-Gallardo, A.; Vázquez-Martínez, O.; Díaz-Muñoz, M.; Vázquez-Cuevas, F.G. Increased Purinergic Responses Dependent on P2Y2 Receptors in Hepatocytes from CCl4-Treated Fibrotic Mice. Int. J. Mol. Sci. 2020, 21, 2305. https://doi.org/10.3390/ijms21072305
Velázquez-Miranda E, Molina-Aguilar C, González-Gallardo A, Vázquez-Martínez O, Díaz-Muñoz M, Vázquez-Cuevas FG. Increased Purinergic Responses Dependent on P2Y2 Receptors in Hepatocytes from CCl4-Treated Fibrotic Mice. International Journal of Molecular Sciences. 2020; 21(7):2305. https://doi.org/10.3390/ijms21072305
Chicago/Turabian StyleVelázquez-Miranda, Erandi, Christian Molina-Aguilar, Adriana González-Gallardo, Olivia Vázquez-Martínez, Mauricio Díaz-Muñoz, and Francisco G Vázquez-Cuevas. 2020. "Increased Purinergic Responses Dependent on P2Y2 Receptors in Hepatocytes from CCl4-Treated Fibrotic Mice" International Journal of Molecular Sciences 21, no. 7: 2305. https://doi.org/10.3390/ijms21072305
APA StyleVelázquez-Miranda, E., Molina-Aguilar, C., González-Gallardo, A., Vázquez-Martínez, O., Díaz-Muñoz, M., & Vázquez-Cuevas, F. G. (2020). Increased Purinergic Responses Dependent on P2Y2 Receptors in Hepatocytes from CCl4-Treated Fibrotic Mice. International Journal of Molecular Sciences, 21(7), 2305. https://doi.org/10.3390/ijms21072305





