HPV Infection Significantly Accelerates Glycogen Metabolism in Cervical Cells with Large Nuclei: Raman Microscopic Study with Subcellular Resolution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Subcellular Distribution of Glycogen in Cervical Epithelial Cells
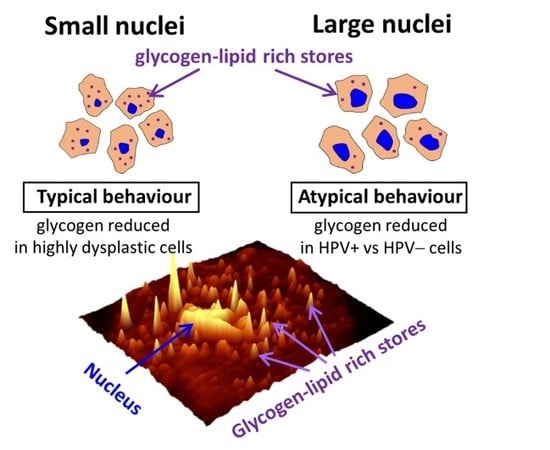
2.2. Glycogen Content Decreases with the Increase of Nuclei Size of Cervical Epithelial Cells in I, IIa, LSIL and HSIL Groups, but Not the Cancer Group
2.3. HPV Accelerates Glycogen Metabolism in Cervical Epithelial Cells
3. Materials and Methods
3.1. Clinical Specimens
3.2. Raman Microscopy
3.3. PCR Reactions
3.4. PAS Staining
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | protein kinase B |
| BrDu | bromodeoxyuridine |
| CAF | cancer-associated fibroblasts |
| FTIR | Fourier Transform Infrared Spectroscopy |
| HPV | human papillomavirus |
| LCR | long control region |
| nPCR | nested PCR |
| P38 MAPK kinase | P38 mitogen-activated protein kinase |
| PAS | Periodic acid–Schiff staining |
| PCR | polymerase chain reaction |
| PI3K | phosphoinositide 3-kinase |
| SCC | squamous cell carcinoma |
| Smad | an acronym from the fusion of Caenorhabditis elegans Sma (“small” worm phenotype) genes and the Drosophila Mad (Mothers against decapentaplegic) |
| TGF-β1 | transforming growth factor β1 |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petry, K.U.; Wörmann, B.; Schneider, A. Benefits and risks of cervical cancer screening. Oncol. Res. Treat. 2014, 37 (Suppl. S3), 48–57. [Google Scholar] [CrossRef]
- Li, W.; Qi, Y.; Cui, X.; Huo, Q.; Zhu, L.; Zhang, A.; Tan, M.; Hong, Q.; Yang, Y.; Zhang, H.; et al. Characteristic of HPV Integration in the Genome and Transcriptome of Cervical Cancer Tissues. BioMed Res. Int. 2018, 6242173. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.; Park, J. The Role of HPV E6 and E7 Oncoproteins in HPV-associated Cervical Carcinogenesis. Cancer Res. Treat. 2005, 37, 319–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi-Zarchi, M.; Peighmbari, F.; Karimi, N.; Rohi, M.; Chiti, Z. A comparison of 3 ways of conventional pap smear, liquid-based cytology and colposcopy vs cervical biopsy for early diagnosis of premalignant lesions or cervical cancer in women with abnormal conventional Pap test. Int. J. Biomed. Sci. 2013, 9, 205–210. [Google Scholar] [PubMed]
- Shaw, P.A. The History of Cervical Screening I: The Pap. Test. J. Obstet. Gynaecol. Can. 2000, 22, 110–114. [Google Scholar] [CrossRef]
- Moriarty, A.T.; Nayar, R.; Arnold, T.; Gearries, L.; Renshaw, A.; Thomas, N.; Souers, R. The tahoe study: Bias in the interpretation of papanicolaou test results when human papillomavirus status is known. Arch. Pathol. Lab. Med. 2014, 138, 1182–1185. [Google Scholar] [CrossRef]
- Crothers, B.A.; Booth, C.N.; Darragh, T.M.; Zhao, C.; Souers, R.J.; Thomas, N.; Moriarty, A.T. False-Positive Papanicolaou (PAP) Test Rates in the College of American Pathologists PAP Education and PAP Proficiency Test Programs. Arch. Pathol. Lab. Med. 2014, 138, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Castillo, M.; Astudillo, A.; Clavero, O.; Velasco, J.; Ibanez, R.; de Sanjose, S. Poor Cervical Cancer Screening Attendance and False Negatives. A Call for Organized Screening. PLoS ONE 2016, 11, e0161403. [Google Scholar] [CrossRef]
- Abreu, A.L.P.; Souza, R.P.; Gimenes, F.; Consolaro, M.E.L. A review of methods for detect human Papillomavirusinfection. Virol. J. 2012, 9. [Google Scholar] [CrossRef] [Green Version]
- Ogilvie, G.S.; Niekerk, D.; Van Krajden, M.; Smith, L.W.; Cook, D.; Gondara, L.; Ceballos, K.; Quinlan, D.; Lee, M.; Martin, R.E.; et al. Effect of Screening With HPV Testing vs Cytology for Cervical Intraepithelial Neoplasia. JAMA 2018, 320, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burd, E. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Gomez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S. Human Papillomavirus and Related Diseases Report; ICO/IARC Inf Center HPV and Cancer (HPV Inf Center): Barcelona, Spain, 2019. [Google Scholar]
- Cubie, H.A. Diseases associated with human papillomavirus infection. Virology 2013, 445, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monsonego, J.; Cox, J.T.; Behrens, C.; Sandri, M.; Franco, E.L.; Yap, P.S.; Huh, W. Prevalence of high-risk human papilloma virus genotypes and associated risk of cervical precancerous lesions in a large U.S. screening population: Data from the ATHENA trial. Gynecol. Oncol. 2015, 137, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, N.; Bosch, F.X.; De Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.F.; Meijer, C.J.L.M. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [Green Version]
- De Sanjosé, S.; Brotons, M.; Pavón, M.A. The natural history of human papillomavirus infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef]
- Sørensen, F.B.; Bichel, P.; Jakobsen, A. DNA level and stereologic estimates of nuclear volume in squamous cell carcinomas of the uterine cervix. A comparative study with analysis of prognostic impact. Cancer 1992, 69, 187–199. [Google Scholar] [CrossRef] [Green Version]
- Neumann, F.R.; Nurse, P. Nuclear size control in fission yeast. J. Cell Biol. 2007, 179, 593–600. [Google Scholar] [CrossRef] [Green Version]
- Veltri, R.W. Epithelia Nuclear Size and Shape. Prostate 2011, 68, 1097–1104. [Google Scholar] [CrossRef]
- Jevtić, P.; Edens, L.J.; Li, X.; Nguyen, T.; Chen, P.; Levy, D.L. Concentration-dependent effects of nuclear lamins on nuclear size in xenopus and mammalian cells. J. Biol. Chem. 2015, 290, 27557–27571. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, L.L.; Wiese, C. TPX2 is required for postmitotic nuclear assembly in cell-free Xenopus laevis egg extracts. J. Cell Biol. 2006, 173, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Irish, J.M.; Kotecha, N.; Nolan, G.P. Mapping normal and cancer cell signalling networks: Towards single-cell proteomics. Nat. Rev. Cancer 2006, 6, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bodovitz, S. Single cell analysis: The new frontier in “Omics” Single cell analysis: Needs and applications. Trends Biotechnol. 2010, 28, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, X.; Li, J.; Feng, Y.; Chen, H.; Guo, W.; Zhang, J.; Wang, T.; Xu, X. Low-cytotoxicity fluorescent probes based on anthracene derivatives for hydrogen sulfide detection. Front. Chem. 2018, 6, 202. [Google Scholar] [CrossRef] [PubMed]
- Ostertag, E.; Stefanakis, M.; Rebner, K.; Kessler, R.W. Elastic and inelastic light scattering spectroscopy and its possible use for label-free brain tumor typing. Anal. Bioanal. Chem. 2017, 409, 6613–6623. [Google Scholar] [CrossRef] [PubMed]
- Swain, R.J.; Stevens, M.M. Raman microspectroscopy for non-invasive biochemical analysis of single cells. Biochem. Soc. Trans. 2007, 35, 544–549. [Google Scholar] [CrossRef] [Green Version]
- Czamara, K.; Majzner, K.; Selmi, A.; Baranska, M.; Ozaki, Y.; Kaczor, A. Unsaturated lipid bodies as a hallmark of inflammation studied by Raman 2D and 3D microscopy. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Czamara, K.; Karnas, E.; Majka, Z.; Wojcik, T.; Zuba-Surma, E.; Baranska, M.; Kaczor, A. Impact of cell cycle dynamics on pathology recognition: Raman imaging study. J. Biophotonics 2019, 12, e201800152. [Google Scholar] [CrossRef]
- Czamara, K.; Petko, F.; Baranska, M.; Kaczor, A. Raman microscopy at the subcellular level: A study on early apoptosis in endothelial cells induced by Fas ligand and cycloheximide. Analyst 2016, 141, 1390–1397. [Google Scholar] [CrossRef]
- Brozek-Pluska, B.; Musial, J.; Kordek, R.; Abramczyk, H. Analysis of human colon by raman spectroscopy and imaging-elucidation of biochemical changes in carcinogenesis. Int. J. Mol. Sci. 2019, 20, 3398. [Google Scholar] [CrossRef] [Green Version]
- Kourkoumelis, N.; Balatsoukas, I.; Moulia, V.; Elka, A.; Gaitanis, G.; Bassukas, I.D. Advances in the in vivo Raman spectroscopy of malignant skin tumors using portable instrumentation. Int. J. Mol. Sci. 2015, 16, 14554–14570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnier, F.; Traynor, D.; Kearney, P.; Clarke, C.; Knief, P.; Martin, C.; O’Leary, J.J.; Byrne, H.; Lyng, F. Processing ThinPrep cervical cytological samples for Raman spectroscopic analysis. Anal. Methods 2014, 6, 7831–7841. [Google Scholar] [CrossRef]
- Daniel, A.; Aruna, P.; Ganesan, S.; Joseph, L. Biochemical assessment of human uterine cervix by micro-Raman mapping. Photodiagn. Photodyn. Ther. 2017, 17, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Duraipandian, S.; Traynor, D.; Kearney, P.; Martin, C.; O’Leary, J.J.; Lyng, F.M. Raman spectroscopic detection of high-grade cervical cytology: Using morphologically normal appearing cells. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargis, E.; Tang, Y.W.; Khabele, D.; Mahadevan-Jansen, A. Near-infrared raman microspectroscopy detects high-risk human papillomaviruses. Transl. Oncol. 2012, 5, 172–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, B.R.; Quinn, M.A.; Burden, F.R.; McNaughton, D. An investigation into FTIR spectroscopy as a biodiagnostic tool for cervical cancer. Biospectroscopy 1996, 2, 143–153. [Google Scholar] [CrossRef]
- Golovynska, I.; Kalmukova, O.; Svitina, H.M.; Kyryk, V.M.; Shablii, V.A.; Senchylo, N.V.; Ostrovska, G.W.; Dzerzhinskyi, M.; Stepanov, Y.V.; Golovynskyi, S.; et al. Morpho-Functional Characteristics of Bone Marrow Multipotent Mesenchymal Stromal Cells after Activation or Inhibition of Epidermal Growth Factor and Toll-Like Receptors or Treatment with DNA Intercalator Cisplatin. Cytom. Part A 2019, 95, 24–33. [Google Scholar] [CrossRef]
- Abbiss, J.W. Early diagnosis of Cancer of the Cervix Uteri. Br. Med. J. 1956, 1, 49–51. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Öhlund, D.; Elyada, E.; Tuveson, D. Fibroblast heterogeneity in the cancer wound. J. Exp. Med. 2014, 211, 1503–1523. [Google Scholar] [CrossRef]
- Curtis, M.; Kenny, H.A.; Ashcroft, B.; Salomon, A.R.; Nebreda, A.R.; Lengyel, E. Fibroblasts Mobilize Tumor Cell Glycogen to Promote Proliferation and Metastasis. Cell Metab. 2019, 29, 141–155.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregoire, A.T.; Ledger, W.D.; Moran, M.J. The Glycogen Content of the Human Female Genital Tract in Cycling, Menopausal, and Women with Encometrial and Cervical Carcinoma. Fertil. Steril. 1973, 24, 198–201. [Google Scholar] [CrossRef]
- Kanungo, S.; Wells, K.; Tribett, T.; El-Gharbawy, A. Glycogen metabolism and glycogen storage disorders. Ann. Transl. Med. 2018, 6, 474. [Google Scholar] [CrossRef] [PubMed]
- Hervieu, A.; Kermorgant, S. The role of PI3K in Met driven cancer: A recap. Front. Mol. Biosci. 2018, 5, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sano, H.; Kane, S.; Sano, E.; Miinea, C.P.; Asara, J.M.; Lane, W.S.; Garner, C.W.; Lienhard, G.E. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 2003, 278, 14599–14602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-García, A.; Samsó, P.; Fontova, P.; Simon-Molas, H.; Manzano, A.; Castano, E.; Rosa, J.L.; Martinez-Outshoorn, U.; Ventura, F.; Navarro-Sabate, A.; et al. TGF-β1 targets Smad, p38 MAPK, and PI3K/Akt signaling pathways to induce PFKFB3 gene expression and glycolysis in glioblastoma cells. FEBS J. 2017, 284, 3437–3454. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.K.; Parish, C.R.; Wong, M.L.; Licinio, J.; Blackburn, A.C. Leptin signals via TGFB1 to promote metastatic potential and stemness in breast cancer. PLoS ONE 2017, 12, e0178454. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Paredes, A.; De la Cruz-Hernández, E.; Martínez-Ramírez, I.; Dueñas-González, A.; Lizano, M. E6 variants of human papillomavirus 18 differentially modulate the protein kinase B/phosphatidylinositol 3-kinase (akt/PI3K) signaling pathway. Virology 2009, 383, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Menges, C.W.; Baglia, L.A.; Lapoint, R.; McCance, D.J. Human papillomavirus type 16 E7 up-regulates AKT activity through the retinoblastoma protein. Cancer Res. 2006, 66, 5555–5559. [Google Scholar] [CrossRef] [Green Version]
- Cirkel, C.; Barop, C.; Beyer, D.A. Method comparison between Munich II and III nomenclature for Pap smear samples. Ger. Gynecol. Assoc. 2015, 16, 203–207. [Google Scholar] [CrossRef]
- Griesser, H.; Marquardt, K.; Jordan, B.; Kühn, W.; Neis, K.; Neumann, H.H. Münchner Nomenklatur III: Gynäkologische Zytodiagnostik der Zervix. Frauenarzt 2013, 54, 1042–1048. [Google Scholar]
- Zink, D.; Fischer, A.H.; Nickerson, J.A. Nuclear structure in cancer cells. Nat. Rev. Cancer 2004, 4, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Zois, C.E.; Harris, A.L. Glycogen metabolism has a key role in the cancer microenvironment and provides new targets for cancer therapy. J. Mol. Med. 2016, 94, 137–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolfi, S.C.; Chan, L.L.-Y.; Qiu, J.; Tedeschi, P.M.; Bertino, J.R.; Hirshfield, K.M.; Oltvai, Z.N.; Vazquez, A. The metabolic demands of cancer cells are coupled to their size and protein synthesis rates. Cancer Metab. 2013, 1, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aterman, K.; Norkin, S. The periodic acid-Schiff reaction. Nature 1963, 197, 1306. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Chiang, C.M. E6 oncoprotein represses p53-dependent gene activation via inhibition of protein acetylation independently of inducing p53 degradation. Mol. Cell 2005, 17, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lim, C.; Seo, T.; Kwon, H.; Min, H.; Choe, J. The viral oncogene human papillomavirus E7 deregulates transcriptional silencing by Brm-related gene 1 via molecular interactions. J. Biol. Chem. 2002, 277, 48842–48848. [Google Scholar] [CrossRef] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitarz, K.; Czamara, K.; Bialecka, J.; Klimek, M.; Zawilinska, B.; Szostek, S.; Kaczor, A. HPV Infection Significantly Accelerates Glycogen Metabolism in Cervical Cells with Large Nuclei: Raman Microscopic Study with Subcellular Resolution. Int. J. Mol. Sci. 2020, 21, 2667. https://doi.org/10.3390/ijms21082667
Sitarz K, Czamara K, Bialecka J, Klimek M, Zawilinska B, Szostek S, Kaczor A. HPV Infection Significantly Accelerates Glycogen Metabolism in Cervical Cells with Large Nuclei: Raman Microscopic Study with Subcellular Resolution. International Journal of Molecular Sciences. 2020; 21(8):2667. https://doi.org/10.3390/ijms21082667
Chicago/Turabian StyleSitarz, Katarzyna, Krzysztof Czamara, Joanna Bialecka, Malgorzata Klimek, Barbara Zawilinska, Slawa Szostek, and Agnieszka Kaczor. 2020. "HPV Infection Significantly Accelerates Glycogen Metabolism in Cervical Cells with Large Nuclei: Raman Microscopic Study with Subcellular Resolution" International Journal of Molecular Sciences 21, no. 8: 2667. https://doi.org/10.3390/ijms21082667