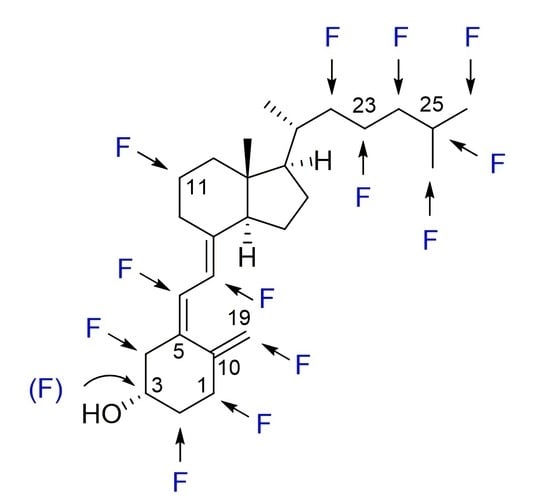
Design and Synthesis of Fluoro Analogues of Vitamin D
Abstract
:1. Introduction
2. A-Ring Fluorinated VD3 Analogues
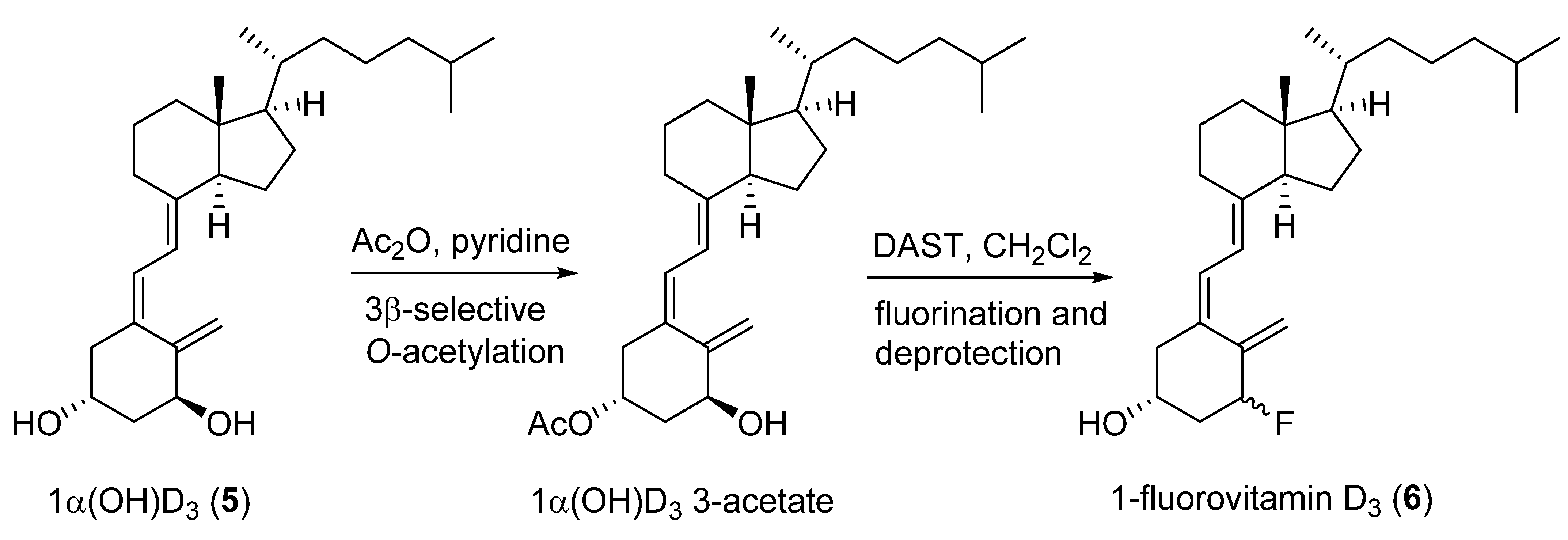
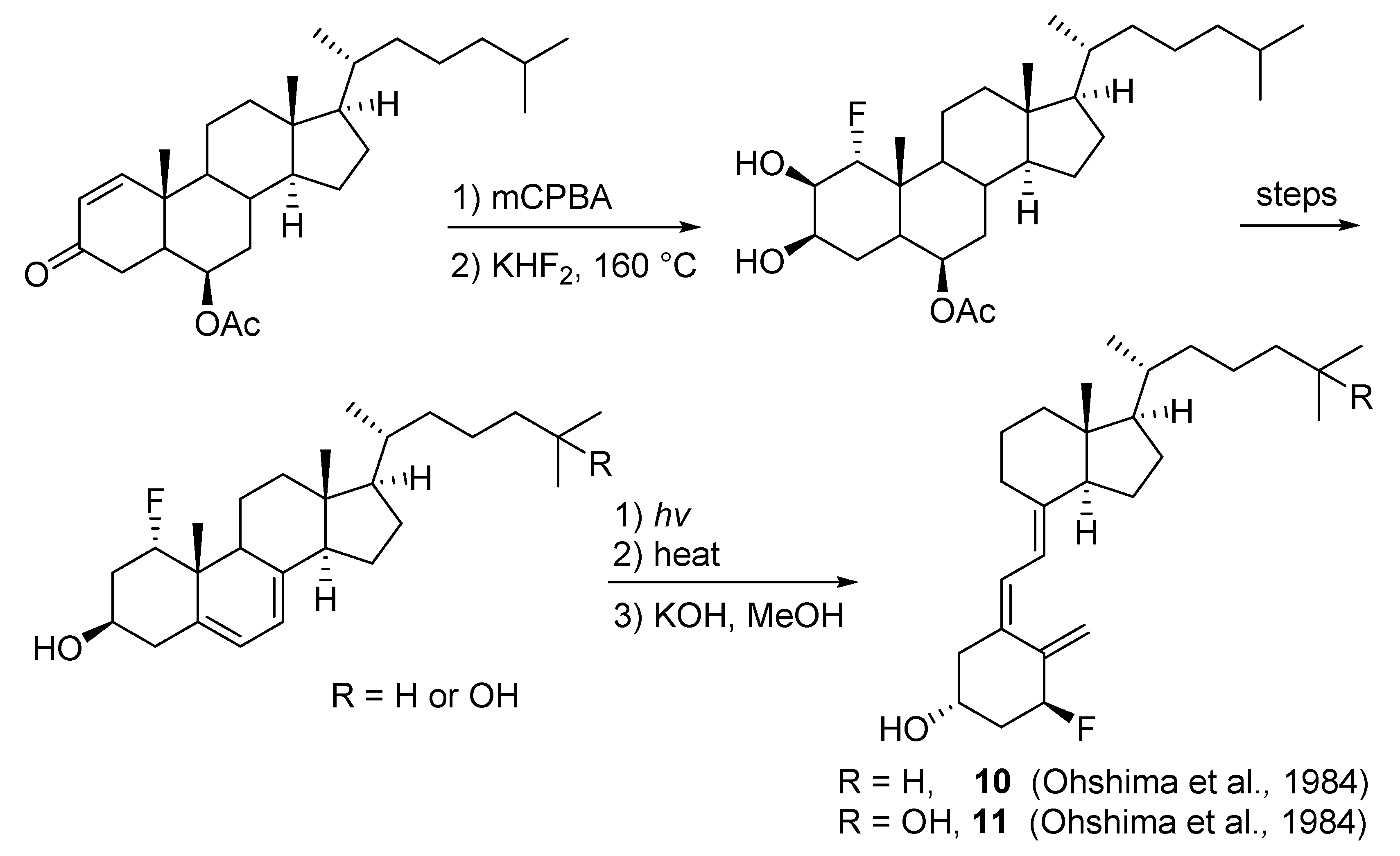
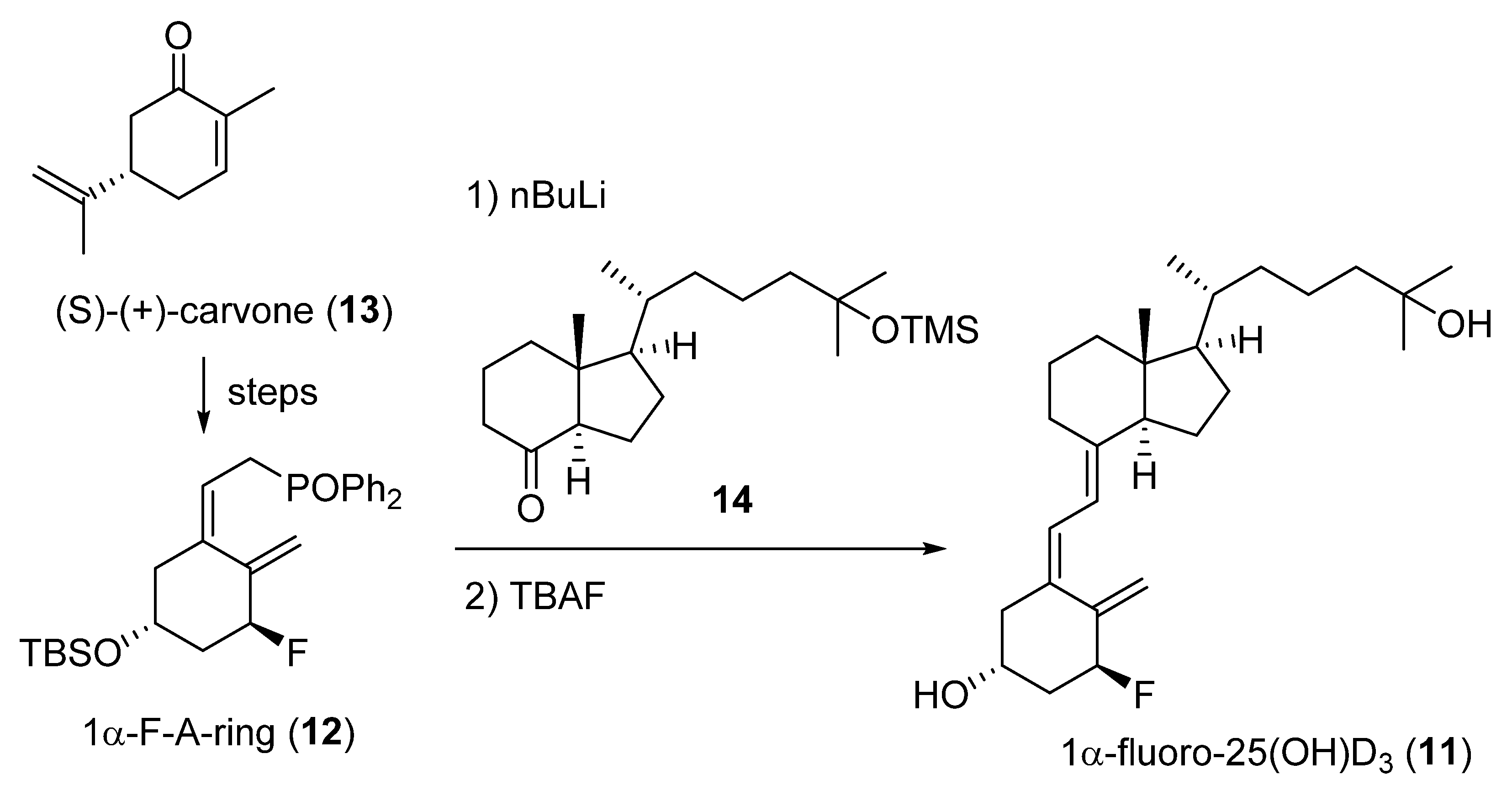
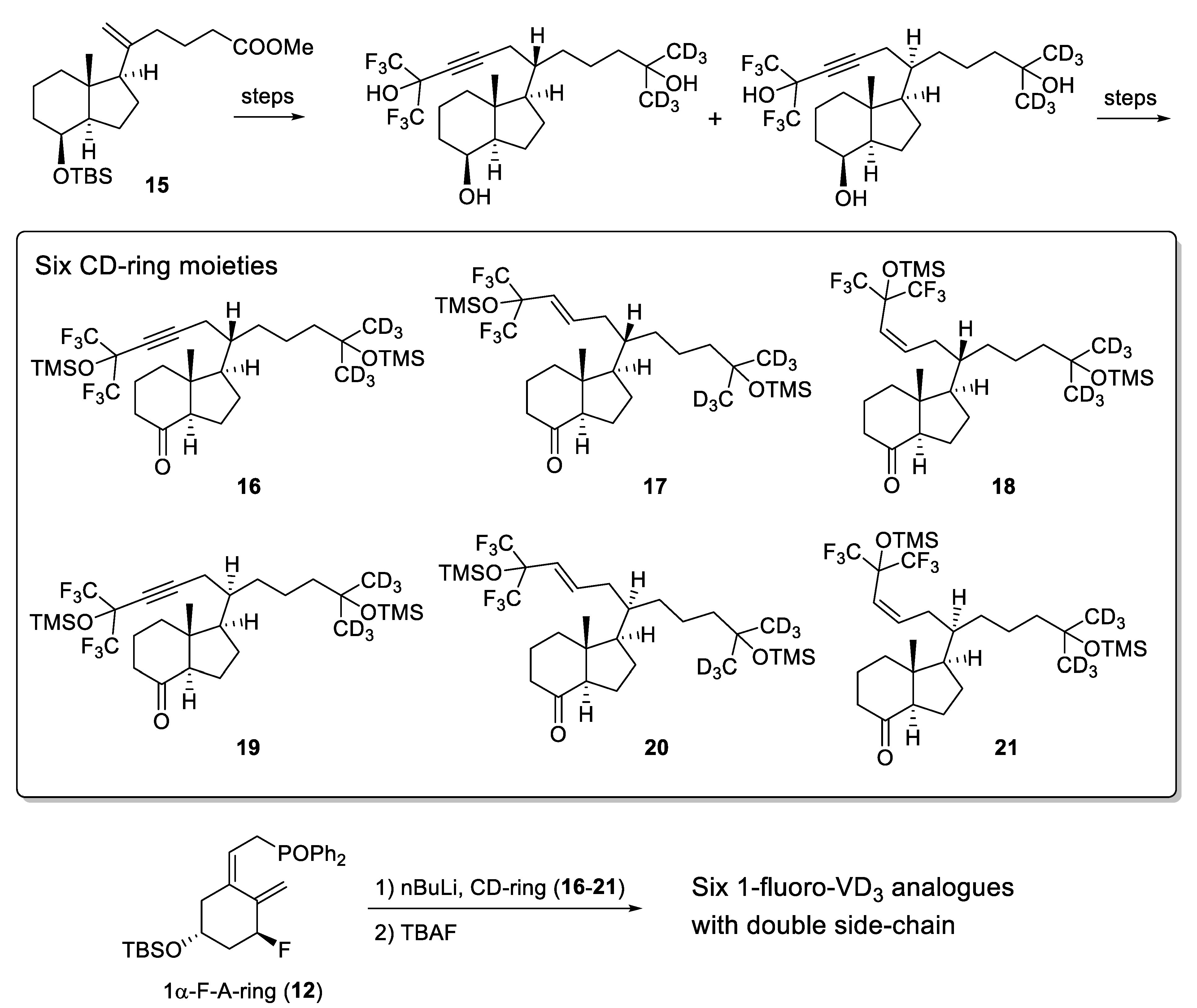
2.1. 1-Fluorinated VD3 Analogues
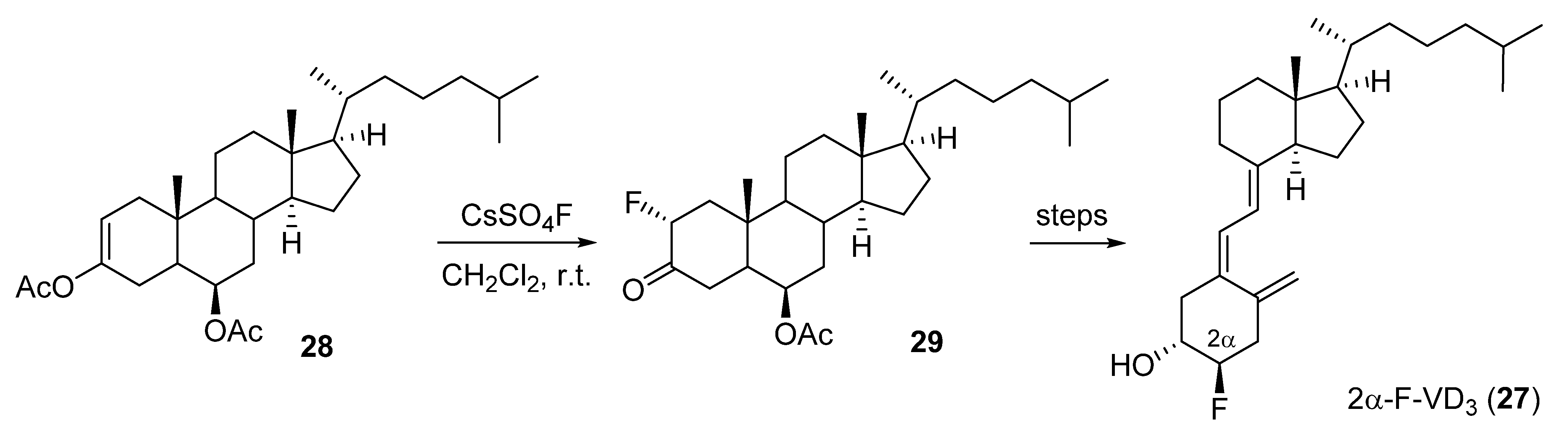
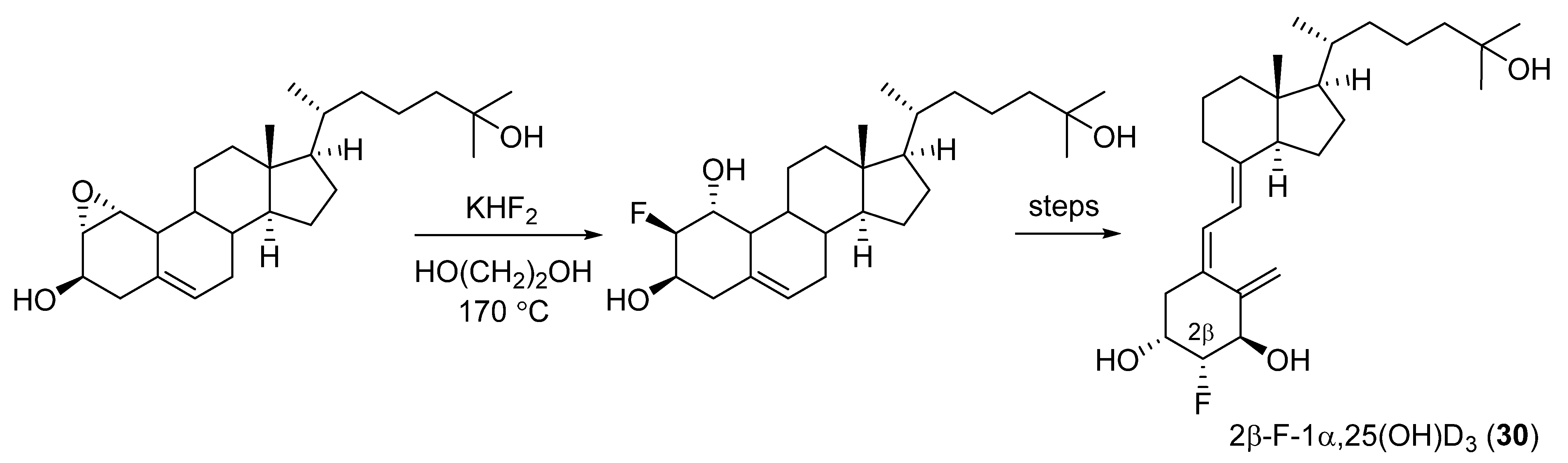
2.2. 2-Fluorinated VD3 Analogues
2.3. 3-Fluorinated VD3 Analogues
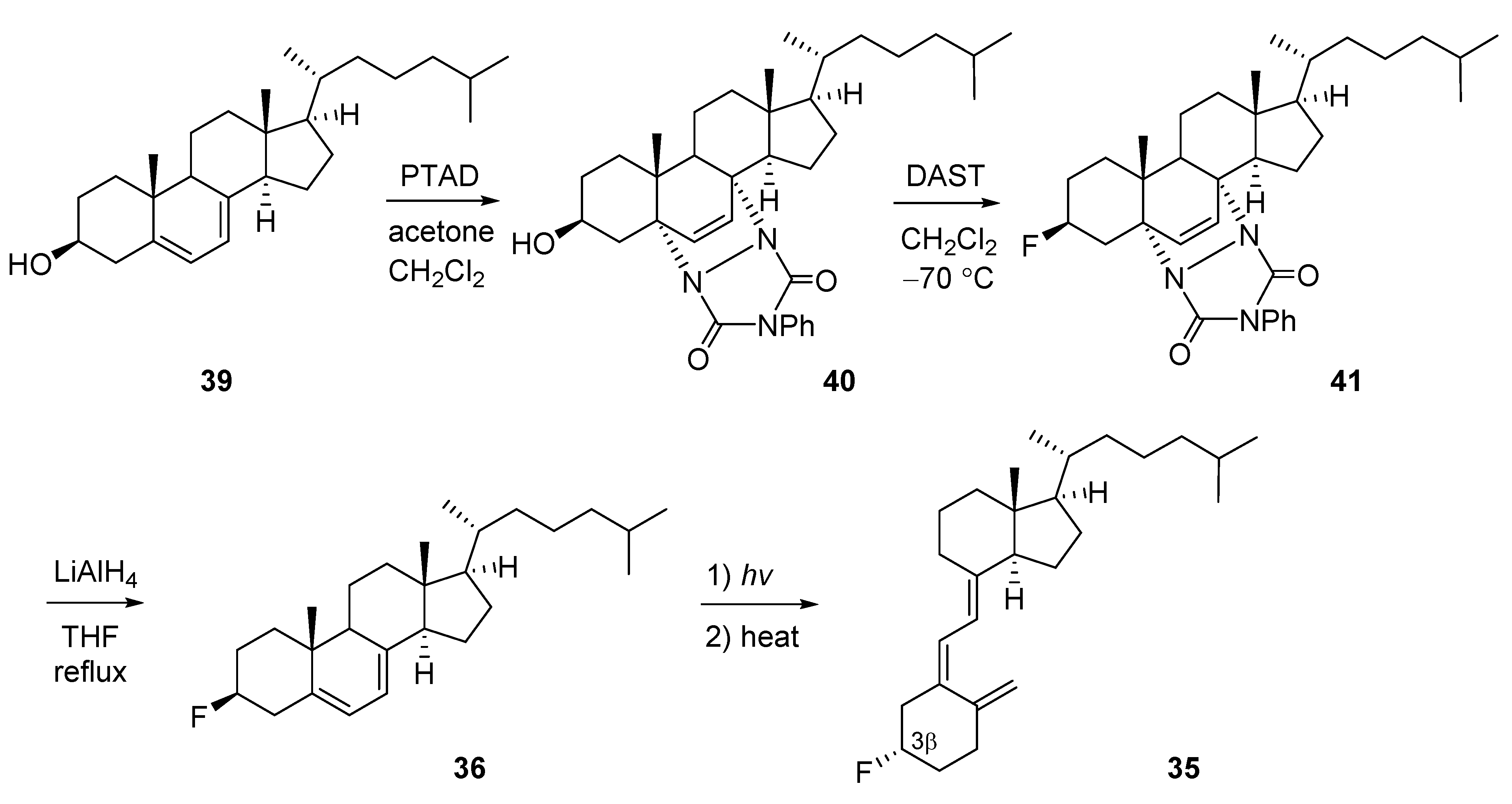
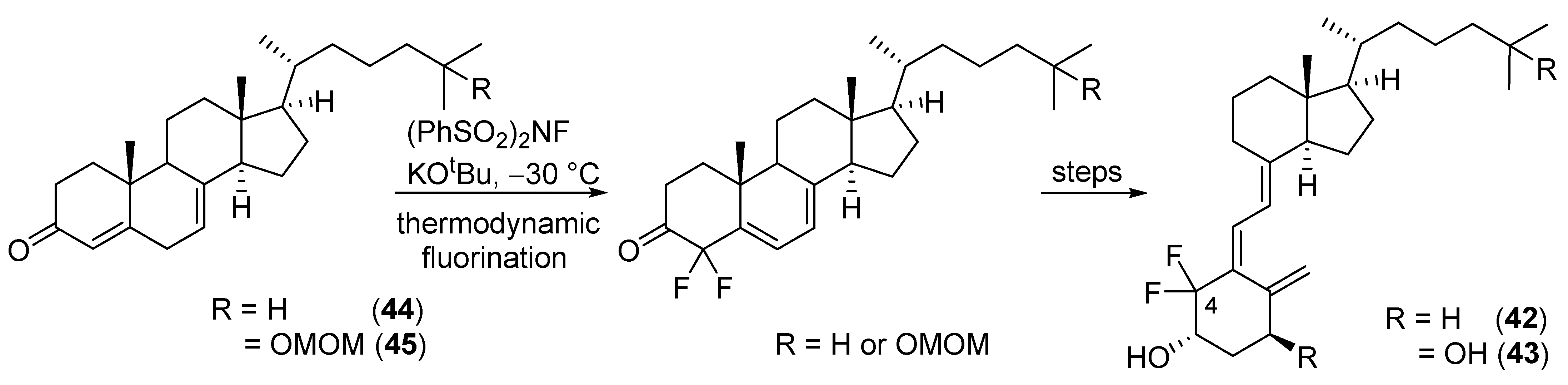
2.4. 4-Fluorinated VD3 Analogues
3. Introduction of a Fluorine Atom into the Triene Part of VD3
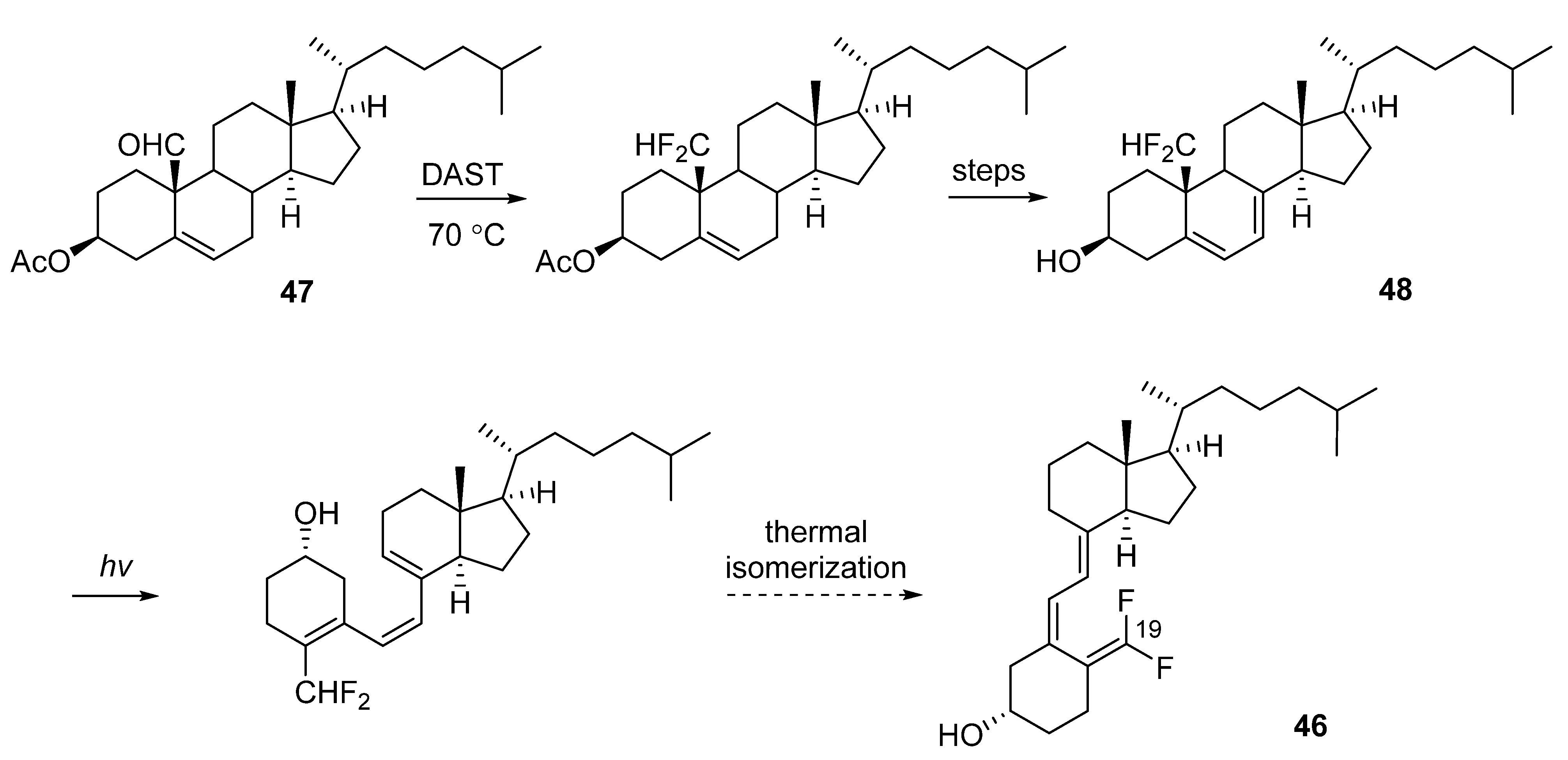
3.1. 19-Fluorinated VD3 Analogues
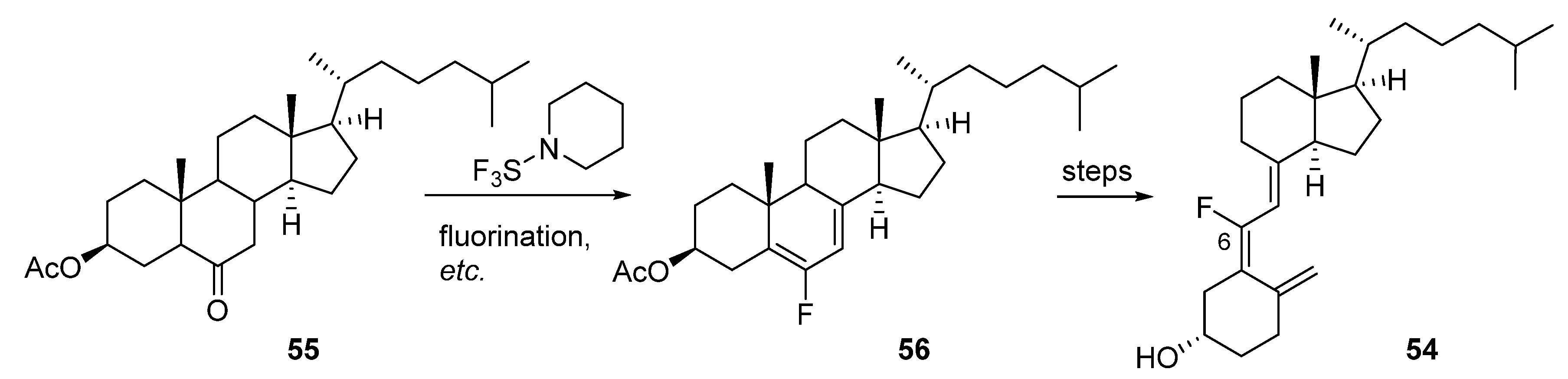
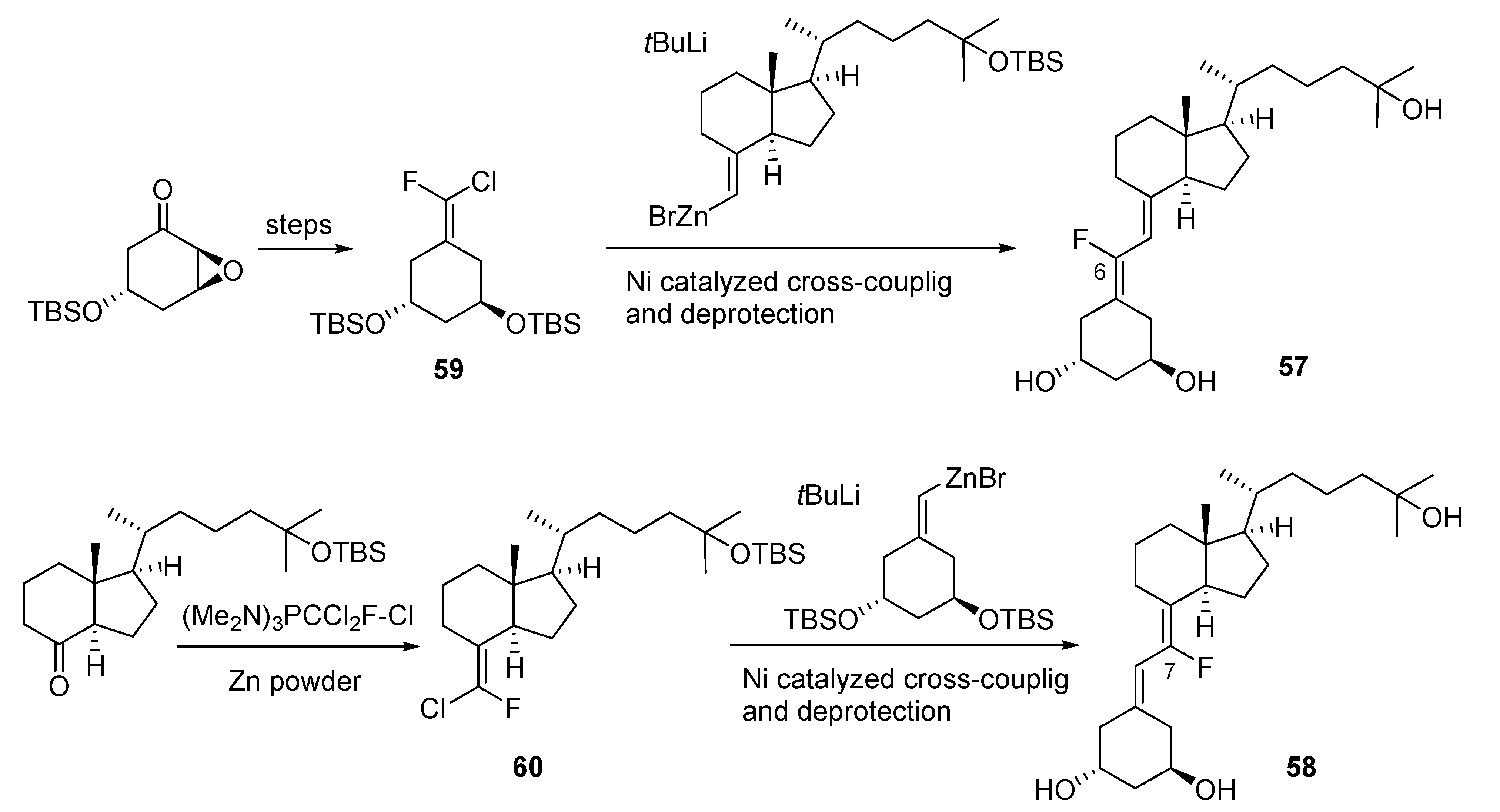
3.2. 6- and 7-Fluorinated VD3 Analogues
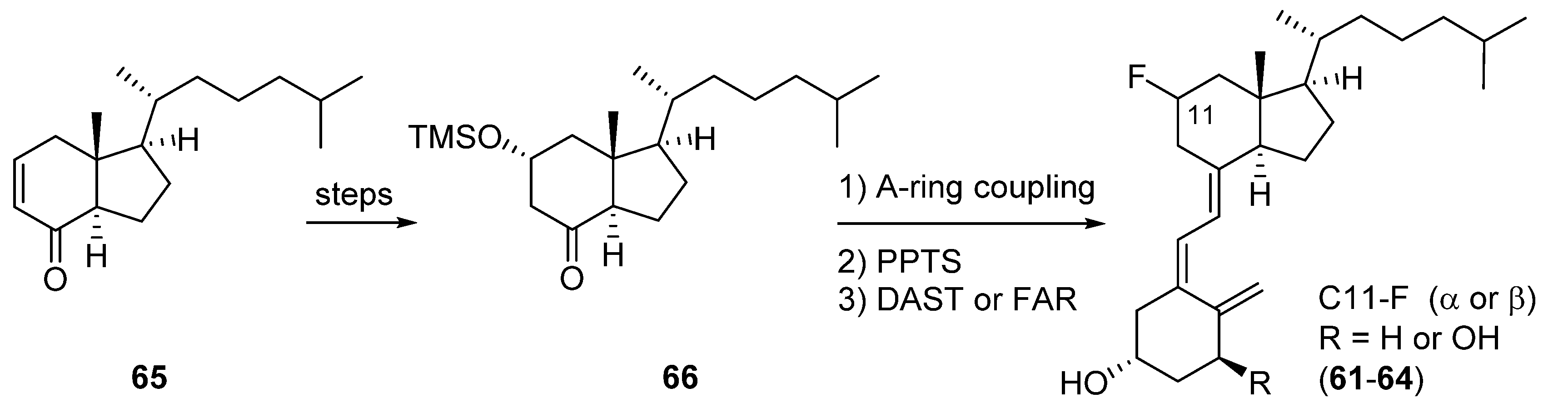
4. CD-Ring Fluorinated VD3 Analogues: 11-Fluorinated VD3 Analogues
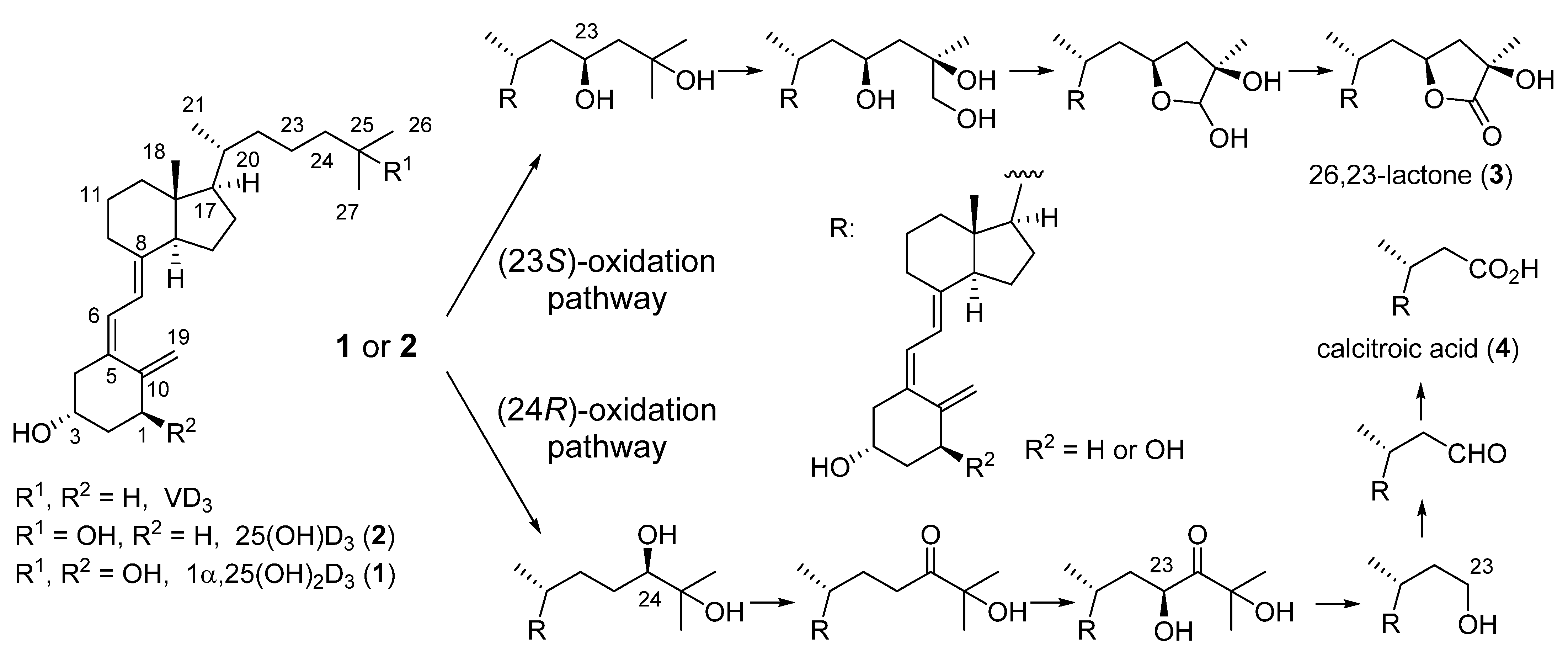
5. Side-Chain Fluorinated VD3 Analogues
5.1. 22-Fluorinated VD3 Analogues
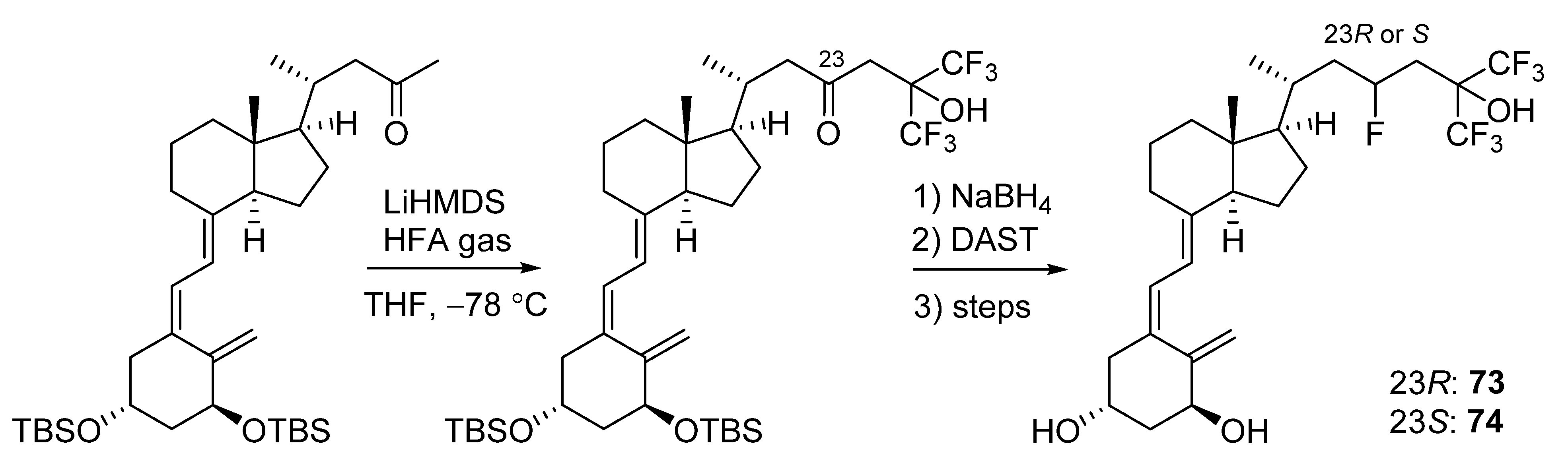
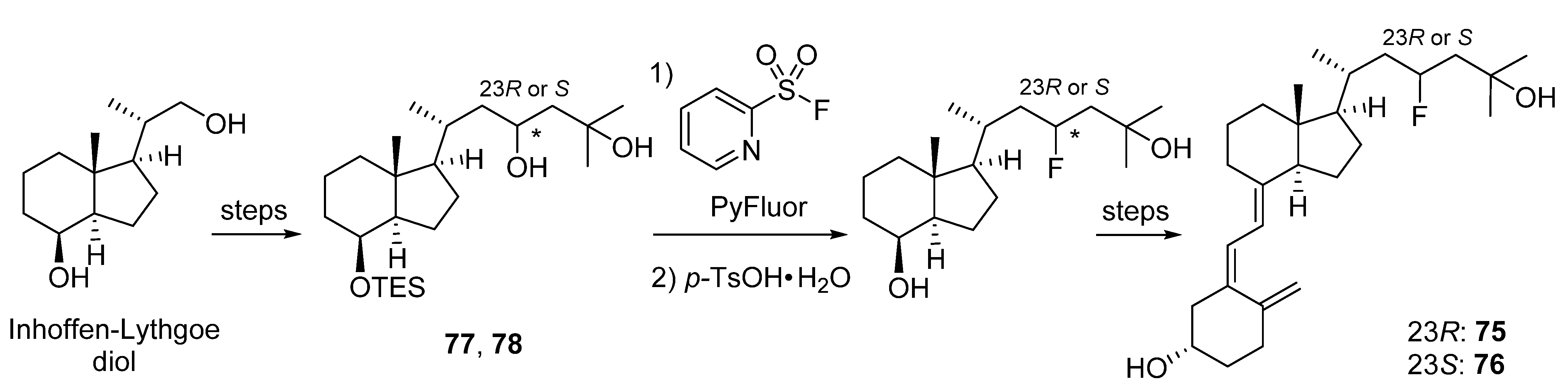
5.2. 23-Fluorinated VD3 Analogues
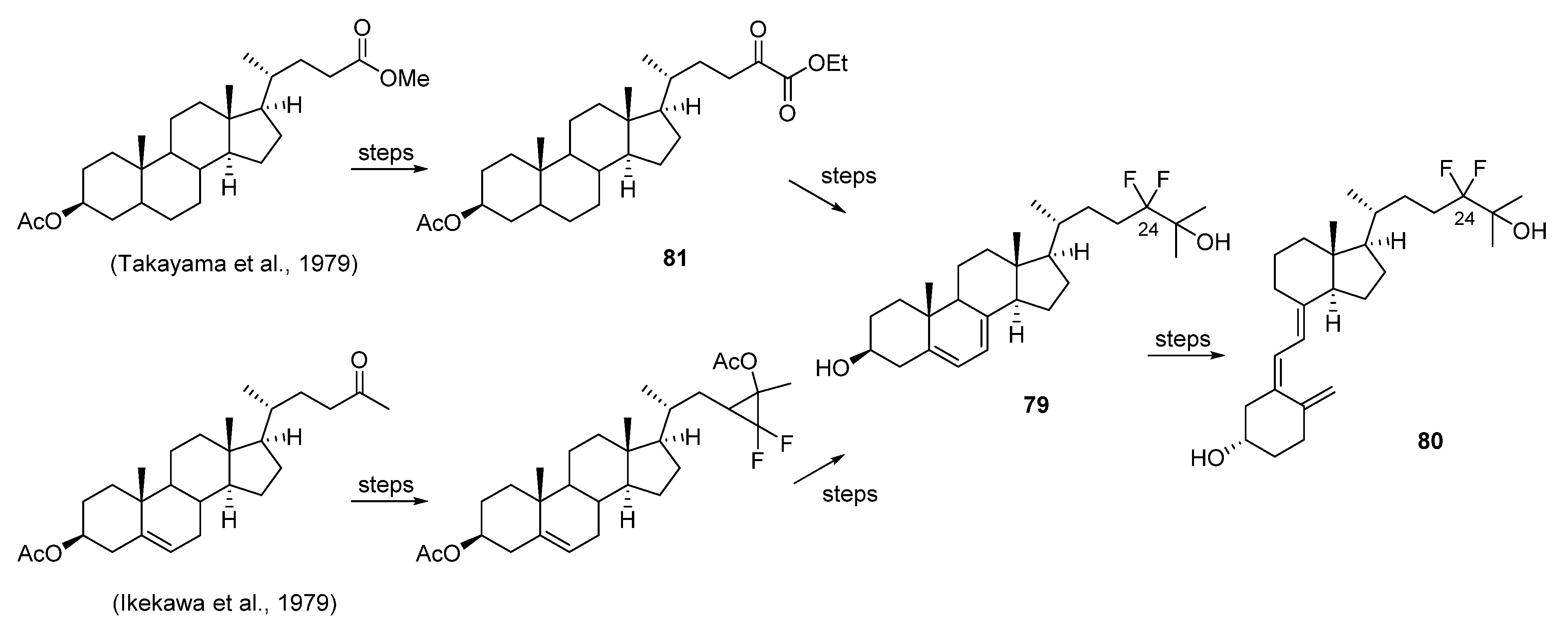
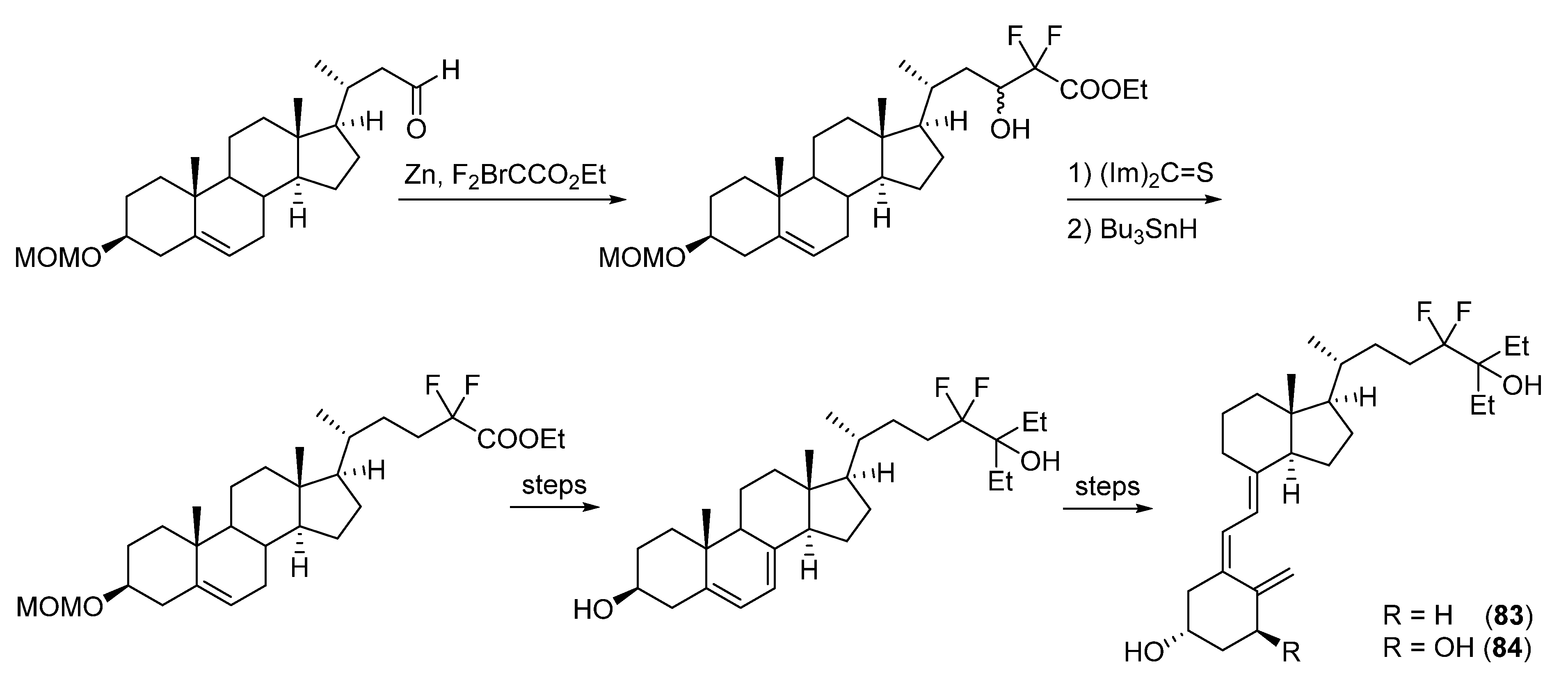
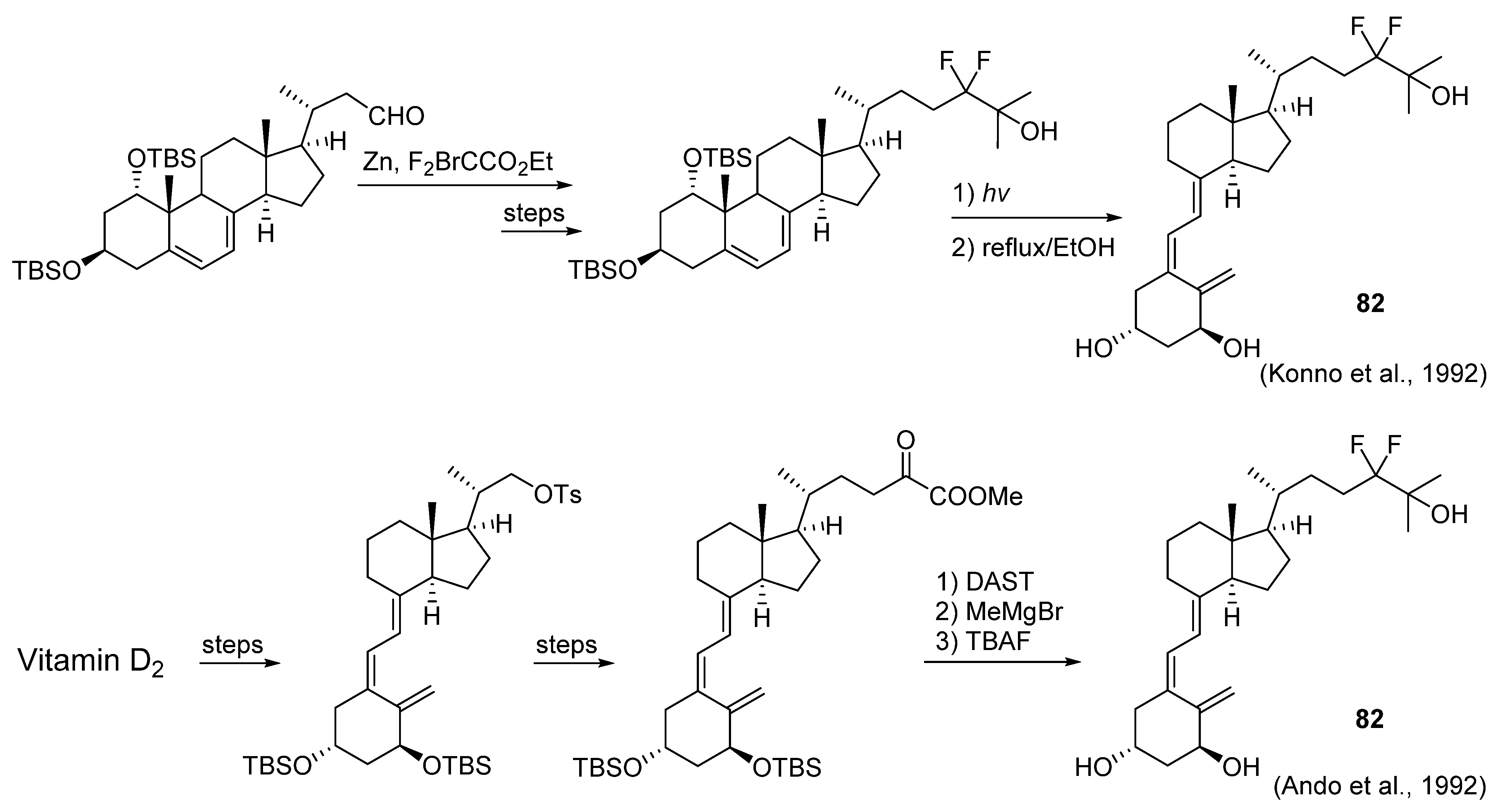
5.3. 24-Fluorinated VD3 Analogues
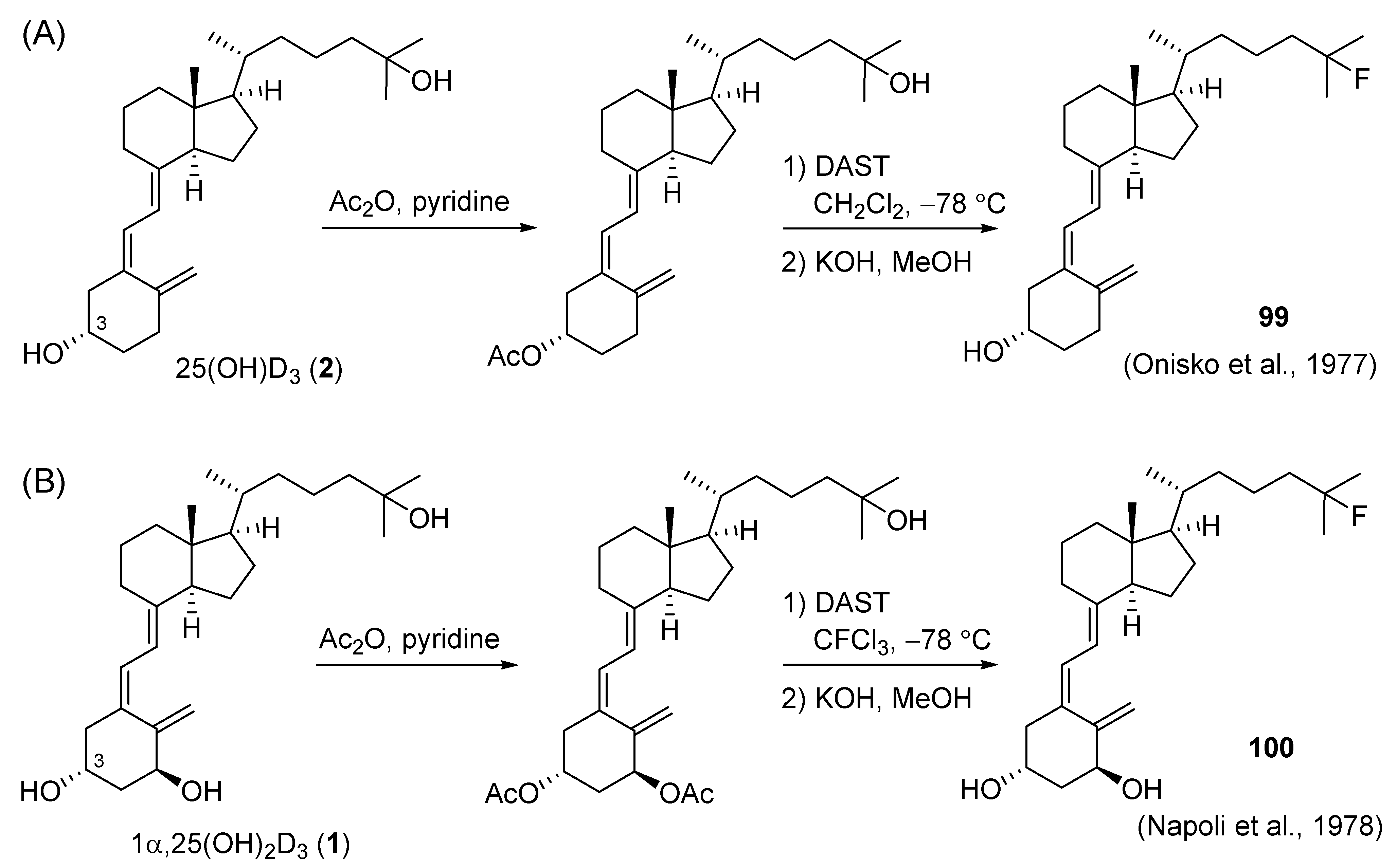
5.4. 25-Fluorinated VD3 Analogues
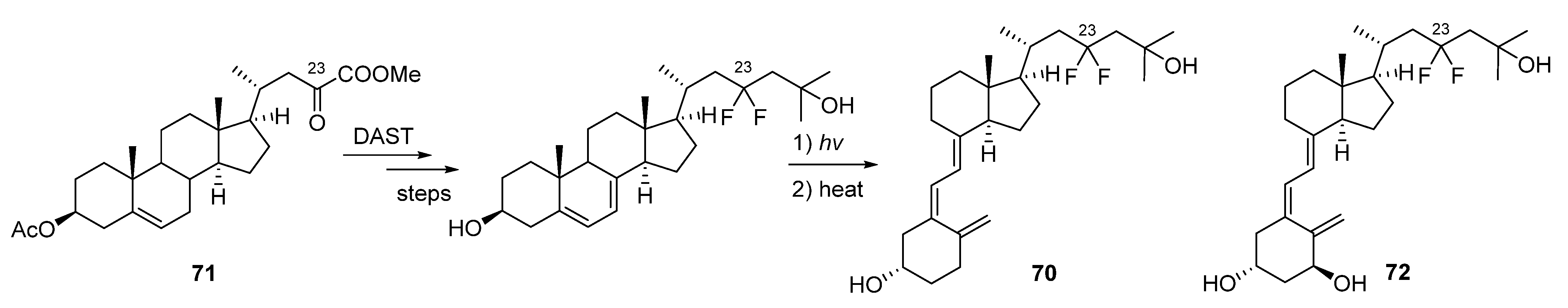
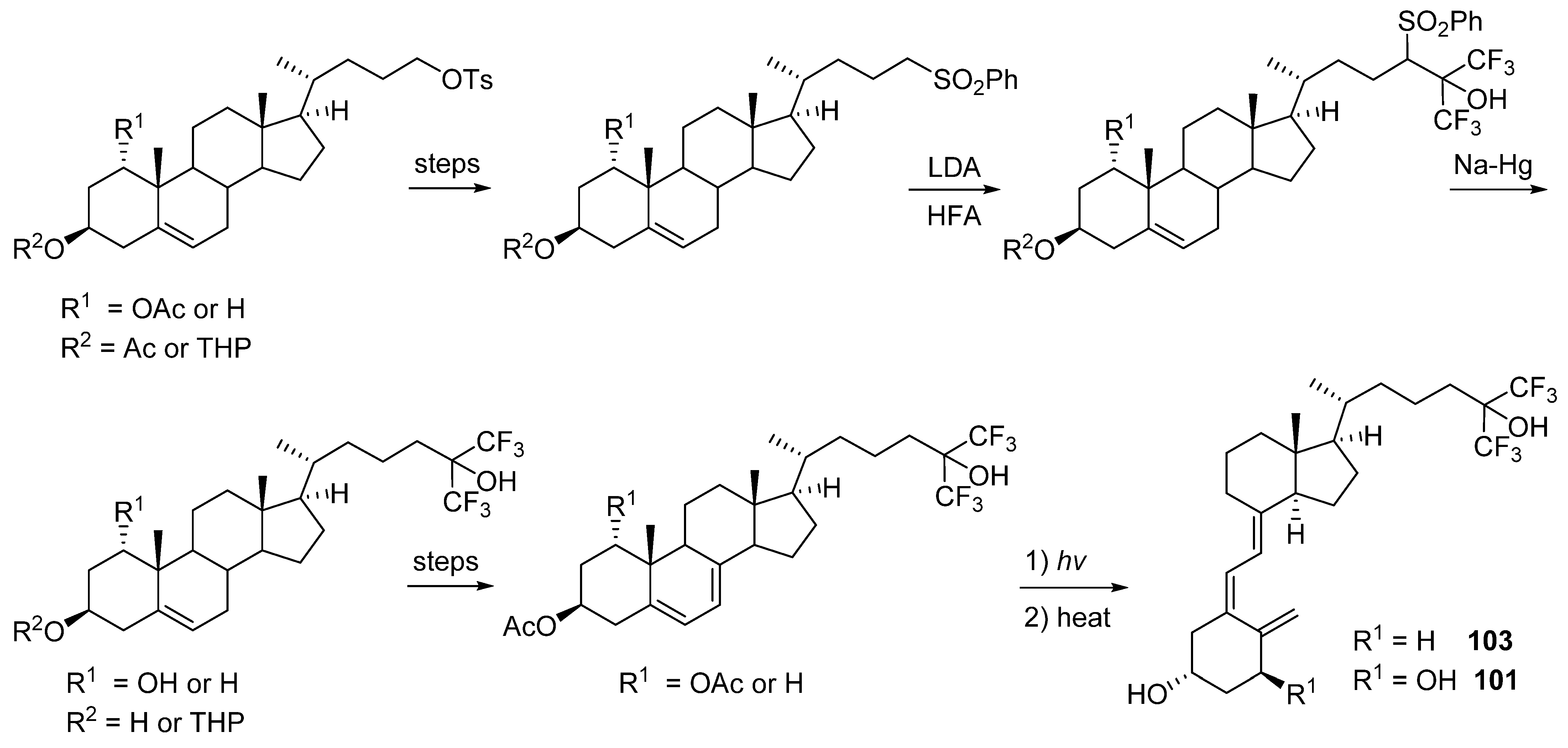
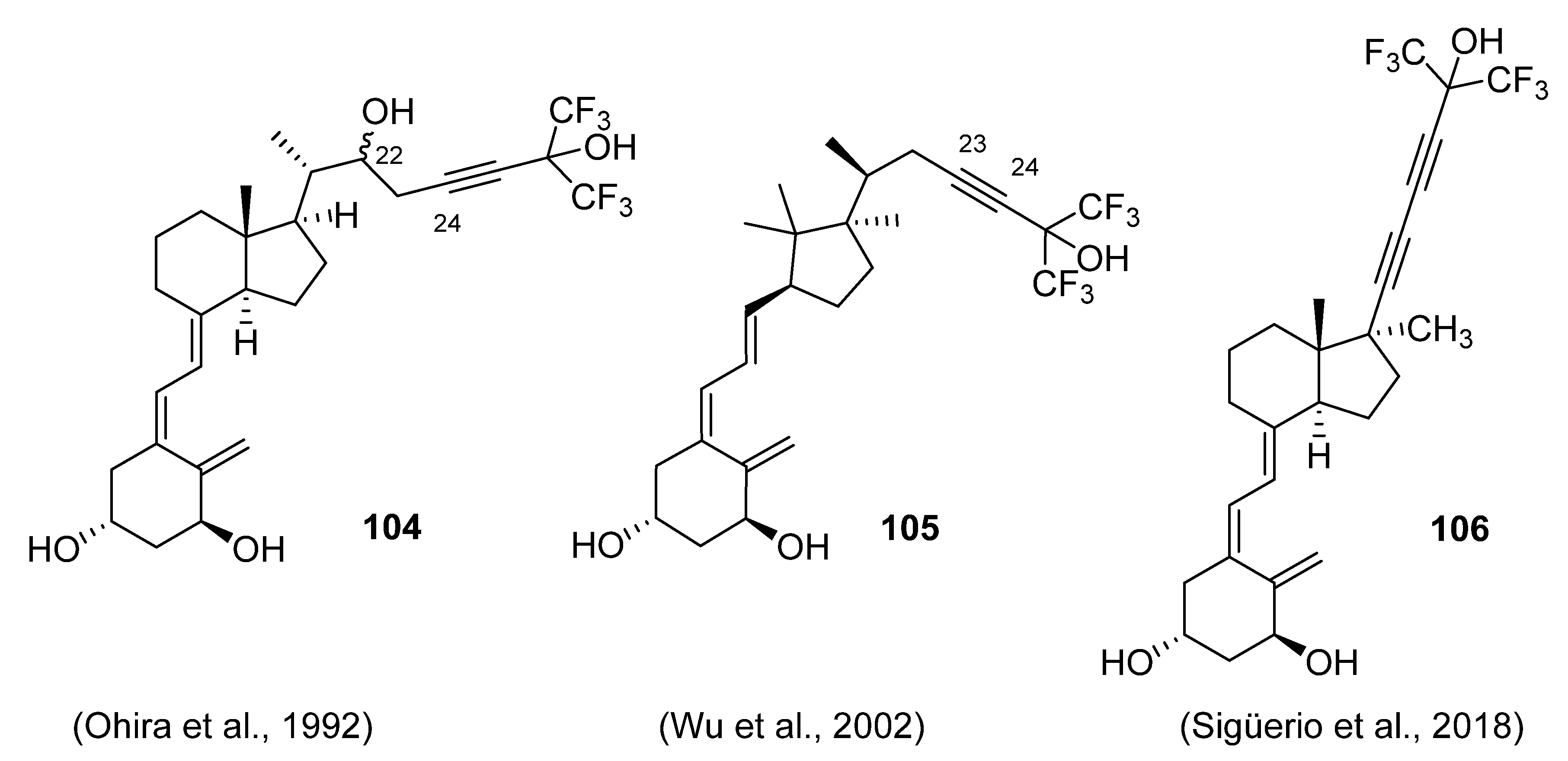
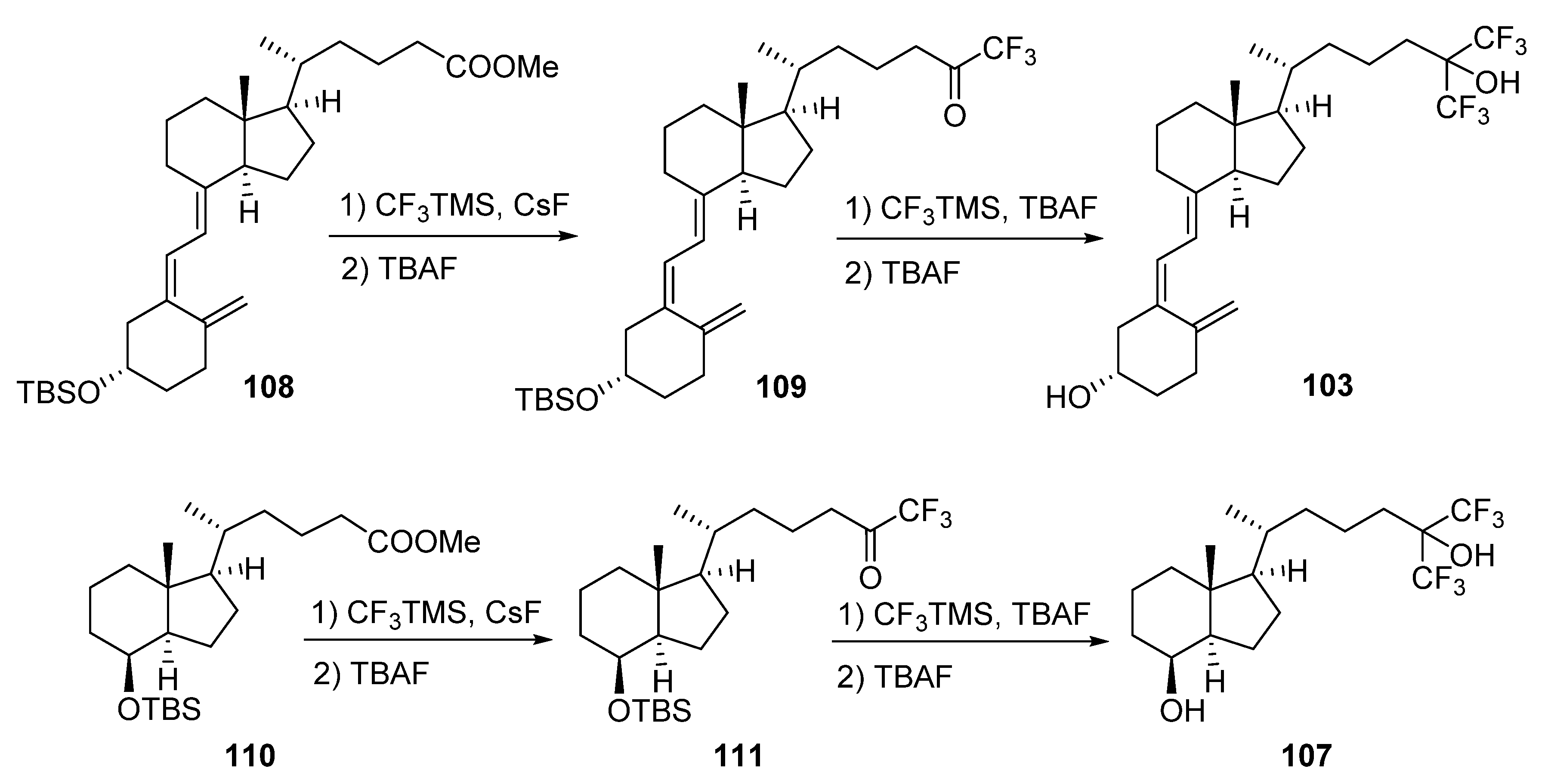
5.5. 26,27-Hexafluorinated VD3 Analogues
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.M.; Shu, Y.Z.; Zhuo, X.; Meanwell, N.A. Metabolic and pharmaceutical aspects of fluorinated compounds. J. Med. Chem. 2020, 63, 6315–6386. [Google Scholar] [CrossRef] [PubMed]
- Böhm, H.-J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Müller, K.; Obst-Sander, U.; Stahl, M. Fluorine in medicinal chemistry. Chembiochem 2004, 5, 637–643. [Google Scholar] [CrossRef]
- Kirk, K.L. Fluorine in medicinal chemistry: Recent therapeutic applications of fluorinated small molecules. J. Fluor. Chem. 2006, 127, 1013–1029. [Google Scholar] [CrossRef]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.L.; Del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in pharmaceutical industry: Fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Han, J.; Fustero, S.; Medio-Simon, M.; Sedgwick, D.M.; Santi, C.; Ruzziconi, R.; Soloshonok, V.A. Fluorine-containing drugs approved by the FDA in 2018. Chemistry 2019, 25, 11797–11819. [Google Scholar] [CrossRef]
- Bégué, J.-P.; Bonnet-Delpon, D. Recent advances (1995–2005) in fluorinated pharmaceuticals based on natural products. J. Fluor. Chem. 2006, 127, 992–1012. [Google Scholar] [CrossRef]
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzym. Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef] [Green Version]
- Berkowitz, D.B.; Karukurichi, K.R.; De La Salud-Bea, R.; Nelson, D.L.; McCune, C.D. Use of fluorinated functionality in enzyme inhibitor development: Mechanistic and analytical advantages. J. Fluor. Chem. 2008, 129, 731–742. [Google Scholar] [CrossRef] [Green Version]
- Maienfisch, P.; Hall, R.G. The importance of fluorine in the life science industry. Chimia 2004, 58, 93–98. [Google Scholar] [CrossRef]
- Prchalová, E.; Štěpánek, O.; Smrček, S.; Kotora, M. Medicinal applications of perfluoroalkylated chain-containing compounds. Future Med. Chem. 2014, 6, 1201–1229. [Google Scholar] [CrossRef]
- Kukhar’, V.P.; Soloshonok, V.A. Aliphatic fluorine-containing amino acids. Russ. Chem. Rev. 1991, 60, 850–864. [Google Scholar] [CrossRef]
- Liu, P.; Sharon, A.; Chu, C.K. Fluorinated nucleosides: Synthesis and biological implication. J. Fluor. Chem. 2008, 129, 743–766. [Google Scholar] [CrossRef] [Green Version]
- Isanbor, C.; O’Hagan, D. Fluorine in medicinal chemistry: A review of anti-cancer agents. J. Fluor. Chem. 2006, 127, 303–319. [Google Scholar] [CrossRef]
- Al-Harthy, T.; Zoghaib, W.; Abdel-Jalil, R. Importance of fluorine in benzazole compounds. Molecules 2020, 25, 4677. [Google Scholar] [CrossRef]
- Krafft, M.P. Fluorocarbons and fluorinated amphiphiles in drug delivery and biomedical research. Adv. Drug Deliv. Rev. 2001, 47, 209–228. [Google Scholar] [CrossRef]
- Sakaki, T.; Kagawa, N.; Yamamoto, K.; Inouye, K. Metabolism of vitamin D3 by cytochromes P450. Front. Biosci. 2005, 10, 119–134. [Google Scholar]
- Sakaki, T.; Sawada, N.; Komai, K.; Shiozawa, S.; Yamada, S.; Yamamoto, K.; Ohyama, Y.; Inouye, K. Dual metabolic pathway of 25-hydroxyvitamin D3 catalyzed by human CYP24. Eur. J. Biochem. 2000, 267, 6158–6165. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, K.; Nishikawa, M.; Okamoto, K.; Horibe, K.; Mano, H.; Yamaguchi, M.; Okon, R.; Nakagawa, K.; Tsugawa, N.; Okano, T.; et al. Elucidation of metabolic pathways of 25-hydroxyvitamin D3 mediated by Cyp24A1 and Cyp3A using Cyp24a1 knockout rats generated by CRISPR/Cas9 system. J. Biol. Chem. 2021, 296, 100668. [Google Scholar] [CrossRef]
- Rochel, N.; Wurtz, J.M.; Mitschler, A.; Klaholz, B.; Moras, D. The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol. Cell 2000, 5, 173–179. [Google Scholar] [CrossRef]
- Napoli, J.L.; Fivizzani, M.A.; Schnoes, H.K.; DeLuca, H.F. 1-Fluorovitamin D3, a vitamin D3 analogue more active on bone-calcium mobilization than on intestinal-calcium transport. Biochemistry 1979, 18, 1641–1646. [Google Scholar] [CrossRef]
- Paaren, H.E.; Fivizzani, M.A.; Schnoes, H.K.; DeLuca, H.F. 1α,25-Difluorovitamin D3: An inert vitamin D analog. Arch. Biochem. Biophys. 1981, 209, 579–583. [Google Scholar] [CrossRef]
- Ohshima, E.; Takatsuto, S.; Ikekawa, N.; De Luca, H.F. Synthesis of 1α-fluorovitamin D3. Chem. Pharm. Bull. 1984, 32, 3518–3524. [Google Scholar] [CrossRef] [Green Version]
- Ohshima, E.; Sai, H.; Takatsuto, S.; Ikekawa, N.; Kobayashi, Y.; Tanaka, Y.; DeLuca, H.F. Synthesis and biological activity of 1α-fluoro-25-hydroxyvitamin D3. Chem. Pharm. Bull. 1984, 32, 3525–3531. [Google Scholar] [CrossRef] [Green Version]
- Shiuey, S.-J.; Kulesha, I.; Baggiolini, E.G.; Uskoković, M.R. Total synthesis of 1α-fluoro-25-hydroxycholecalciferol and -ergocalciferol. J. Org. Chem. 1990, 55, 243–247. [Google Scholar] [CrossRef]
- Kiegiel, J.; Wovkulich, P.M.; Uskoković, M.R. Chemical conversion of vitamin D3 to its 1,25-dihydroxy metabolite. Tetrahedron Lett. 1991, 32, 6057–6060. [Google Scholar] [CrossRef]
- Maehr, H.; Lee, H.J.; Perry, B.; Suh, N.; Uskoković, M.R. Calcitriol derivatives with two different side chains at C-20. V. Potent inhibitors of mammary carcinogenesis and inducers of leukemia differentiation. J. Med. Chem. 2009, 52, 5505–5519. [Google Scholar] [CrossRef] [Green Version]
- Nagata, A.; Akagi, Y.; Asano, L.; Kotake, K.; Kawagoe, F.; Mendoza, A.; Masoud, S.S.; Usuda, K.; Yasui, K.; Takemoto, Y.; et al. Synthetic chemical probes that dissect vitamin D activities. ACS Chem. Biol. 2019, 14, 2851–2858. [Google Scholar] [CrossRef]
- Ono, K.; Yoshida, A.; Saito, N.; Fujishima, T.; Honzawa, S.; Suhara, Y.; Kishimoto, S.; Sugiura, T.; Waku, K.; Takayama, H.; et al. Efficient synthesis of 2-modified 1α,25-dihydroxy-19-norvitamin D3 with Julia olefination: High potency in induction of differentiation on HL-60 cells. J. Org. Chem. 2003, 68, 7407–7415. [Google Scholar] [CrossRef]
- Oshida, J.-I.; Morisaki, M.; Ikekawa, N. Synthesis of 2β-fluoro-1α-hydroxyvitamin D3. Tetrahedron Lett. 1980, 21, 1755–1756. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Nakazawa, M.; Kumadaki, I.; Taguchi, T.; Ohshima, E.; Ikekawa, N.; Tanaka, Y.; DeLuca, H.F. Studies on organic fluorine compounds. L. Synthesis and biological activity of 2α-fluorovitamin D3. Chem. Pharm. Bull. 1986, 34, 1568–1572. [Google Scholar] [CrossRef] [Green Version]
- Scheddin, D.; Mayer, H.; Schönecker, B.; Gliesing, S.; Reichenbächer, M. Synthesis and biological activities of 2β-chloro-, 2β-fluoro-, and 2β-methoxy-1α,25-dihydroxyvitamin D3. Steroids 1998, 63, 633–643. [Google Scholar] [CrossRef]
- Mikami, K.; Ohba, S.; Ohmura, H.; Kubodera, N.; Nakagawa, K.; Okano, T. Asymmetric catalytic ene-cyclization approach to 2-fluoro-19-nor-1,25-dihydroxyvitamin D3 A-ring analog with significant transactivation activity. Chirality 2001, 13, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Okano, T.; Ozono, K.; Kato, S.; Kubodera, N.; Ohba, S.; Itoh, Y.; Mikami, K. Catalytic asymmetric synthesis and anticancer effects of the novel non-calcemic analog of vitamin D, 2α-fluoro-19-nor-22-oxa-1α,25-dihydroxyvitamin D3 in metastatic lung carcinoma. J. Fluor. Chem. 2007, 128, 654–667. [Google Scholar] [CrossRef]
- Posner, G.H.; Woodard, B.T.; Crawford, K.R.; Peleg, S.; Brown, A.J.; Dolan, P.; Kensler, T.W. 2,2-Disubstituted analogues of the natural hormone 1α,25-dihydroxyvitamin D3: Chemistry and biology. Bioorg. Med. Chem. 2002, 10, 2353–2365. [Google Scholar] [CrossRef]
- Yakhimovich, R.I.; Klimashevskii, V.M.; Segal, G.M. Synthesis and biological activity of 3α-fluoro-9,10-secocholesta-5,7,10(19)-triene, the fluoro derivative of vitamin D3. Khimiko-Farmatsevticheskii Zhurnal 1976, 10, 58–64. [Google Scholar] [CrossRef]
- Sheves, M.; Sialom, B.; Mazur, Y. Stereoselective conversion of vitamin D3 into its 3β-halogenated derivatives. The synthesis of a 1α-hydroxy-3β-fluorovitamin D3 analogue. J. Chem. Soc. Chem. Commun. 1978, 554–555. [Google Scholar] [CrossRef]
- Revelle, L.K.; Londowski, J.M.; Kost, S.B.; Corradino, R.A.; Kumar, R. Synthesis and biological activity of 3β-fluorovitamin D3: Comparison of the biological activity of 3β-fluorovitamin D3 and 3-deoxyvitamin D3. J. Steroid Biochem. 1985, 22, 469–474. [Google Scholar] [CrossRef]
- Shimizu, M.; Iwasaki, Y.; Yamada, S. 4,4-Difluoro-1α,25-dihydroxyvitamin D3: Analog to probe A-ring conformation in vitamin D-receptor complex. Tetrahedron Lett. 1999, 40, 1697–1700. [Google Scholar] [CrossRef]
- Sialom, B.; Mazur, Y. Influence of fluorine and oxygen atoms at C-19 on the previtamin D-vitamin D interconversion. J. Org. Chem. 1980, 45, 2201–2204. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Shimizu, M.; Hirosawa, T.; Yamada, S. Regioselective synthesis of 19-fluorovitamin D via fluorination of vitamin D-sulfur dioxide adducts. Tetrahedron Lett. 1996, 37, 6753–6754. [Google Scholar] [CrossRef]
- Shimizu, M.; Iwasaki, Y.; Ohno, A.; Yamada, S. Synthesis of (10Z)- and (10E)-19-fluoro-1α,25-dihydroxyvitamin D3: Compounds to probe vitamin D conformation in receptor complex by 19F-NMR. Chem. Pharm. Bull. 2000, 48, 1484–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, M.; Ohno, A.; Yamada, S. (10Z)- and (10E)-19-fluoro-1α,25-dihydroxyvitamin D3: An improved synthesis via 19-nor-10-oxo-vitamin D. Chem. Pharm. Bull. 2001, 49, 312–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dauben, W.G.; Kohler, B.; Roesle, A. Synthesis of 6-fluorovitamin D3. J. Org. Chem. 1985, 50, 2007–2010. [Google Scholar] [CrossRef]
- Wilhelm, F.; Dauben, W.G.; Kohler, B.; Roesle, A.; Norman, A.W. 6-Fluoro-vitamin D3: A new antagonist of the biological actions of vitamin D3 and its metabolites which interacts with the intestinal receptor for 1α,25(OH)2-vitamin D3. Arch. Biochem. Biophys. 1984, 233, 127–132. [Google Scholar] [CrossRef]
- Takenouchi, K.; Ishizuka, S.; Miura, D.; Sato, F.; Hanazawa, T. 6,7-Substituted 19-norvitamin D3 derivatives and pharmaceuticals containing them. Jpn. Kokai Tokkyo Koho. JP 2004-175763. 2004. Available online: https://patents.google.com/patent/US7530024 (accessed on 26 July 2021).
- Zhu, G.-D.; Haver, D.V.; Jurriaans, H.; De Clercq, P.J. 11-Fluoro-1α-hydroxyvitamin D3: The quest for experimental evidence of the folded vitamin D conformation. Tetrahedron 1994, 50, 7049–7060. [Google Scholar] [CrossRef]
- Gill, H.S.; Londowski, J.M.; Corradino, R.A.; Kumar, R. The synthesis and biological activity of 22-fluorovitamin D3: A new vitamin D analog. Steroids 1986, 48, 93–108. [Google Scholar] [CrossRef]
- Taguchi, T.; Mitsuhashi, S.; Yamanouchi, A.; Kobayashi, Y.; Sai, H.; Ikekawa, N. Synthesis of 23,23-difluoro-25-hydroxyvitamin D3. Tetrahedron Lett. 1984, 25, 4933–4936. [Google Scholar] [CrossRef]
- Nakada, M.; Tanaka, Y.; DeLuca, H.F.; Kobayashi, Y.; Ikekawa, N. Biological activities and binding properties of 23,23-difluoro-25-hydroxyvitamin D3 and its 1α-hydroxy derivative. Arch. Biochem. Biophys. 1985, 241, 173–178. [Google Scholar] [CrossRef]
- Ikeda, M.; Matsumura, H.; Sawada, N.; Hashimoto, K.; Tanaka, T.; Noguchi, T.; Hayashi, M. Synthesis and biological evaluations of C-23-modified 26,26,26,27,27,27-F6-vitamin D3 analogues. Bioorg. Med. Chem. 2000, 8, 1809–1817. [Google Scholar] [CrossRef]
- Kawagoe, F.; Yasuda, K.; Mototani, S.; Sugiyama, T.; Uesugi, M.; Sakaki, T.; Kittaka, A. Synthesis and CYP24A1-dependent metabolism of 23-fluorinated vitamin D3 analogues. ACS Omega 2019, 4, 11332–11337. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Ohmori, M.; Takayama, H. Synthesis of 24,24-difluoro-25-hydroxyvitamin D3. Tetrahedron Lett. 1979, 20, 1859–1862. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Taguchi, T.; Terada, T.; Oshida, J.-I.; Morisaki, M.; Ikekawa, N. Synthesis of 24,24-difluoro- and 24ξ-fluoro-25-hydroxyvitamin D3. Tetrahedron Lett. 1979, 20, 2023–2026. [Google Scholar] [CrossRef]
- Tanaka, Y.; DeLuca, H.F.; Schnoes, H.K.; Ikekawa, N.; Kobayashi, Y. 24,24-difluoro-1,25-dihydroxyvitamin D3: In vitro production, isolation, and biological activity. Arch. Biochem. Biophys. 1980, 199, 473–478. [Google Scholar] [CrossRef]
- Gill, H.S.; Londowski, J.M.; Corradino, R.A.; Zinsmeister, A.R.; Kumar, R. Synthesis and biological activity of novel vitamin D analogues: 24,24-difluoro-25-hydroxy-26,27-dimethylvitamin D3 and 24,24-difluoro-1α,25-dihydroxy-26,27-dimethylvitamin D3. J. Med. Chem 1990, 33, 480–490. [Google Scholar] [CrossRef]
- Konno, K.; Ojima, K.; Hayashi, T.; Takayama, H. An alternative and efficient synthesis of 24,24-difluoro-1α,25-dihydroxyvitamin D3. Chem. Pharm. Bull. 1992, 40, 1120–1124. [Google Scholar] [CrossRef] [Green Version]
- Ando, K.; Kondo, F.; Koike, F.; Takayama, H. An improved synthesis of 24,24-difluoro-1α,25-dihydroxyvitamin D3 from vitamin D2. Chem. Pharm. Bull. 1992, 40, 1662–1664. [Google Scholar] [CrossRef] [Green Version]
- Kondo, F.; Maki, S.; Konno, K.; Takayama, H. The first synthesis of 24,24-difluoro-1α-hydroxyvitamin D3 by means of radical deoxygenation of alcohols. Chem. Pharm. Bull. 1996, 44, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, H.; Hosotani, R.; Miyamoto, Y.; Nakano, Y.; Yamamoto, K.; Yamada, S.; Shinki, T.; Suda, T.; Yamaguchi, K.; Konno, K.; et al. Stereoselective synthesis and structural establishment of (25S)-24,24-difluoro-1α,25,26-trihydroxyvitamin D3, a major metabolite of 24,24-difluoro-1α,25-dihydroxyvitamin D3. Tetrahedron 1998, 54, 14705–14724. [Google Scholar] [CrossRef]
- Flores, A.; Massarelli, I.; Thoden, J.B.; Plum, L.A.; DeLuca, H.F. A methylene group on C-2 of 24,24-difluoro-19-nor-1α,25-dihydroxyvitamin D3 markedly increases bone calcium mobilization in nivo. J. Med. Chem. 2015, 58, 9731–9741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
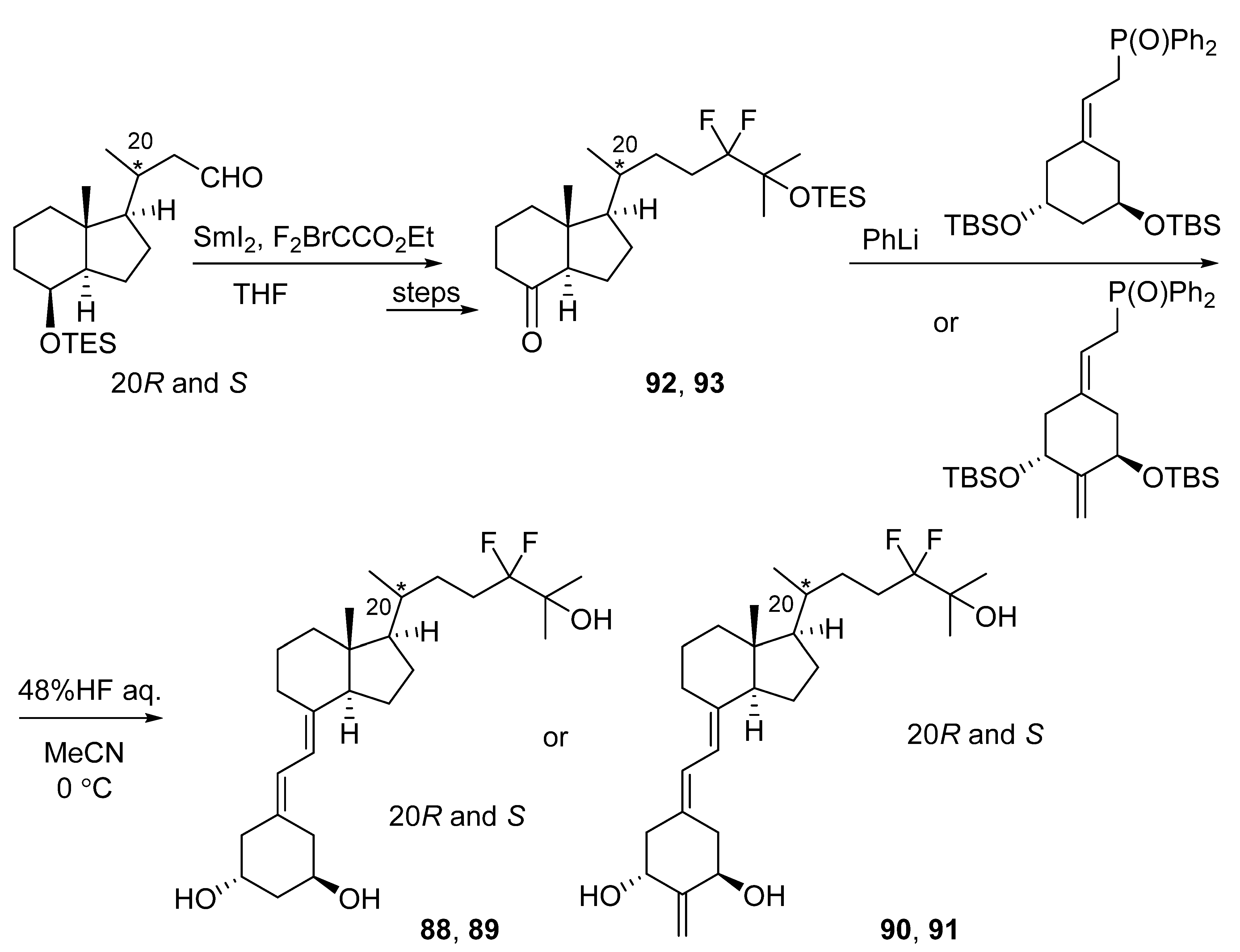
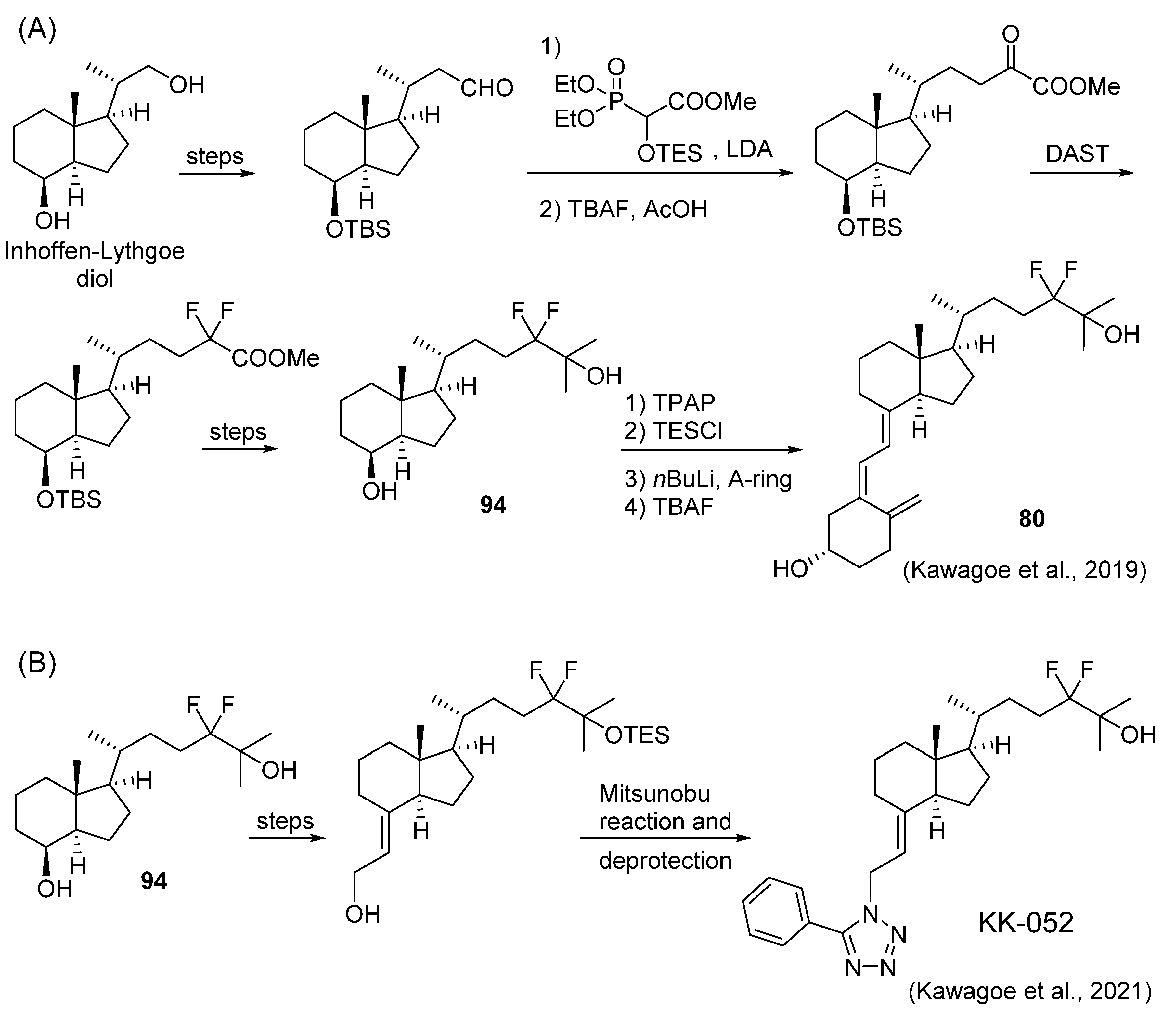
- Kawagoe, F.; Mototani, S.; Yasuda, K.; Nagasawa, K.; Uesugi, M.; Sakaki, T.; Kittaka, A. Introduction of fluorine atoms to vitamin D3 side-chain and synthesis of 24,24-difluoro-25-hydroxyvitamin D3. J. Steroid Biochem. Mol. Biol. 2019, 195, 105477. [Google Scholar] [CrossRef]
- Kawagoe, F.; Mendoza, A.; Hayata, Y.; Asano, L.; Kotake, K.; Mototani, S.; Kawamura, S.; Kurosaki, S.; Akagi, Y.; Takemoto, Y.; et al. Discovery of a vitamin D receptor-silent vitamin D derivative that impairs sterol regulatory element-binding protein in vivo. J. Med. Chem. 2021, 64, 5689–5709. [Google Scholar] [CrossRef]
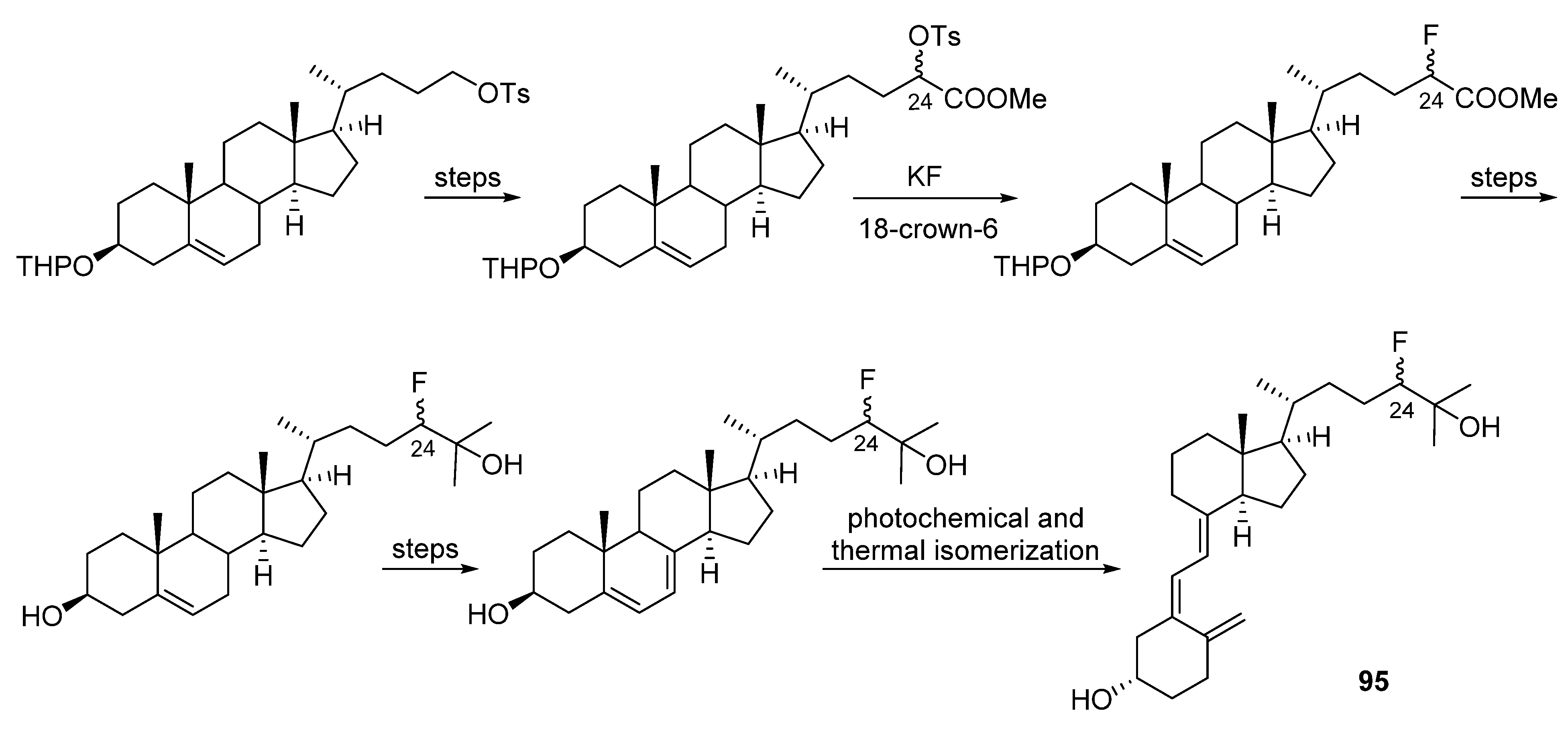
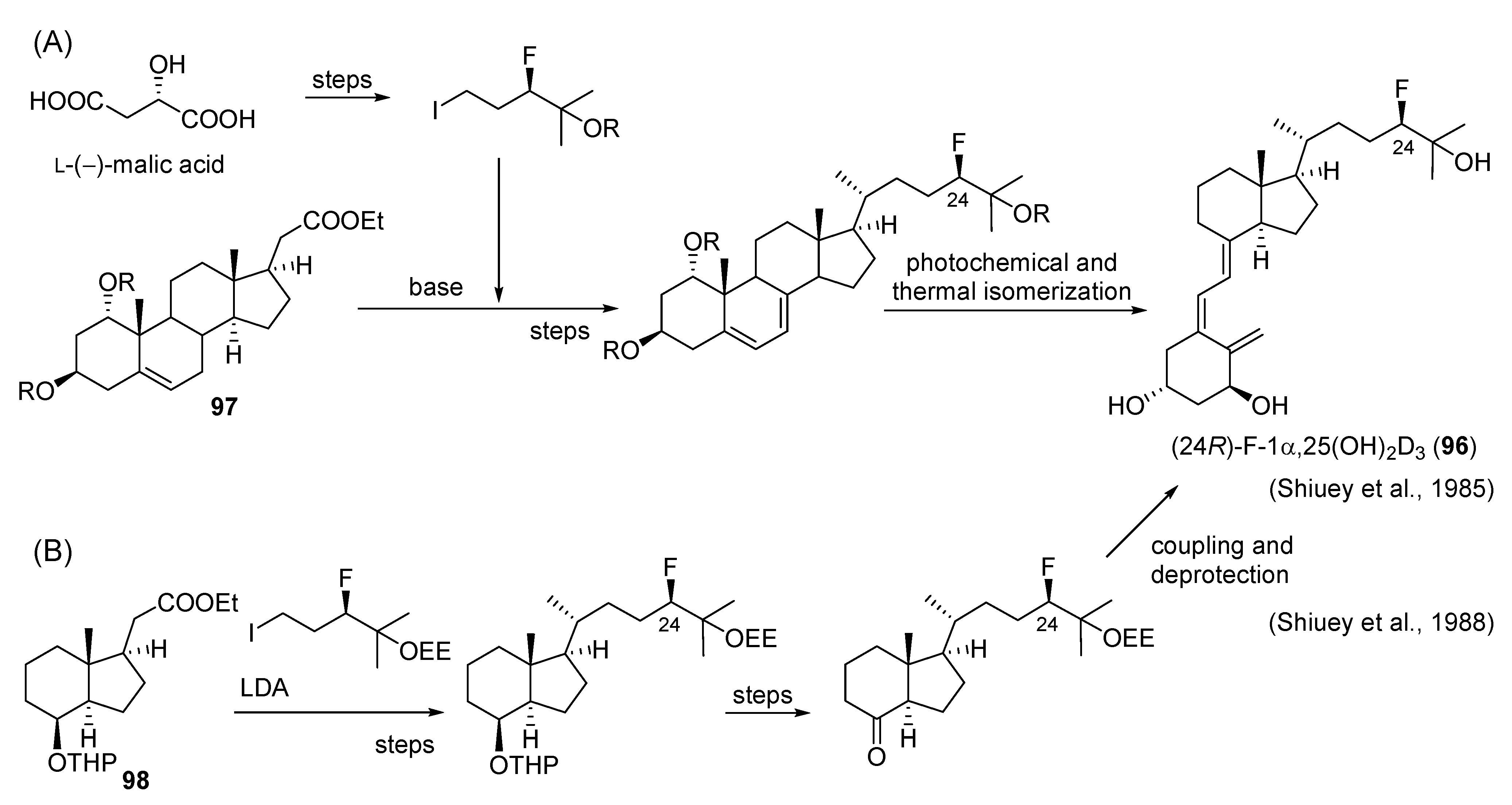
- Shiuey, S.-J.; Partridge, J.J.; Chadha, N.K.; Boris, A.; Uskoković, M.R. Stereospecific synthesis of 1α,25-dihydroxy-24R-fluorocholecalciferol (Ro23-0233). In Vitamin D, Chemical, Biochemical and Clinical Update; Walter De Gruyter: Berlin, Germany, 1985; pp. 765–766. [Google Scholar]
- Shiuey, S.-J.; Partridge, J.J.; Uskoković, M.R. Triply convergent synthesis of 1α,25-dihydroxy-24(R)-fluorocholecalciferol. J. Org. Chem. 1988, 53, 1040–1046. [Google Scholar] [CrossRef]
- Onisko, B.L.; Schnoes, H.K.; DeLuca, H.F. Synthesis of potential vitamin D antagonists. Tetrahedron Lett. 1977, 18, 1107–1108. [Google Scholar] [CrossRef]
- Napoli, J.L.; Fivizzani, M.A.; Hamstra, A.H.; Schnoes, H.K.; DeLuca, H.F.; Stern, P.H. The synthesis and activity in vitro of 25-masked 1α-hydroxylated vitamin D3 analogs. Steroids 1978, 32, 453–466. [Google Scholar] [CrossRef]
- Leyssens, C.; Verlinden, L.; Verstuyf, A. The future of vitamin D analogs. Front. Physiol. 2014, 5, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maestro, M.A.; Molnár, F.; Carlberg, C. Vitamin D and its synthetic analogs. J. Med. Chem. 2019, 62, 6854–6875. [Google Scholar] [CrossRef]
- Tanaka, Y.; DeLuca, H.F.; Kobayashi, Y.; Ikekawa, N. 26,26,26,27,27,27-Hexafluoro-1,25-dihydroxyvitamin D3: A highly potent, long-lasting analog of 1,25-dihydroxyvitamin D3. Arch. Biochem. Biophys. 1984, 229, 348–354. [Google Scholar] [CrossRef]
- Harada, M.; Miyahara, T.; Miyata, M.; Tomita, I.; Okayachi, H.; Ikemoto, Y.; Higuchi, S.; Otomo, S.; Kozuka, H.; Ikekawa, N. Effects on cultured neonatal mouse calvaria of 1α,25-dihydroxyvitamin D3, 26,26,26,27,27,27-hexafluoro-1α,25-dihydroxyvitamin D3 and 26,26,26,27,27,27-hexafluoro-1α,23S,25-trihydroxyvitamin D3. Bone Miner. 1992, 18, 41–49. [Google Scholar] [CrossRef]
- Inaba, M.; Okuno, S.; Nishizawa, Y.; Imanishi, Y.; Katsumata, T.; Sugata, I.; Morii, H. Effect of substituting fluorine for hydrogen at C-26 and C-27 on the side chain of 1,25-dihydroxyvitamin D3. Biochem. Pharmacol. 1993, 45, 2331–2336. [Google Scholar] [CrossRef]
- Sakaki, T.; Sawada, N.; Abe, D.; Komai, K.; Shiozawa, S.; Nonaka, Y.; Nakagawa, K.; Okano, T.; Ohta, M.; Inouye, K. Metabolism of 26,26,26,27,27,27-F6-1α,25-dihydroxyvitamin D3 by CYP24: Species-based difference between humans and rats. Biochem. Pharmacol. 2003, 65, 1957–1965. [Google Scholar] [CrossRef]
- Kasai, N.; Sakaki, T.; Shinkyo, R.; Ikushiro, S.; Iyanagi, T.; Ohta, M.; Inouye, K. Metabolism of 26,26,26,27,27,27-F6-1α,23S,25-trihydroxyvitamin D3 by human UDP-glucuronosyltransferase 1A3*. Drug Metab. Dispos. 2005, 33, 102–107. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Taguchi, T.; Kanuma, N. Synthesis of 26,26,26,27,27,27-hexafluoro-25-hydroxyvitamin D3. J. Chem. Soc. Chem. Commun. 1980, 459–460. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Taguchi, T.; Mitsuhashi, S.; Eguchi, T.; Ohshima, E.; Ikekawa, N. Studies on organic fluorine compounds. XXXIX. Studies on steroids. LXXIX. Synthesis of 1α,25-dihydroxy-26,26,26,27,27,27-hexafluorovitamin D3. Chem. Pharm. Bull. 1982, 30, 4297–4304. [Google Scholar] [CrossRef] [Green Version]
- Ohira, Y.; Taguchi, T.; Iseki, K.; Kobayashi, Y. Preparation of (22S)- and (22R)-24-homo-26, 26, 26, 27, 27, 27-hexafluoro-1,22,25-trihydroxy-24-yne-vitamin D3. Chem. Pharm. Bull. 1992, 40, 1647–1649. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Sabbe, K.; De Clercq, P.; Vandewalle, M.; Bouillon, R.; Verstuyf, A. Vitamin D3: Synthesis of seco C-9,11,21-trisnor-17-methyl-1α,25-dihydroxyvitamin D3 analogues. Bioorg. Med. Chem. Lett. 2002, 12, 1629–1632. [Google Scholar] [CrossRef]
- Sigüeiro, R.; Maestro, M.A.; Mouriño, A. Synthesis of side-chain locked analogs of 1α,25-dihydroxyvitamin D3 bearing a C17 methyl group. Org. Lett. 2018, 20, 2641–2644. [Google Scholar] [CrossRef]
- Kawagoe, F.; Sugiyama, T.; Uesugi, M.; Kittaka, A. Recent developments for introducing a hexafluoroisopropanol unit into the vitamin D side chain. J. Steroid Biochem. Mol. Biol. 2018, 177, 250–254. [Google Scholar] [CrossRef]
- Asano, L.; Watanabe, M.; Ryoden, Y.; Usuda, K.; Yamaguchi, T.; Khambu, B.; Takashima, M.; Sato, S.; Sakai, J.; Nagasawa, K.; et al. Vitamin D metabolite, 25-hydroxyvitamin D, regulates lipid metabolism by inducing degradation of SREBP/SCAP. Cell Chem. Biol. 2017, 24, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawagoe, F.; Mototani, S.; Kittaka, A. Design and Synthesis of Fluoro Analogues of Vitamin D. Int. J. Mol. Sci. 2021, 22, 8191. https://doi.org/10.3390/ijms22158191
Kawagoe F, Mototani S, Kittaka A. Design and Synthesis of Fluoro Analogues of Vitamin D. International Journal of Molecular Sciences. 2021; 22(15):8191. https://doi.org/10.3390/ijms22158191
Chicago/Turabian StyleKawagoe, Fumihiro, Sayuri Mototani, and Atsushi Kittaka. 2021. "Design and Synthesis of Fluoro Analogues of Vitamin D" International Journal of Molecular Sciences 22, no. 15: 8191. https://doi.org/10.3390/ijms22158191