Injectable Thixotropic β–Cyclodextrin–Functionalized Hydrogels Based on Guanosine Quartet Assembly
Abstract
:1. Introduction
2. Results and Discussion
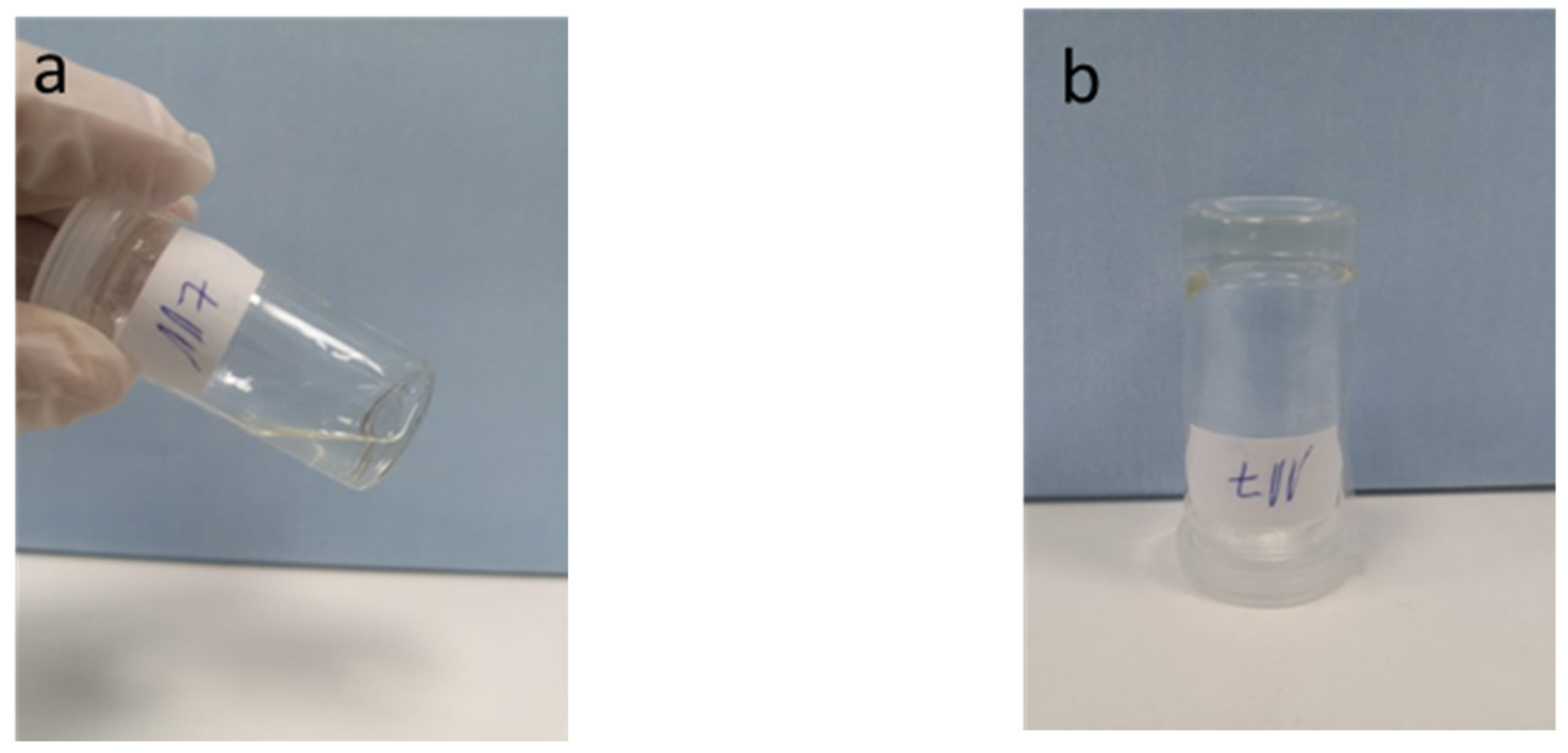
2.1. Synthesis of G4–CD Hydrogels
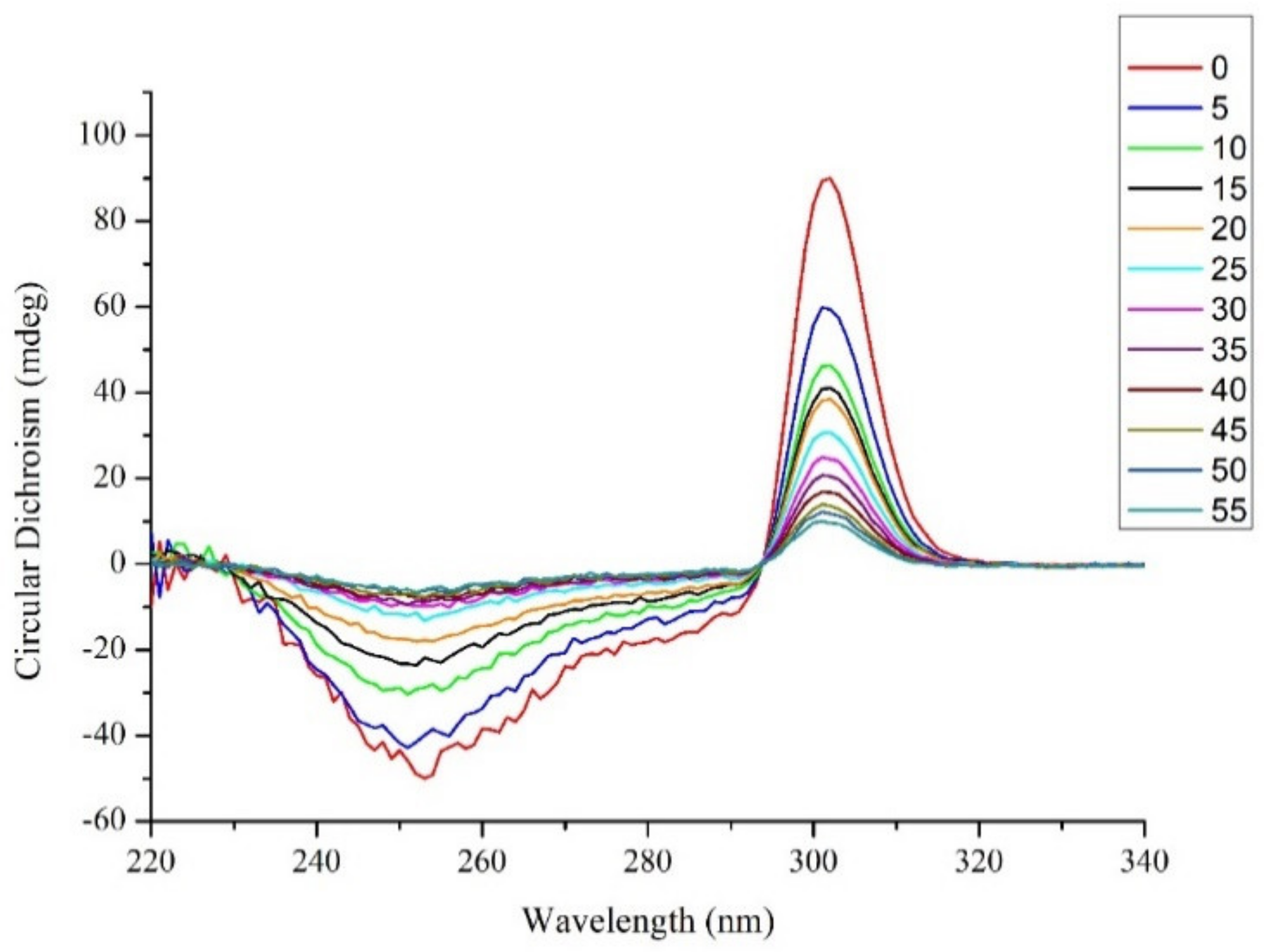
2.2. Circular Dichroism Investigations of G4–CD_1–7 Hydrogels
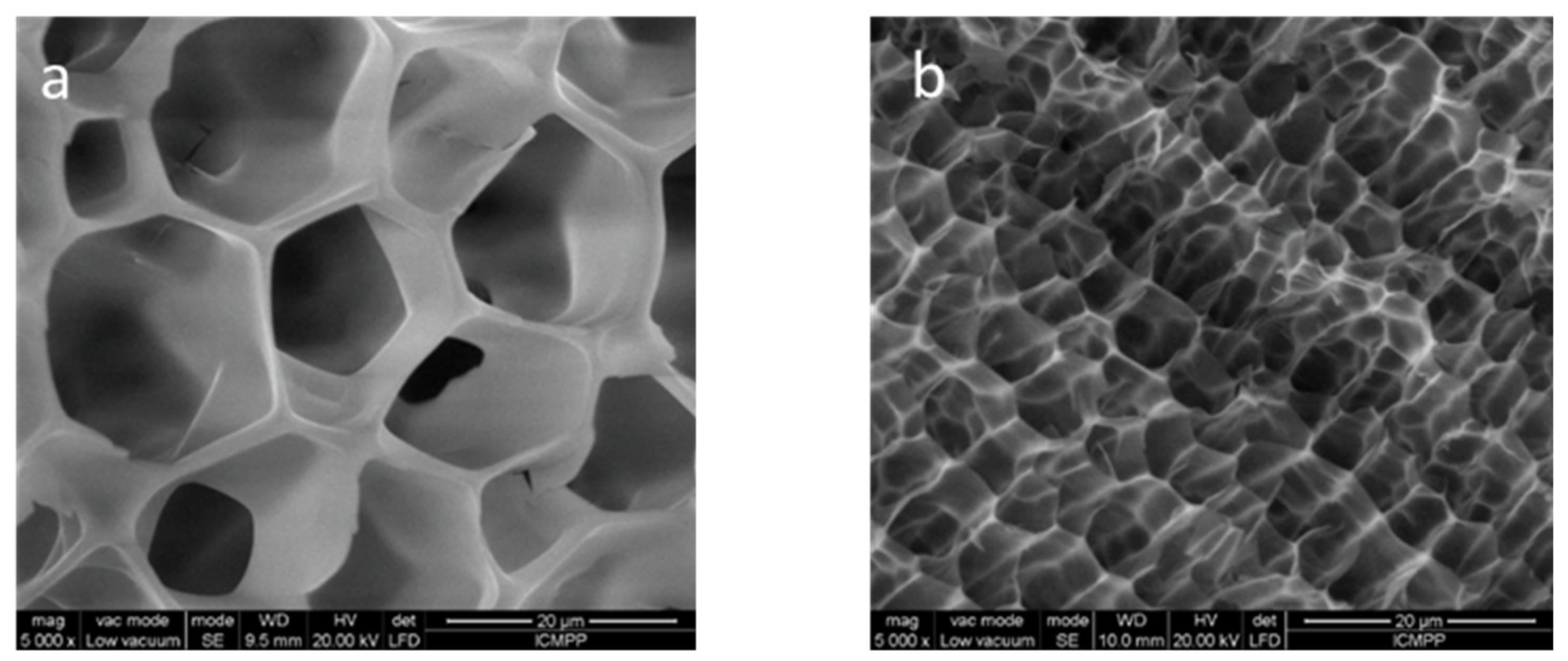
2.3. Scanning Electron Microscopy
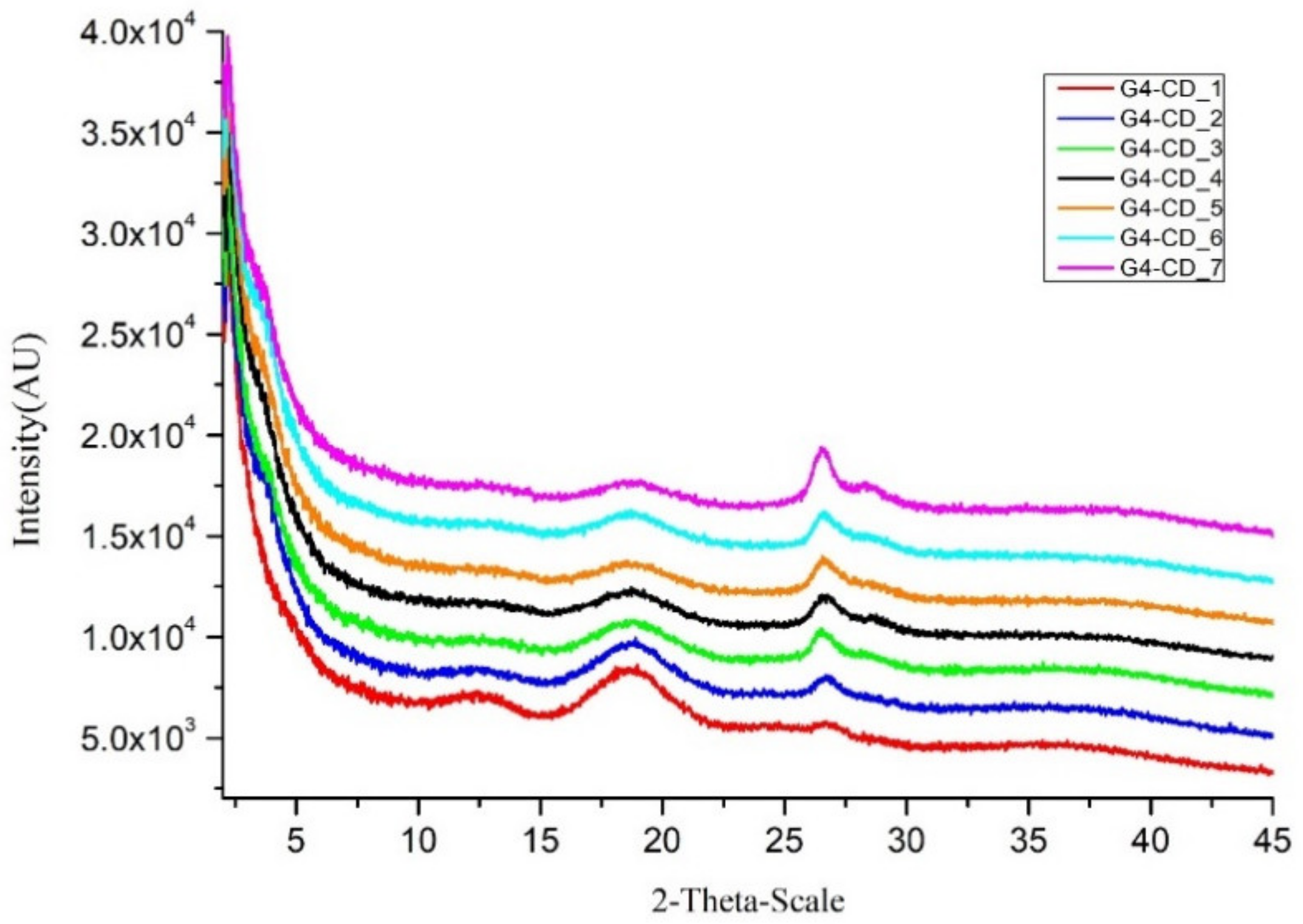
2.4. X–ray Diffraction
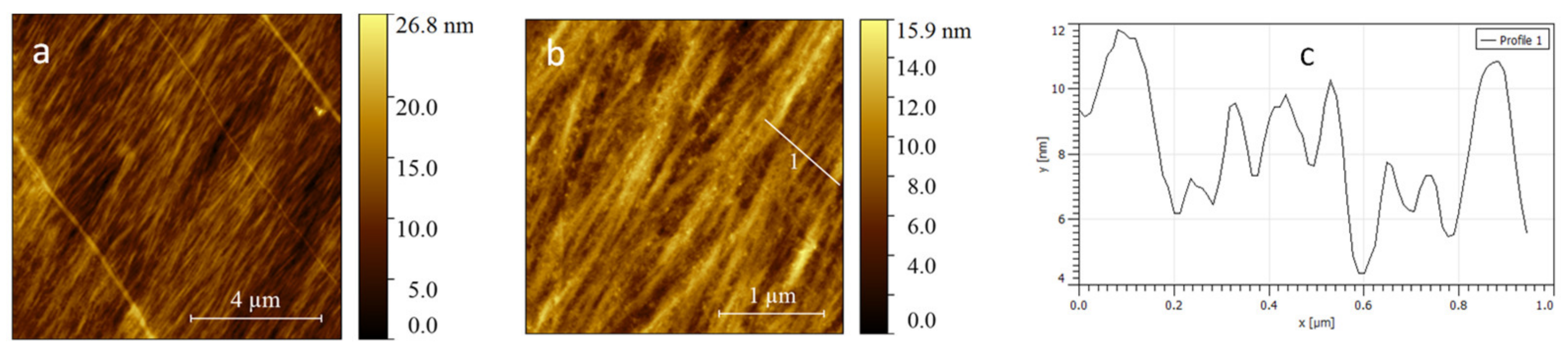
2.5. Atomic Force Microscopy
2.6. Rheology Investigations
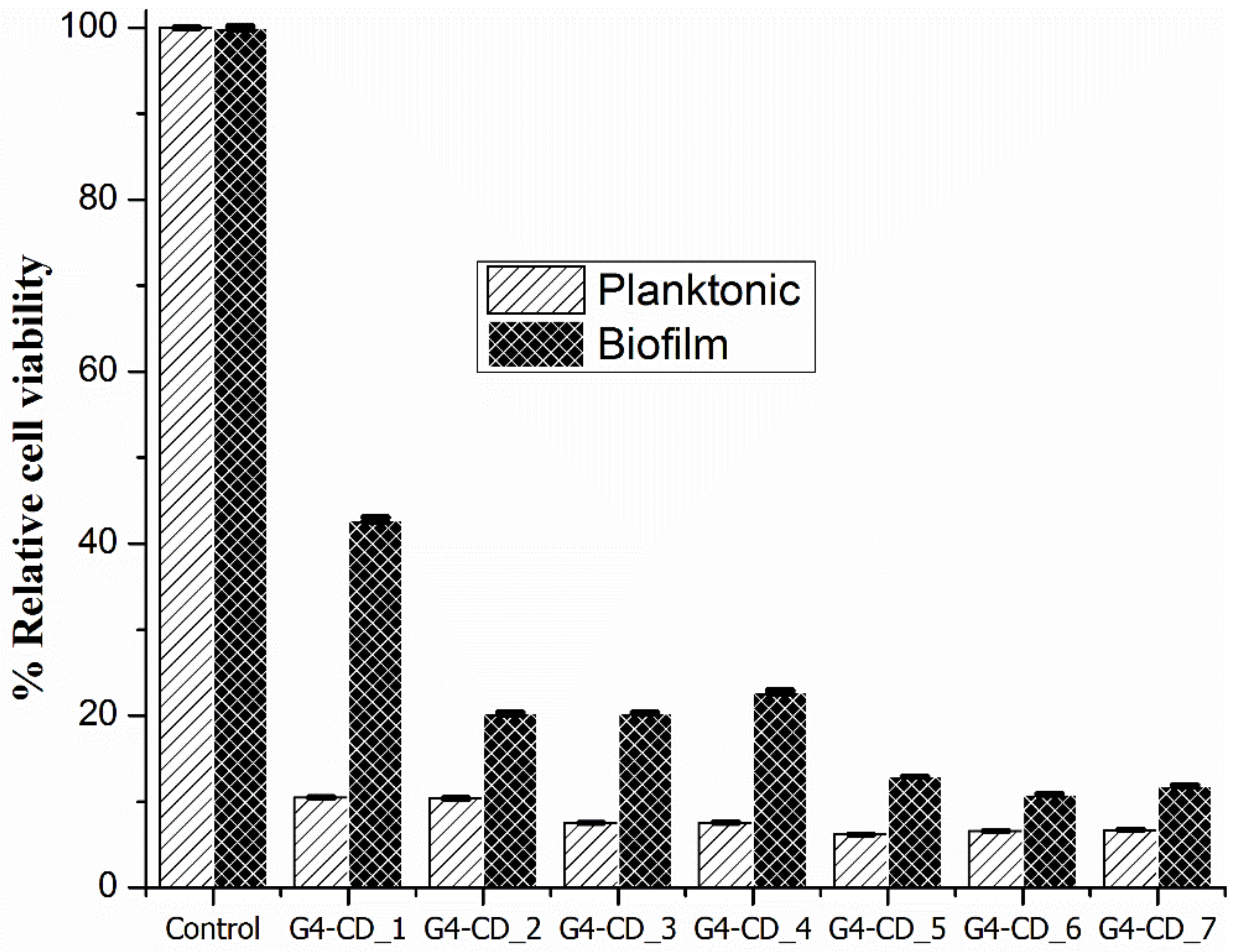
2.7. Antimicrobial Properties
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Guanosine–Based β–CD Hydrogels (G4–CD)
3.3. Characterizations
3.3.1. Surface Analyses
3.3.2. Circular Dichroism
3.3.3. X–ray Diffraction
3.3.4. Rheological Measurements of the Hydrogels
3.4. Antimicrobial Property of the Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding

Conflicts of Interest
References
- Seliktar, D. Designing Cell-Compatible Hydrogels for Biomedical Applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef]
- Correa, S.; Grosskopf, A.K.; Lopez Hernandez, H.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021. [Google Scholar] [CrossRef]
- Yu, L.; Ding, J. Injectable Hydrogels as Unique Biomedical Materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Zhang, L.; Wu, L.; Chen, Y.; Xie, D.; Chen, W. Hydrogel Cryopreservation System: An Effective Method for Cell Storage. Int. J. Mol. Sci. 2018, 19, 3330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muir, V.G.; Burdick, J.A. Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels. Chem. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for Protein Delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef] [PubMed]
- Jervis, P.J.; Amorim, C.; Pereira, T.; Martins, J.A.; Ferreira, P.M.T. Dehydropeptide Supramolecular Hydrogels and Nanostructures as Potential Peptidomimetic Biomedical Materials. Int. J. Mol. Sci. 2021, 22, 2528. [Google Scholar] [CrossRef]
- Domiński, A.; Konieczny, T.; Kurcok, P. α–Cyclodextrin–Based Polypseudorotaxane Hydrogels. Materials 2020, 13, 133. [Google Scholar] [CrossRef] [Green Version]
- Moore, A.N.; Hartgerink, J.D. Self-Assembling Multidomain Peptide Nanofibers for Delivery of Bioactive Molecules and Tissue Regeneration. Acc. Chem. Res. 2017, 50, 714–722. [Google Scholar] [CrossRef]
- Cai, L.; Liu, S.; Guo, J.; Jia, Y.-G. Polypeptide-Based Self-Healing Hydrogels: Design and Biomedical Applications. Acta Biomater. 2020, 113, 84–100. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Ji, H.; Qian, Y.; Wang, Q.; Liu, X.; Zhao, W.; Zhao, C. Heparin-Based and Heparin–Inspired Hydrogels: Size-Effect, Gelation and Biomedical Applications. J. Mater. Chem. B 2019, 7, 1186–1208. [Google Scholar] [CrossRef] [PubMed]
- Machín, R.; Isasi, J.R.; Vélaz, I. β–Cyclodextrin Hydrogels as Potential Drug Delivery Systems. Carbohydr. Polym. 2012, 87, 2024–2030. [Google Scholar] [CrossRef]
- Zhang, B.; He, J.; Shi, M.; Liang, Y.; Guo, B. Injectable Self-Healing Supramolecular Hydrogels with Conductivity and Photo–Thermal Antibacterial Activity to Enhance Complete Skin Regeneration. Chem. Eng. J. 2020, 400, 125994. [Google Scholar] [CrossRef]
- Loethen, S.; Kim, J.-M.; Thompson, D.H. Biomedical Applications of Cyclodextrin Based Polyrotaxanes. Polym. Rev. 2007, 47, 383–418. [Google Scholar] [CrossRef]
- Webber, M.J.; Appel, E.A.; Meijer, E.W.; Langer, R. Supramolecular Biomaterials. Nat. Mater. 2016, 15, 13–26. [Google Scholar] [CrossRef]
- Skilling, K.J.; Citossi, F.; Bradshaw, T.D.; Ashford, M.; Kellam, B.; Marlow, M. Insights into Low Molecular Mass Organic Gelators: A Focus on Drug Delivery and Tissue Engineering Applications. Soft Matter 2013, 10, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, S.; Guerin, T.; Toth, I.; Stephenson, R. Recent Advances in Self-Assembled Peptides: Implications for Targeted Drug Delivery and Vaccine Engineering. Adv. Drug Deliv. Rev. 2016, 110, 169–187. [Google Scholar] [CrossRef] [Green Version]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Rezaei Kolahchi, A.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of Hydrogel Biomaterials with Controlled Mechanical Properties for Tissue Engineering Applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef]
- Taylor, D.L.; Panhuis, M. In Het Self-Healing Hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Li, Q.; Yang, L.; Liu, H.; Yan, R.; Xiao, L.; Liu, H.; Wang, J.; Yang, B.; et al. Transparent Conductive Supramolecular Hydrogels with Stimuli–Responsive Properties for On-Demand Dissolvable Diabetic Foot Wound Dressings. Macromol. Rapid Commun. 2020, 41, 2000441. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Zhou, C.H.; Wang, J.; Tong, D.S.; Yu, W.H.; Wang, H. Recent Advances in Clay Mineral-Containing Nanocomposite Hydrogels. Soft Matter 2015, 11, 9229–9246. [Google Scholar] [CrossRef] [Green Version]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrin-Based Hydrogels toward Improved Wound Dressings. Crit. Rev. Biotechnol. 2014, 34, 328–337. [Google Scholar] [CrossRef]
- Pricope, G.; Ursu, E.L.; Sardaru, M.; Cojocaru, C.; Clima, L.; Marangoci, N.; Danac, R.; Mangalagiu, I.I.; Simionescu, B.C.; Pinteala, M.; et al. Novel Cyclodextrin-Based PH-Sensitive Supramolecular Host-Guest Assembly for Staining Acidic Cellular Organelles. Polym. Chem. 2018, 9, 968–975. [Google Scholar] [CrossRef]
- Marangoci, N.; Timpu, D.; Corciova, A.; Mircea, C.; Petrovici, A.-R.; Nicolescu, A.; Ursu, E.-L.; Nastasa, V.; Bostanaru, A.-C.; Mares, M.; et al. β–Cyclodextrin as a Functional Excipient Used for Enhancing the Diminazene Aceturate Bioavailability. Pharmaceutics 2019, 11, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorica, C.; Palumbo, F.S.; Pitarresi, G.; Puleio, R.; Condorelli, L.; Collura, G.; Giammona, G. A Hyaluronic Acid/Cyclodextrin Based Injectable Hydrogel for Local Doxorubicin Delivery to Solid Tumors. Int. J. Pharm. 2020, 589, 119879. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Keddie, D.J.; Kannappan, V.; Gibson, H.; Khalil, I.R.; Kowalczuk, M.; Martin, C.; Shuai, X.; Radecka, I. Production and Characterisation of Bacterial Cellulose Hydrogels Loaded with Curcumin Encapsulated in Cyclodextrins as Wound Dressings. Eur. Polym. J. 2019, 118, 437–450. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Z.; Liu, X.; Ni, X.; Li, J. Gelatin–Based Hydrogels with β–Cyclodextrin as a Dual Functional Component for Enhanced Drug Loading and Controlled Release. RSC Adv. 2013, 3, 25041–25049. [Google Scholar] [CrossRef]
- Rotaru, A.; Pricope, G.; Plank, T.N.; Clima, L.; Ursu, E.L.; Pinteala, M.; Davis, J.T.; Barboiu, M. G-Quartet Hydrogels for Effective Cell Growth Applications. Chem. Commun. 2017, 53, 12668–12671. [Google Scholar] [CrossRef]
- Ursu, E.-L.; Gavril, G.; Morariu, S.; Pinteala, M.; Barboiu, M.; Rotaru, A. Single-Walled Carbon Nanotubes-G-Quadruple Hydrogel Nanocomposite Matrixes for Cell Support Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110800. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.T. G–Quartets 40 Years Later: From 5′-GMP to Molecular Biology and Supramolecular Chemistry. Angew. Chem. Int. Ed. 2004, 43, 668–698. [Google Scholar] [CrossRef] [PubMed]
- Peters, G.M.; Skala, L.P.; Plank, T.N.; Hyman, B.J.; Manjunatha Reddy, G.N.; Marsh, A.; Brown, S.P.; Davis, J.T. A G4·K+ Hydrogel Stabilized by an Anion. J. Am. Chem. Soc. 2014, 136, 12596–12599. [Google Scholar] [CrossRef] [Green Version]
- Cafferty, B.J.; Gállego, I.; Chen, M.C.; Farley, K.I.; Eritja, R.; Hud, N.V. Efficient Self–Assembly in Water of Long Noncovalent Polymers by Nucleobase Analogues. J. Am. Chem. Soc. 2013, 135, 2447–2450. [Google Scholar] [CrossRef]
- Wenz, G.; Han, B.-H.; Müller, A. Cyclodextrin Rotaxanes and Polyrotaxanes. Chem. Rev. 2006, 106, 782–817. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tian, H. Bright Functional Rotaxanes. Chem. Soc. Rev. 2009, 39, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y. Cyclodextrin–Based Bioactive Supramolecular Assemblies. Chem. Soc. Rev. 2010, 39, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Sardaru, M.-C.; Carp, O.; Ursu, E.-L.; Craciun, A.-M.; Cojocaru, C.; Silion, M.; Kovalska, V.; Mangalagiu, I.; Danac, R.; Rotaru, A. Cyclodextrin Encapsulated PH Sensitive Dyes as Fluorescent Cellular Probes: Self–Aggregation and In Vitro Assessments. Molecules 2020, 25, 4397. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, A.; Chen, X.; Shen, X.Y.; Tong, L.; Hu, R.; Sun, J.Z.; Tang, B.Z. Specific Recognition of β–Cyclodextrin by a Tetraphenylethene Luminogen through a Cooperative Boronic Acid/Diol Interaction. Chem. Eur. J. 2011, 17, 14736–14740. [Google Scholar] [CrossRef]
- Nishiyabu, R.; Kubo, Y.; James, T.D.; Fossey, J.S. Boronic Acid Building Blocks: Tools for Sensing and Separation. Chem. Commun. 2011, 47, 1106–1123. [Google Scholar] [CrossRef]
- Yang, T.; Ji, R.; Deng, X.-X.; Du, F.-S.; Li, Z.-C. Glucose-Responsive Hydrogels Based on Dynamic Covalent Chemistry and Inclusion Complexation. Soft Matter 2014, 10, 2671–2678. [Google Scholar] [CrossRef]
- Houston, T.A. Developing High-Affinity Boron-Based Receptors for Cell–Surface Carbohydrates. ChemBioChem 2010, 11, 954–957. [Google Scholar] [CrossRef]
- Freeman, R.; Finder, T.; Bahshi, L.; Willner, I. β–Cyclodextrin-Modified CdSe/ZnS Quantum Dots for Sensing and Chiroselective Analysis. Nano Lett. 2009, 9, 2073–2076. [Google Scholar] [CrossRef]
- Kejnovská, I.; Renčiuk, D.; Palacký, J.; Vorlíčková, M. CD Study of the G-Quadruplex Conformation. Methods Mol. Biol. 2019, 2035, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Panda, M.; Walmsley, J.A. Circular Dichroism Study of Supramolecular Assemblies of Guanosine 5′-Monophosphate. J. Phys. Chem. B 2011, 115, 6377–6383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Largy, E.; Mergny, J.-L.; Gabelica, V. Role of Alkali Metal Ions in G-Quadruplex Nucleic Acid Structure and Stability. Met. Ions Life Sci. 2016, 16, 203–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, J.T.; Spada, G.P. Supramolecular Architectures Generated by Self-Assembly of Guanosine Derivatives. Chem. Soc. Rev. 2007, 36, 296–313. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospinning of Nanofibers from Non-Polymeric Systems: Electrospun Nanofibers from Native Cyclodextrins. J. Colloid Interface Sci. 2013, 404, 1–7. [Google Scholar] [CrossRef]
- Saenger, W.; Jacob, J.; Gessler, K.; Steiner, T.; Hoffmann, D.; Sanbe, H.; Koizumi, K.; Smith, S.M.; Takaha, T. Structures of the Common Cyclodextrins and Their Larger AnaloguesBeyond the Doughnut. Chem. Rev. 1998, 98, 1787–1802. [Google Scholar] [CrossRef]
- Shigemitsu, H.; Kida, T. Preparation of Nano- and Microstructures through Molecular Assembly of Cyclic Oligosaccharides. Polym. J. 2018, 50, 541–550. [Google Scholar] [CrossRef]
- Rusa, C.; Bullions, T.; Fox, J.; Porbeni, F.; Wang, X.; Tonelli, A. Inclusion Compound Formation with a New Columnar Cyclodextrin Host. Langmuir 2002, 18, 10016–10023. [Google Scholar] [CrossRef]
- Ortiz, M.; de Kee, D.; Carreau, P.J. Rheology of Concentrated Poly(Ethylene Oxide) Solutions. J. Rheol. 1994, 38, 519–539. [Google Scholar] [CrossRef]
- Yamamura, H.; Suzuki, K.; Uchibori, K.; Miyagawa, A.; Kawai, M.; Ohmizo, C.; Katsu, T. Mimicking an Antimicrobial Peptide Polymyxin B by Use of Cyclodextrin. Chem. Commun. 2011, 48, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Karginov, V.A.; Nestorovich, E.M.; Schmidtmann, F.; Robinson, T.M.; Yohannes, A.; Fahmi, N.E.; Bezrukov, S.M.; Hecht, S.M. Inhibition of S. Aureus α–Hemolysin and B. Anthracis Lethal Toxin by β–Cyclodextrin Derivatives. Bioorg. Med. Chem. 2007, 15, 5424–5431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athanassiou, G.; Michaleas, S.; Lada-Chitiroglou, E.; Tsitsa, T.; Antoniadou-Vyza, E. Antimicrobial activity of beta-lactam antibiotics against clinical pathogens after molecular inclusion in several cyclodextrins. A novel approach to bacterial resistance. J. Pharm. Pharmacol. 2003, 55, 291–300. [Google Scholar] [CrossRef]
- Bhargava, S.; Agrawel, G.P. Preparation & characterization of solid inclusion complex of cefpodoxime proxetil with beta-cyclodextrin. Curr. Drug Deliv. 2008, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska, M.; Mizera, M.; Szymanowska-Powałowska, D.; Lewandowska, K.; Błaszczak, W.; Goscianska, J.; Pietrzak, R.; Cielecka-Piontek, J. b-Cyclodextrin complexation as an effective drug delivery system for meropenem. Eur. J. Pharm. Biopharm. 2016, 99, 24–34. [Google Scholar] [CrossRef]
- Gwyddion 2.59. Available online: http://gwyddion.net/ (accessed on 28 June 2021).
- Bauer, A.W.; Perry, D.M.; Kirby, W.M.M. Single-Disk Antibiotic-Sensitivity Testing of Staphylococci: An Analysis of Technique and Results. Arch. Int. Med. 1959, 104, 208–216. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ; Image Processing and Analysis in Java, ImageJ version 1.52t. Available online: https://imagej.nih.gov/ij/ (accessed on 25 May 2021).
- Addinsoft. XLSTAT Statistical and Data Analysis Solution. 2021. Available online: https://www.xlstat.com (accessed on 25 May 2021).


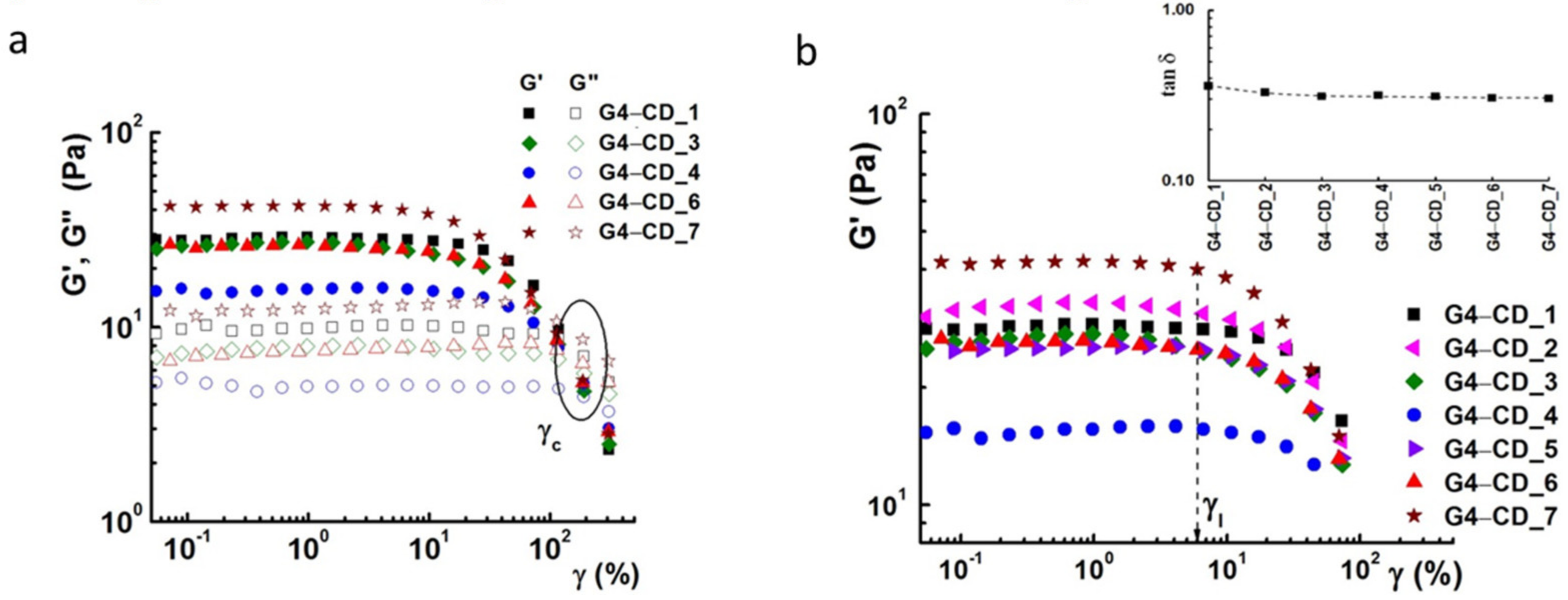

| Sample | G’ a(Pa) | G” a(Pa) | Tan δ a (=G”/G’) | η0 b | ηreg c (%) | Structure Recovery d (%) |
|---|---|---|---|---|---|---|
| G4–CD_1 | 28.3 | 10.2 | 0.36 | 87.58 ± 1.1 | 60.16 | 55.68 |
| G4–CD_2 | 29.8 | 9.8 | 0.33 | 63.27 ± 0.7 | 69.04 | 57.52 |
| G4–CD_3 | 20.8 | 6.38 | 0.32 | 82.15 ± 1.2 | 60.54 | 93.42 |
| G4–CD_4 | 15.6 | 5.02 | 0.31 | 55.53 ± 0.7 | 71.17 | 91.42 |
| G4–CD_5 | 25.4 | 7.94 | 0.31 | 98.45 ± 1.1 | 44.77 | 92.22 |
| G4–CD_6 | 25.3 | 7.73 | 0.31 | 74.06 ± 0.5 | 60.24 | 83.02 |
| G4–CD_7 | 41.6 | 12.6 | 0.31 | 217.36 ± 0.9 | 41.08 | 93.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardaru, M.-C.; Rosca, I.; Morariu, S.; Ursu, E.-L.; Ghiarasim, R.; Rotaru, A. Injectable Thixotropic β–Cyclodextrin–Functionalized Hydrogels Based on Guanosine Quartet Assembly. Int. J. Mol. Sci. 2021, 22, 9179. https://doi.org/10.3390/ijms22179179
Sardaru M-C, Rosca I, Morariu S, Ursu E-L, Ghiarasim R, Rotaru A. Injectable Thixotropic β–Cyclodextrin–Functionalized Hydrogels Based on Guanosine Quartet Assembly. International Journal of Molecular Sciences. 2021; 22(17):9179. https://doi.org/10.3390/ijms22179179
Chicago/Turabian StyleSardaru, Monica-Cornelia, Irina Rosca, Simona Morariu, Elena-Laura Ursu, Razvan Ghiarasim, and Alexandru Rotaru. 2021. "Injectable Thixotropic β–Cyclodextrin–Functionalized Hydrogels Based on Guanosine Quartet Assembly" International Journal of Molecular Sciences 22, no. 17: 9179. https://doi.org/10.3390/ijms22179179







