Stereo-Specific Modulation of the Extracellular Calcium-Sensing Receptor in Colon Cancer Cells
Abstract
:1. Introduction
2. Results
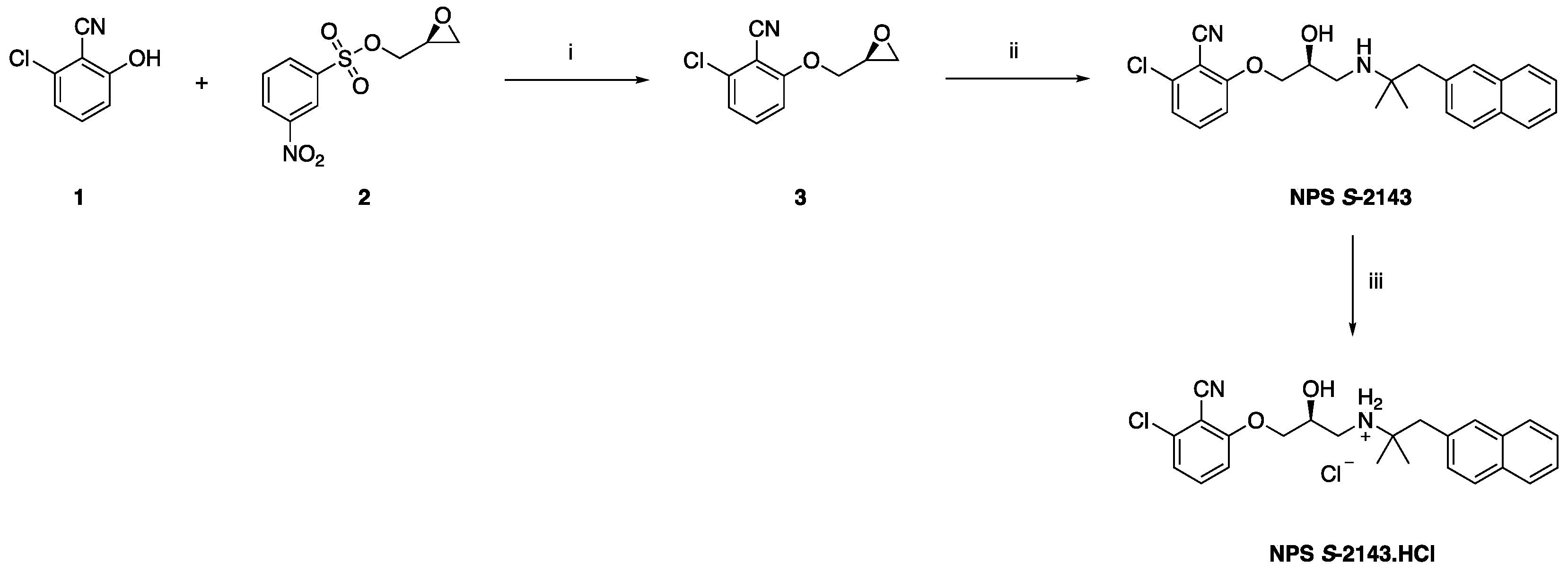
2.1. Synthetic Chemistry
2.2. Crystal Structure of NSP S-2143.HCl
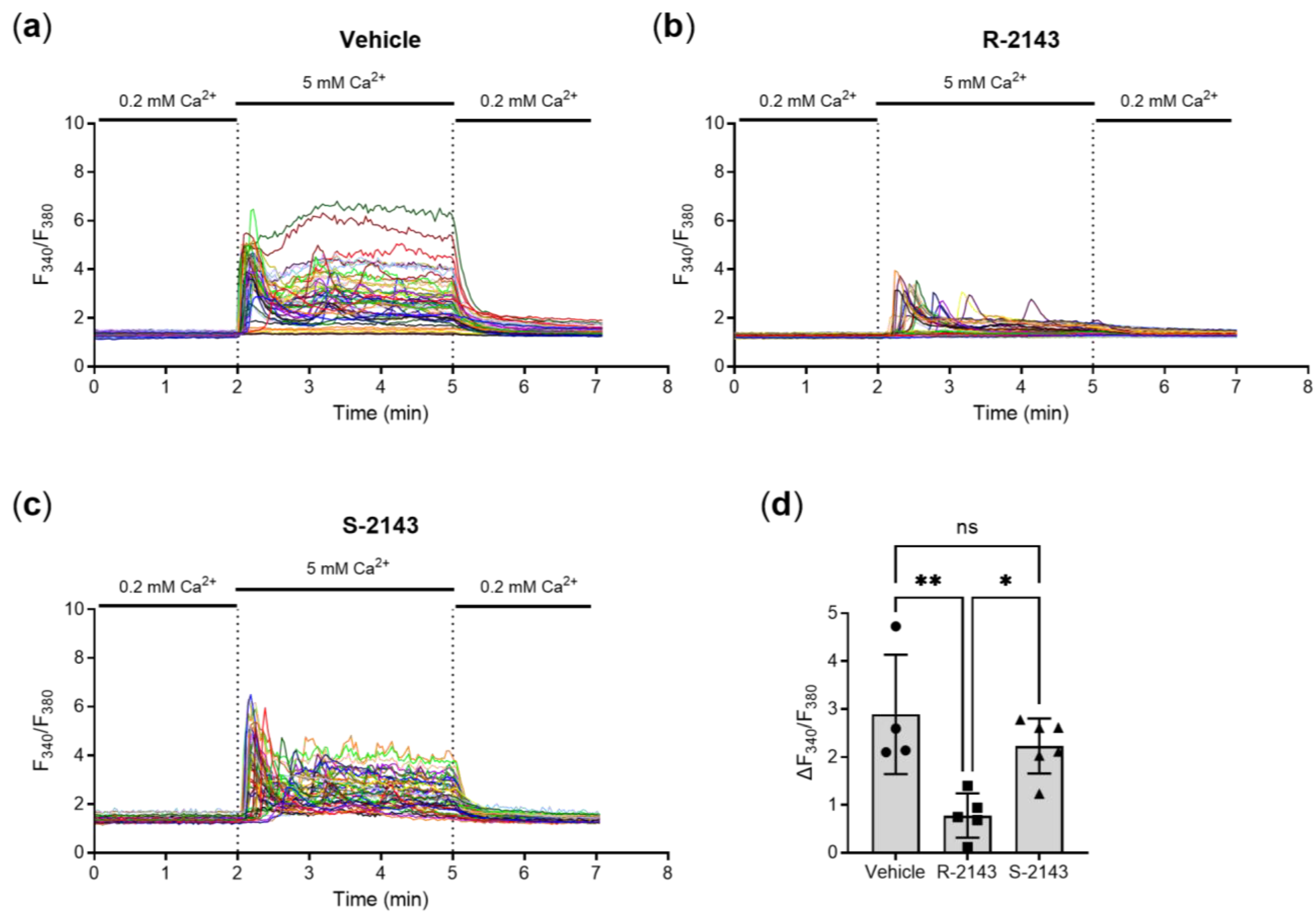
2.3. Enantiospecific Inhition of the CaSR via NPS 2143
2.4. CaSR Gene Induction and Pro-Inflammatory Responses in Colon Cancer Cells Are Mediated through the CaSR
3. Discussion
4. Materials and Methods
4.1. Preparation of NPS S-2143—Synthetic Chemistry
4.1.1. Synthesis of (S)-2-chloro-6-(oxiran-2-ylmethoxy)benzonitrile 3
4.1.2. Synthesis of (S)-2-chloro-6-(3-((2,2-dimethyl-3-(naphthalen-2-yl)propyl)amino)-2-hydroxypropoxy) benzonitrile (NPS S-2143)
4.1.3. Synthesis of (S)-2-chloro-6-(3-((2,2-dimethyl-3-(naphthalen-2-yl)propyl)amino)-2-hydroxypropoxy) benzonitrile hydrochloride (NPS S-2143.HCl)
4.2. Crystal Structure Determination
4.3. Other Compounds and Reagents
4.4. Calcium Imaging Experiments
4.5. Colon Cancer Cells
4.6. Compound Treatments
4.7. Reverse Transcription Real Time PCR (RT-qPCR)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, E.M.; Gamba, G.; Riccardi, D.; Lombardi, M.; Butters, R.; Kifor, O.; Sun, A.; Hediger, M.A.; Lytton, J.; Hebert, S.C. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 1993, 366, 575–580. [Google Scholar] [CrossRef]
- Brown, E.M. Role of the calcium-sensing receptor in extracellular calcium homeostasis. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Hannan, F.M.; Kallay, E.; Chang, W.; Brandi, M.L.; Thakker, R.V. The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases. Nat. Rev. Endocrinol. 2018, 15, 33–51. [Google Scholar] [CrossRef]
- Leach, K.; Hannan, F.M.; Josephs, T.M.; Keller, A.N.; Møller, T.C.; Ward, D.T.; Kallay, E.; Mason, R.S.; Thakker, R.V.; Riccardi, D.; et al. International Union of Basic and Clinical Pharmacology. CVIII. Calcium-Sensing Receptor Nomenclature, Pharmacology, and Function. Pharmacol. Rev. 2020, 72, 558–604. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.F.; Van Wagenen, B.C.; Balandrin, M.F. Discovery and Development of Calcimimetic and Calcilytic Compounds. Prog. Med. Chem. 2018, 57, 1–86. [Google Scholar] [CrossRef] [PubMed]
- Iamartino, L.; Elajnaf, T.; Kallay, E.; Schepelmann, M. Calcium-sensing receptor in colorectal inflammation and cancer: Current insights and future perspectives. World J. Gastroenterol. 2018, 24, 4119–4131. [Google Scholar] [CrossRef]
- Mine, Y.; Zhang, H. Anti-inflammatory Effects of Poly-l-lysine in Intestinal Mucosal System Mediated by Calcium-Sensing Receptor Activation. J. Agric. Food Chem. 2015, 63, 10437–10447. [Google Scholar] [CrossRef]
- Zhang, H.; Kovacs-Nolan, J.; Kodera, T.; Eto, Y.; Mine, Y. γ-Glutamyl cysteine and γ-glutamyl valine inhibit TNF-α signaling in intestinal epithelial cells and reduce inflammation in a mouse model of colitis via allosteric activation of the calcium-sensing receptor. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2015, 1852, 792–804. [Google Scholar] [CrossRef] [Green Version]
- Iamartino, L.; Elajnaf, T.; Gall, K.; David, J.; Manhardt, T.; Heffeter, P.; Grusch, M.; Derdak, S.; Baumgartner-Parzer, S.; Schepelmann, M.; et al. Effects of pharmacological calcimimetics on colorectal cancer cells over-expressing the human calcium-sensing receptor. Biochim. Biophys. Acta (BBA) Bioenerg. 2020, 1867, 118836. [Google Scholar] [CrossRef] [PubMed]
- Elajnaf, T.; Iamartino, L.; Mesteri, I.; Müller, C.; Bassetto, M.; Manhardt, T.; Baumgartner-Parzer, S.; Kallay, E.; Schepelmann, M. Nutritional and Pharmacological Targeting of the Calcium-Sensing Receptor Influences Chemically Induced Colitis in Mice. Nutrients 2019, 11, 3072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Block, G.A.; Bushinsky, D.A.; Cheng, S.; Cunningham, J.; Dehmel, B.; Drueke, T.B.; Ketteler, M.; KewalRamani, R.; Martin, K.J.; Moe, S.M.; et al. Effect of Etelcalcetide vs Cinacalcet on Serum Parathyroid Hormone in Patients Receiving Hemodialysis with Secondary Hyperparathyroidism: A Randomized Clinical Trial. JAMA 2017, 317, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.F.; Steffey, M.E.; Hammerland, L.G.; Hung, B.C.P.; Van Wagenen, B.C.; DelMar, E.G.; Balandrin, M.F. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc. Natl. Acad. Sci. USA 1998, 95, 4040–4045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquis, R.W.; Lago, A.M.; Callahan, J.F.; Trout, R.E.L.; Gowen, M.; DelMar, E.G.; Van Wagenen, B.C.; Logan, S.; Shimizu, S.; Fox, J.; et al. Antagonists of the Calcium Receptor I. Amino Alcohol-Based Parathyroid Hormone Secretagogues. J. Med. Chem. 2009, 52, 3982–3993. [Google Scholar] [CrossRef] [PubMed]
- Yarova, P.L.; Huang, P.; Schepelmann, M.W.; Bruce, R.; Ecker, R.; Nica, R.; Telezhkin, V.S.; Traini, D.; Dos Reis, L.G.; Kidd, E.J.; et al. Characterization of Negative Allosteric Modulators of the Calcium-Sensing Receptor for Repurposing as a Treatment of Asthma. J. Pharmacol. Exp. Ther. 2021, 376, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Cayzac, S.H.; Rocher, A.; Obeso, A.; Gonzalez, C.; Riccardi, D.; Kemp, P. Spermine attenuates carotid body glomus cell oxygen sensing by inhibiting L-type Ca2+ channels. Respir. Physiol. Neurobiol. 2011, 175, 80–89. [Google Scholar] [CrossRef]
- Finney, B.A.; Del Moral, P.M.; Wilkinson, W.J.; Cayzac, S.; Cole, M.; Warburton, D.; Kemp, P.J.; Riccardi, D. Regulation of mouse lung development by the extracellular calcium-sensing receptor, CaR. J. Physiol. 2008, 586, 6007–6019. [Google Scholar] [CrossRef]
- Grant, M.P.; Stepanchick, A.; Cavanaugh, A.; Breitwieser, G.E. Agonist-Driven Maturation and Plasma Membrane Insertion of Calcium-Sensing Receptors Dynamically Control Signal Amplitude. Sci. Signal. 2011, 4, ra78. [Google Scholar] [CrossRef]
- Subramanian, S.; Rhodes, J.M.; Hart, A.C.; Tam, B.; Roberts, C.L.; Smith, S.L.; Corkill, J.E.; Winstanley, C.; Virji, M.; Campbell, B.J. Characterization of epithelial IL-8 response to inflammatory bowel disease mucosal E. coli and its inhibition by mesalamine. Inflamm. Bowel Dis. 2008, 14, 162–175. [Google Scholar] [CrossRef]
- Martin-Viñas, J.J.; Quigley, E.M.M. Immune response in irritable bowel syndrome: A systematic review of systemic and mucosal inflammatory mediators. J. Dig. Dis. 2016, 17, 572–581. [Google Scholar] [CrossRef]
- Pedersen, G. Development, validation and implementation of an in vitro model for the study of metabolic and immune func-tion in normal and inflamed human colonic epithelium. Dan Med. J. 2015, 62, B4973. [Google Scholar]
- Yarova, P.L.; Stewart, A.L.; Sathish, V.; Britt, R.D., Jr.; Thompson, M.A.; Lowe, A.; Freeman, M.; Aravamudan, B.; Kita, H.; Brennan, S.; et al. Calcium-sensing receptor antagonists abrogate airway hyperresponsiveness and inflammation in allergic asthma. Sci. Transl. Med. 2015, 7, 284ra60. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, E. Calcimimetic and calcilytic drugs: Just for parathyroid cells? Cell Calcium 2004, 35, 283–289. [Google Scholar] [CrossRef]
- Cheng, S.X.; Okuda, M.; Hall, A.E.; Geibel, J.P.; Hebert, S.C. Expression of calcium-sensing receptor in rat colonic epithelium: Evidence for modulation of fluid secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G240–G250. [Google Scholar] [CrossRef] [PubMed]
- Geibel, J.; Sritharan, K.; Geibel, R.; Geibel, P.; Persing, J.S.; Seeger, A.; Roepke, T.K.; Deichstetter, M.; Prinz, C.; Cheng, S.X.; et al. Calcium-sensing receptor abrogates secretagogue- induced increases in intestinal net fluid secretion by enhancing cyclic nucleotide destruction. Proc. Natl. Acad. Sci. USA 2006, 103, 9390–9397. [Google Scholar] [CrossRef] [Green Version]
- MacLeod, R.J. Extracellular calcium-sensing receptor/PTH knockout mice colons have increased Wnt/β-catenin signaling, reduced non-canonical Wnt signaling, and increased susceptibility to azoxymethane-induced aberrant crypt foci. Lab. Investig. 2013, 93, 520–527. [Google Scholar] [CrossRef]
- Cheng, S.X.; Lightfoot, Y.L.; Yang, T.; Zadeh, M.; Tang, L.; Sahay, B.; Wang, G.P.; Owen, J.L.; Mohamadzadeh, M. Epithelial CaSR deficiency alters intestinal integrity and promotes proinflammatory immune responses. FEBS Lett. 2014, 588, 4158–4166. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Qiu, L.; Song, H.; Dang, N. NPS-2143 (hydrochloride) inhibits melanoma cancer cell proliferation and induces autophagy and apoptosis. Méd. Sci. 2018, 34, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, E.F.; Goodman, W.G. Calcimimetic and Calcilytic Drugs: Feats, Flops, and Futures. Calcif. Tissue Int. 2016, 98, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement withSHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Ward, D.T.; Mughal, M.Z.; Ranieri, M.; Dvorak-Ewell, M.M.; Valenti, G.; Riccardi, D. Molecular and clinical analysis of a neonatal severe hyperparathyroidism case caused by a stop mutation in the calcium-sensing receptor extracellular domain representing in effect a human ‘knockout’. Eur. J. Endocrinol. 2013, 169, K1–K7. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schepelmann, M.; Kupper, N.; Sladczyk, M.; Mansfield, B.; Manhardt, T.; Piatek, K.; Iamartino, L.; Riccardi, D.; Kariuki, B.M.; Bassetto, M.; et al. Stereo-Specific Modulation of the Extracellular Calcium-Sensing Receptor in Colon Cancer Cells. Int. J. Mol. Sci. 2021, 22, 10124. https://doi.org/10.3390/ijms221810124
Schepelmann M, Kupper N, Sladczyk M, Mansfield B, Manhardt T, Piatek K, Iamartino L, Riccardi D, Kariuki BM, Bassetto M, et al. Stereo-Specific Modulation of the Extracellular Calcium-Sensing Receptor in Colon Cancer Cells. International Journal of Molecular Sciences. 2021; 22(18):10124. https://doi.org/10.3390/ijms221810124
Chicago/Turabian StyleSchepelmann, Martin, Nadja Kupper, Marta Sladczyk, Bethan Mansfield, Teresa Manhardt, Karina Piatek, Luca Iamartino, Daniela Riccardi, Benson M. Kariuki, Marcella Bassetto, and et al. 2021. "Stereo-Specific Modulation of the Extracellular Calcium-Sensing Receptor in Colon Cancer Cells" International Journal of Molecular Sciences 22, no. 18: 10124. https://doi.org/10.3390/ijms221810124





