Therapeutic Effect of an Antibody-Derived Peptide in a Galleria mellonella Model of Systemic Candidiasis
Abstract
:1. Introduction
2. Results
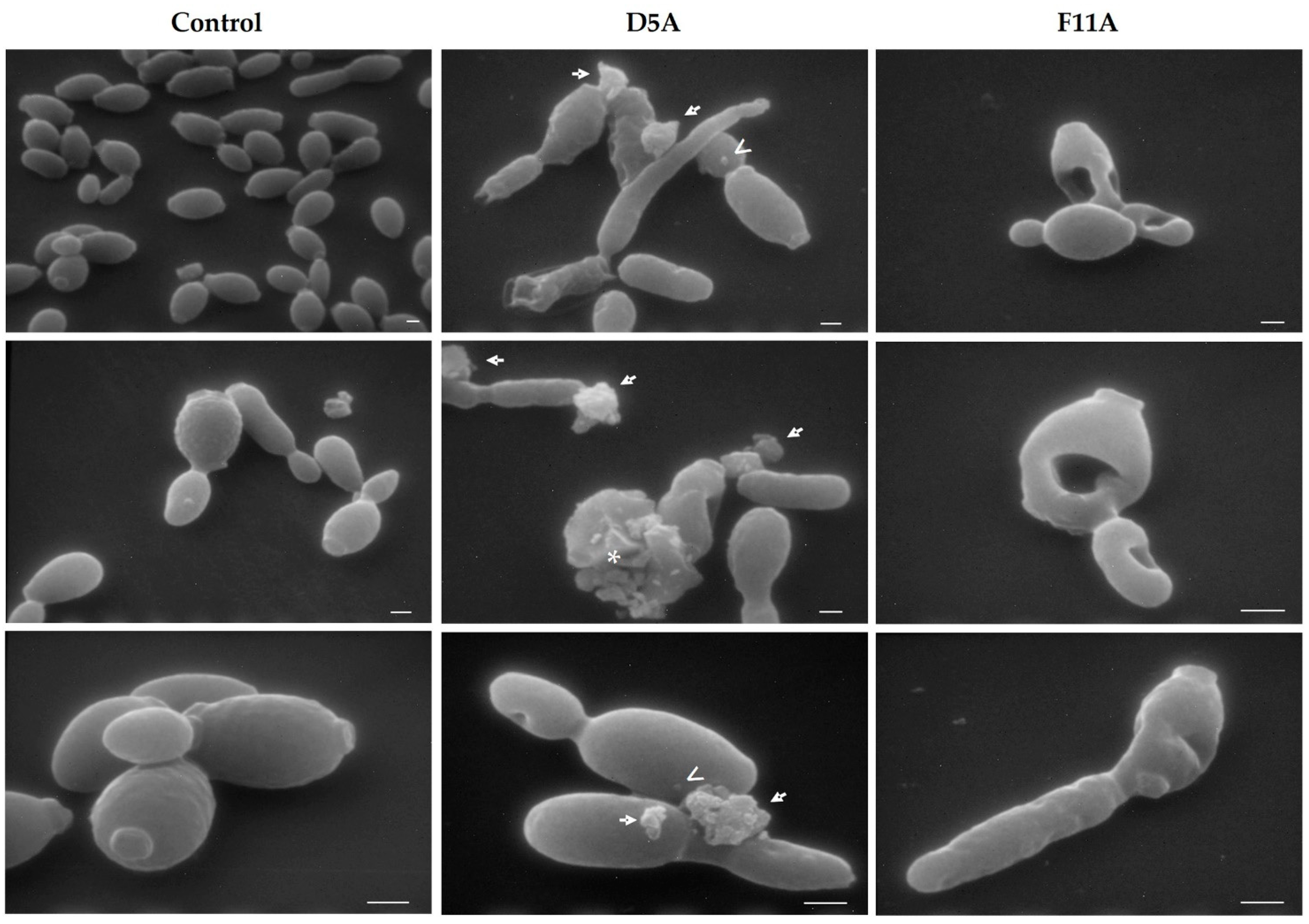
2.1. Visualisation of Peptides’ Direct Antifungal Effects on C. albicans Cells by Scanning Electron Microscopy (SEM)
2.2. In Vivo Toxicity and Therapeutic Activity of the Investigated Peptides
2.3. Host Immunomodulation by Investigated Peptides
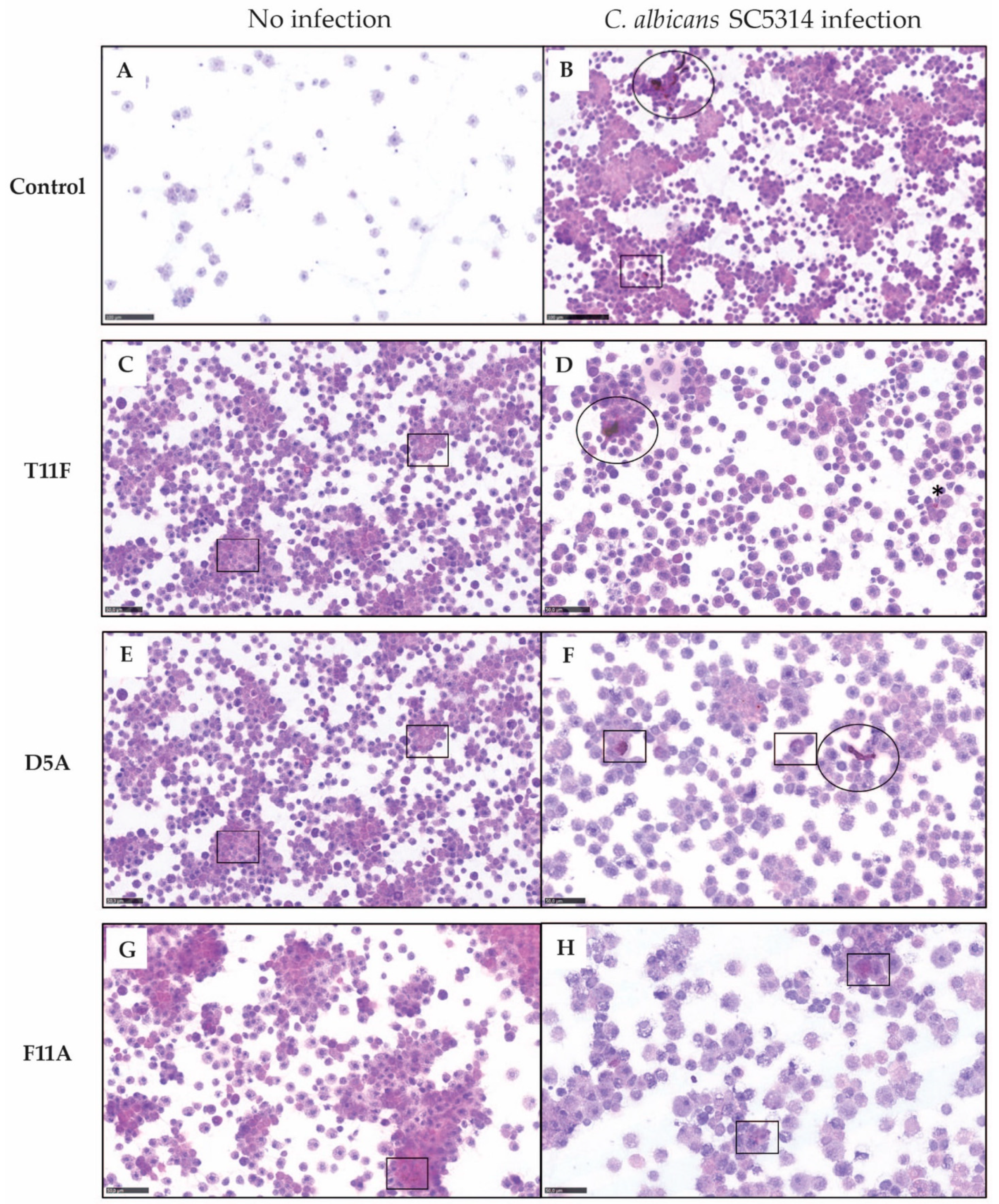
2.3.1. Hemocyte Analysis
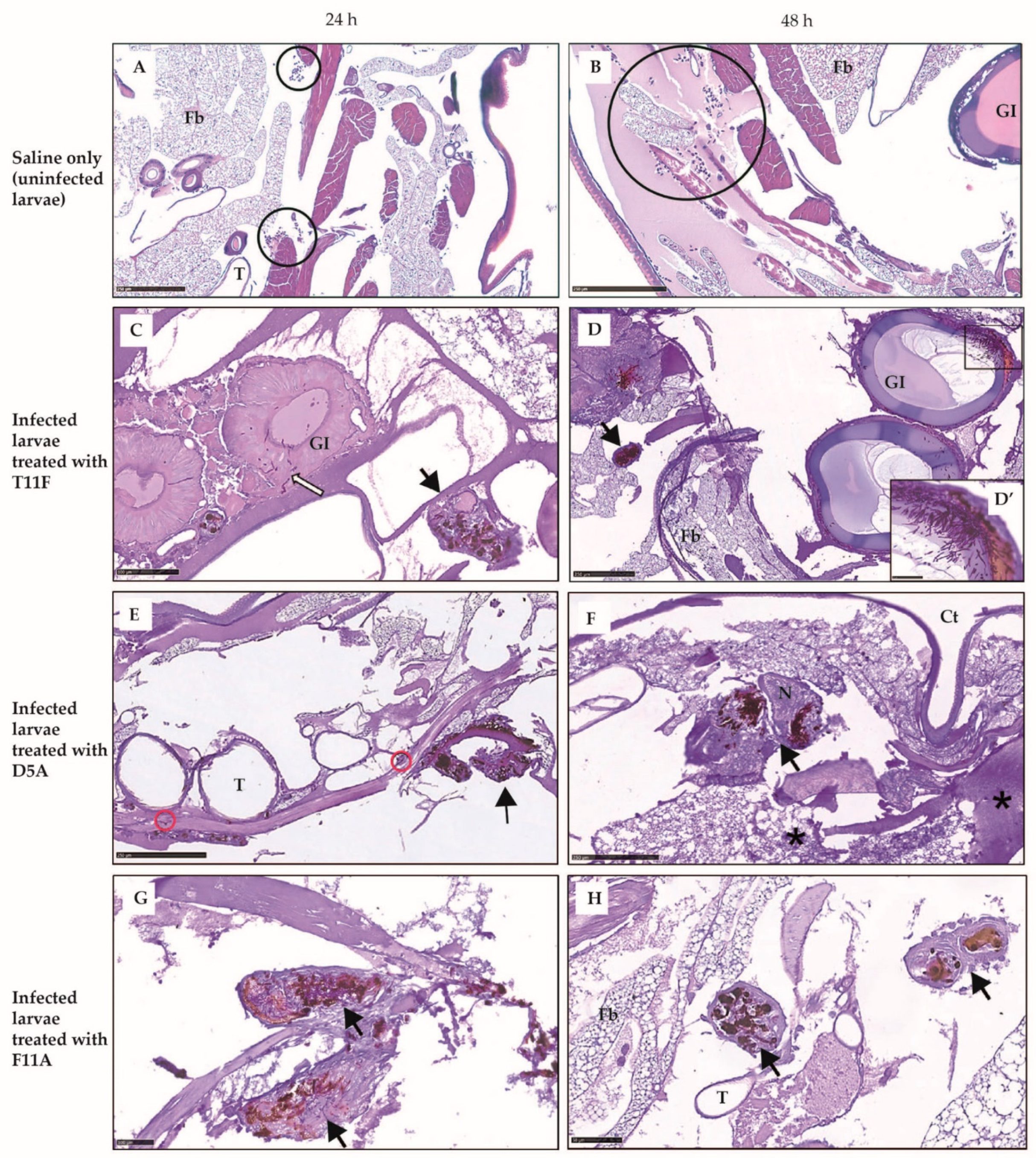
2.3.2. Larval Histology
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis
4.2. SEM Studies
4.3. Evaluation of In Vivo Toxicity and Therapeutic Activity of the Investigated Peptides
4.4. Evaluation of In Vivo Immunomodulatory Activity of the Investigated Peptides
4.4.1. Hemocyte Analysis
4.4.2. Larval Histology
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi. 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.; Rodrigues, M.L.; Coelho, C. The Still Underestimated Problem of Fungal Diseases Worldwide. Front. Microbiol. 2019, 10, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats Posed by the Fungal Kingdom to Humans, Wildlife, and Agriculture. mBio 2020, 11, e00449–e00520. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Bromley, M.J. How to bolster the antifungal pipeline. Science 2015, 347, 1414–1416. [Google Scholar] [CrossRef] [Green Version]
- Chaves, A.F.A.; Xander, P.; Romera, L.M.D.M.; Fonseca, F.L.A.; Batista, W.L. What is the elephant in the room when considering new therapies for fungal diseases? Crit. Rev. Microbiol. 2021, 47, 275–289. [Google Scholar] [CrossRef]
- Robbins, N.; Wright, G.D.; Cowen, L.E. Antifungal Drugs: The Current Armamentarium and Development of New Agents. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Rauseo, A.M.; Coler-Reilly, A.; Larson, L.; Spec, A. Hope on the Horizon: Novel Fungal Treatments in Development. Open Forum Infect. Dis. 2020, 7, ofaa016. [Google Scholar] [CrossRef] [Green Version]
- Mota Fernandes, C.; Dasilva, D.; Haranahalli, K.; McCarthy, J.B.; Mallamo, J.; Ojima, I.; Del Poeta, M. The Future of Antifungal Drug Therapy: Novel Compounds and Targets. Antimicrob. Agents Chemother. 2021, 65, e01719–e01720. [Google Scholar] [CrossRef]
- Rossato, L.; Camargo dos Santos, M.; Vitale, R.G.; de Hoog, S.; Ishida, K. Alternative treatment of fungal infections: Synergy with non-antifungal agents. Mycoses 2021, 64, 232–244. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, F.; Zeng, M.; Mao, Y.; Song, Z. Drug repurposing strategies in the development of potential antifungal agents. Appl. Microbiol. Biotechnol. 2021, 105, 5259–5279. [Google Scholar] [CrossRef]
- Li, D.; She, X.; Calderone, R. The antifungal pipeline: The need is established. Are there new compounds? FEMS Yeast Res. 2020, 20, foaa023. [Google Scholar] [CrossRef]
- Johnson, M.D. Antifungals in Clinical Use and the Pipeline. Infect. Dis. Clin. N. Am. 2021, 35, 341–371. [Google Scholar] [CrossRef]
- Waterer, G. Advances in anti-fungal therapies. Mycopathologia 2021, 1–8. [Google Scholar] [CrossRef]
- Basso, V.; Tran, D.Q.; Ouellette, A.J.; Selsted, M.E. Host Defense Peptides as Templates for Antifungal Drug Development. J. Fungi 2020, 6, 241. [Google Scholar] [CrossRef]
- Mithoor, D.; Madhu, K.M.; Dhanasekhar, R.; Ranjith, K.; Preetam, G.; Vasco, A.; Debmalya, B. Clinical Applications of Antimicrobial Peptides (AMPs): Where do we Stand Now? Protein Pept. Lett. 2020, 27, 120–134. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Beuerman, R.W.; Dua, H.S.; Lakshminarayanan, R.; Mohammed, I. Strategies in Translating the Therapeutic Potentials of Host Defense Peptides. Front. Immunol. 2020, 11, 983. [Google Scholar] [CrossRef] [PubMed]
- Pachón-Ibáñez, M.E.; Smani, Y.; Pachón, J.; Sánchez-Céspedes, J. Perspectives for clinical use of engineered human host defense antimicrobial peptides. FEMS Microbiol. Rev. 2017, 41, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Buda De Cesare, G.; Cristy, S.A.; Garsin, D.A.; Lorenz, M.C. Antimicrobial Peptides: A New Frontier in Antifungal Therapy. mBio 2020, 11, e02123–e02220. [Google Scholar] [CrossRef]
- Deepika, S.; Gopal Singh, B. Recent Updates on Antifungal Peptides. Mini-Rev. Med. Chem. 2020, 20, 260–268. [Google Scholar] [CrossRef]
- Fernández de Ullivarri, M.; Arbulu, S.; Garcia-Gutierrez, E.; Cotter, P.D. Antifungal Peptides as Therapeutic Agents. Front. Cell. Infect. Microbiol. 2020, 10, 105. [Google Scholar] [CrossRef]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef]
- Li, T.; Li, L.; Du, F.; Sun, L.; Shi, J.; Long, M.; Chen, Z. Activity and Mechanism of Action of Antifungal Peptides from Microorganisms: A Review. Molecules 2021, 26, 3438. [Google Scholar] [CrossRef]
- Ryu, M.; Park, J.; Yeom, J.-H.; Joo, M.; Lee, K. Rediscovery of antimicrobial peptides as therapeutic agents. J. Microbiol. 2021, 59, 113–123. [Google Scholar] [CrossRef]
- Bobek, L.A.; Situ, H. MUC7 20-Mer: Investigation of antimicrobial activity, secondary structure, and possible mechanism of antifungal action. Antimicrob. Agents Chemother. 2003, 47, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Liepke, C.; Baxmann, S.; Heine, C.; Breithaupt, N.; Ständker, L.; Forssmann, W.-G. Human hemoglobin-derived peptides exhibit antimicrobial activity: A class of host defense peptides. J. Chromatogr. B 2003, 791, 345–356. [Google Scholar] [CrossRef]
- Den Hertog, A.L.; van Marle, J.; van Veen, H.A.; Van’t Hof, W.; Bolscher, J.G.; Veerman, E.C.; Nieuw Amerongen, A.V. Candidacidal effects of two antimicrobial peptides: Histatin 5 causes small membrane defects, but LL-37 causes massive disruption of the cell membrane. Biochem. J. 2005, 388, 689–695. [Google Scholar] [CrossRef] [Green Version]
- Viejo-Diaz, M.; Andres, M.T.; Fierro, J.F. Different anti-Candida activities of two human lactoferrin-derived peptides, Lfpep and kaliocin-1. Antimicrob. Agents Chemother. 2005, 49, 2583–2588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lis, M.; Liu, T.T.; Barker, K.S.; Rogers, P.D.; Bobek, L.A. Antimicrobial peptide MUC7 12-mer activates the calcium/calcineurin pathway in Candida albicans. FEMS Yeast Res. 2010, 10, 579–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puri, S.; Edgerton, M. How does it kill? Understanding the candidacidal mechanism of salivary Histatin 5. Eukaryot. Cell 2014, 13, 958–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarsini, M.; Tomasinsig, L.; Arzese, A.; D’Este, F.; Oro, D.; Skerlavaj, B. Antifungal activity of cathelicidin peptides against planktonic and biofilm cultures of Candida species isolated from vaginal infections. Peptides 2015, 71, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Ciociola, T.; Zanello, P.P.; D’Adda, T.; Galati, S.; Conti, S.; Magliani, W.; Giovati, L. A peptide found in human serum, derived from the C-terminus of albumin, shows antifungal activity in vitro and in vivo. Microorganisms 2020, 8, 1627. [Google Scholar] [CrossRef] [PubMed]
- Van der Weerden, N.L.; Bleackley, M.R.; Anderson, M.A. Properties and mechanisms of action of naturally occurring antifungal peptides. Cell Mol. Life Sci. 2013, 70, 3545–3570. [Google Scholar] [CrossRef] [PubMed]
- Ciociola, T.; Giovati, L.; Conti, S.; Magliani, W.; Santinoli, C.; Polonelli, L. Natural and synthetic peptides with antifungal activity. Future Med. Chem. 2016, 8, 1413–1433. [Google Scholar] [CrossRef] [PubMed]
- Magliani, W.; Giovati, L.; Ciociola, T.; Sperindè, M.; Santinoli, C.; Conti, G.; Conti, S.; Polonelli, L. Antibodies as a source of anti-infective peptides: An update. Future Microbiol. 2015, 10, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Ciociola, T.; Giovati, L.; Conti, S.; Magliani, W. Anti-Infective Antibody-Derived Peptides Active against Endogenous and Exogenous Fungi. Microorganisms 2021, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Polonelli, L.; Ciociola, T.; Magliani, W.; Zanello, P.P.; D’Adda, T.; Galati, S.; De Bernardis, F.; Arancia, S.; Gabrielli, E.; Pericolini, E.; et al. Peptides of the constant region of antibodies display fungicidal activity. PLoS ONE 2012, 7, e34105. [Google Scholar] [CrossRef] [Green Version]
- Pertinhez, T.A.; Ciociola, T.; Giovati, L.; Magliani, W.; Belletti, S.; Polonelli, L.; Conti, S.; Spisni, A. Dissection of the Structural Features of a Fungicidal Antibody-Derived Peptide. Int. J. Mol. Sci. 2018, 19, 3792. [Google Scholar] [CrossRef] [Green Version]
- Moreno-García, M.; Condé, R.; Bello-Bedoy, R.; Lanz-Mendoza, H. The damage threshold hypothesis and the immune strategies of insects. Infect. Genet. Evol. 2014, 24, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Desalermos, A.; Fuchs, B.B.; Mylonakis, E. Selecting an Invertebrate Model Host for the Study of Fungal Pathogenesis. PLOS Pathog. 2012, 8, e1002451. [Google Scholar] [CrossRef] [Green Version]
- Pereira, T.C.; de Barros, P.P.; Fugisaki, L.R.O.; Rossoni, R.D.; Ribeiro, F.C.; de Menezes, R.T.; Junqueira, J.C.; Scorzoni, L. Recent Advances in the Use of Galleria mellonella Model to Study Immune Responses against Human Pathogens. J. Fungi. 2018, 4, 128. [Google Scholar] [CrossRef] [Green Version]
- Nicola, A.M.; Albuquerque, P.; Paes, H.C.; Fernandes, L.; Costa, F.F.; Kioshima, E.S.; Abadio, A.K.R.; Bocca, A.L.; Felipe, M.S. Antifungal drugs: New insights in research & development. Pharmacol. Ther. 2019, 195, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Romani, L.; Puccetti, P. Controlling pathogenic inflammation to fungi. Expert Rev. Anti-Infect. Ther. 2007, 5, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Borghi, E.; Falleni, M.; Perdoni, F.; Tosi, D.; Lappin, D.F.; O’Donnell, L.; Greetham, D.; Ramage, G.; Nile, C. Acetylcholine Protects against Candida albicans Infection by Inhibiting Biofilm Formation and Promoting Hemocyte Function in a Galleria mellonella Infection Model. Eukaryot. Cell 2015, 14, 834–844. [Google Scholar] [CrossRef] [Green Version]
- Simitsopoulou, M.; Roilides, E.; Walsh, T.J. Immunomodulatory Properties of Antifungal Agents on Phagocytic Cells. Immunol. Investig. 2011, 40, 809–824. [Google Scholar] [CrossRef]
- Di Mambro, T.; Guerriero, I.; Aurisicchio, L.; Magnani, M.; Marra, E. The Yin and Yang of Current Antifungal Therapeutic Strategies: How Can We Harness Our Natural Defenses? Front. Pharmacol. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercer, D.K.; O’Neil, D.A. Innate Inspiration: Antifungal Peptides and Other Immunotherapeutics from the Host Immune Response. Front. Immunol. 2020, 11, 2177. [Google Scholar] [CrossRef]
- Auvynet, C.; Rosenstein, Y. Multifunctional host defense peptides: Antimicrobial peptides, the small yet big players in innate and adaptive immunity. FEBS J. 2009, 276, 6497–6508. [Google Scholar] [CrossRef]
- Perdoni, F.; Falleni, M.; Tosi, D.; Cirasola, D.; Romagnoli, S.; Braidotti, P.; Clementi, E.; Bulfamante, G.; Borghi, E. A histological procedure to study fungal infection in the wax moth Galleria mellonella. Eur. J. Histochem. 2014, 58, 2428. [Google Scholar] [CrossRef] [Green Version]
- Stączek, S.; Zdybicka-Barabas, A.; Wiater, A.; Pleszczyńska, M.; Cytryńska, M. Activation of cellular immune response in insect model host Galleria mellonella by fungal α-1,3-glucan. Pathog. Dis. 2020, 78, ftaa062. [Google Scholar] [CrossRef]
- Nile, C.; Falleni, M.; Cirasola, D.; Alghamdi, A.; Anderson, O.F.; Delaney, C.; Ramage, G.; Ottaviano, E.; Tosi, D.; Bulfamante, G.; et al. Repurposing Pilocarpine Hydrochloride for Treatment of Candida albicans Infections. mSphere 2019, 4, e00689–e00718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciociola, T.; Pertinhez, T.A.; Giovati, L.; Sperinde, M.; Magliani, W.; Ferrari, E.; Gatti, R.; D’Adda, T.; Spisni, A.; Conti, S.; et al. Dissecting the Structure-Function Relationship of a Fungicidal Peptide Derived from the Constant Region of Human Immunoglobulins. Antimicrob. Agents Chemother. 2016, 60, 2435–2442. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ottaviano, E.; Borghi, E.; Giovati, L.; Falleni, M.; Tosi, D.; Magliani, W.; Morace, G.; Conti, S.; Ciociola, T. Therapeutic Effect of an Antibody-Derived Peptide in a Galleria mellonella Model of Systemic Candidiasis. Int. J. Mol. Sci. 2021, 22, 10904. https://doi.org/10.3390/ijms222010904
Ottaviano E, Borghi E, Giovati L, Falleni M, Tosi D, Magliani W, Morace G, Conti S, Ciociola T. Therapeutic Effect of an Antibody-Derived Peptide in a Galleria mellonella Model of Systemic Candidiasis. International Journal of Molecular Sciences. 2021; 22(20):10904. https://doi.org/10.3390/ijms222010904
Chicago/Turabian StyleOttaviano, Emerenziana, Elisa Borghi, Laura Giovati, Monica Falleni, Delfina Tosi, Walter Magliani, Giulia Morace, Stefania Conti, and Tecla Ciociola. 2021. "Therapeutic Effect of an Antibody-Derived Peptide in a Galleria mellonella Model of Systemic Candidiasis" International Journal of Molecular Sciences 22, no. 20: 10904. https://doi.org/10.3390/ijms222010904






