Synthesis, Properties and Bioimaging Applications of Silver-Based Quantum Dots
Abstract
:1. Introduction
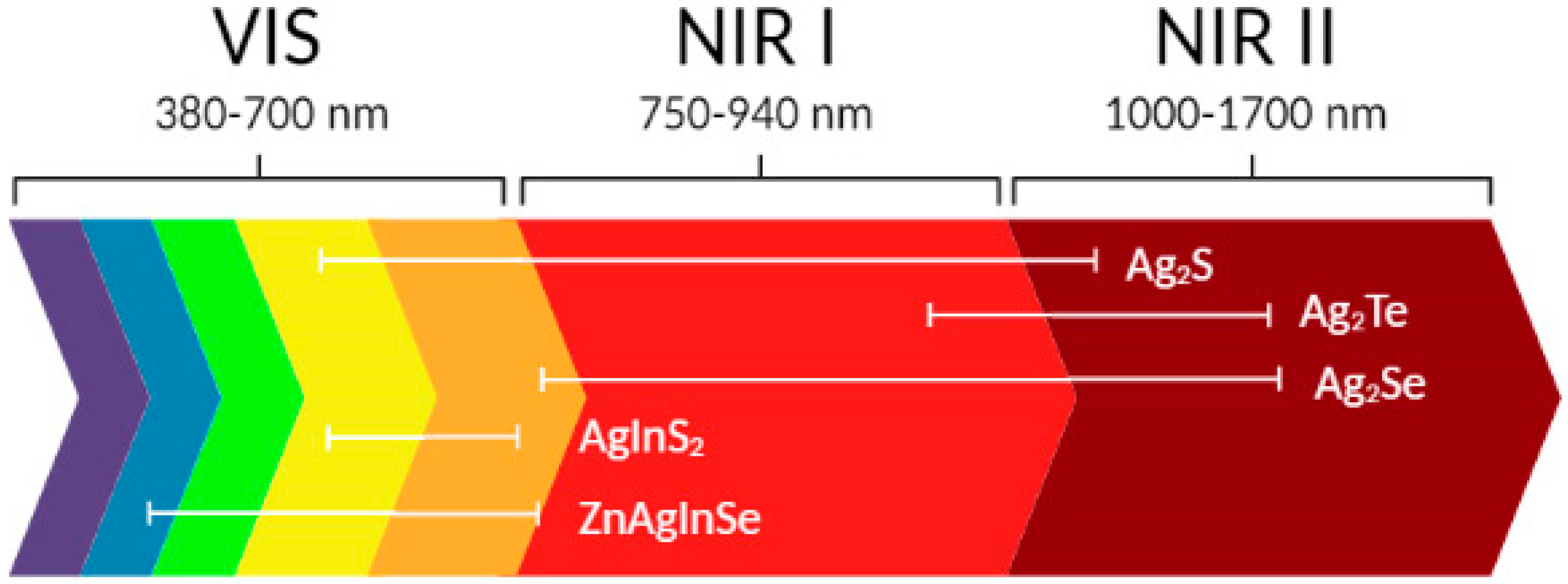
2. Basic Properties of Ag-Based QDs
- Monoclinic α-Ag2S QDs (body-centered cubic, stable at 178 °C and below);
- β-Ag2S (face-centered cubic, stable at 178–600 °C);
- γ-Ag2S (stable at 600 °C and above).
3. Chemical Synthesis of Ag-Based QDs
4. “Green” Synthesis of Ag-Based QDs
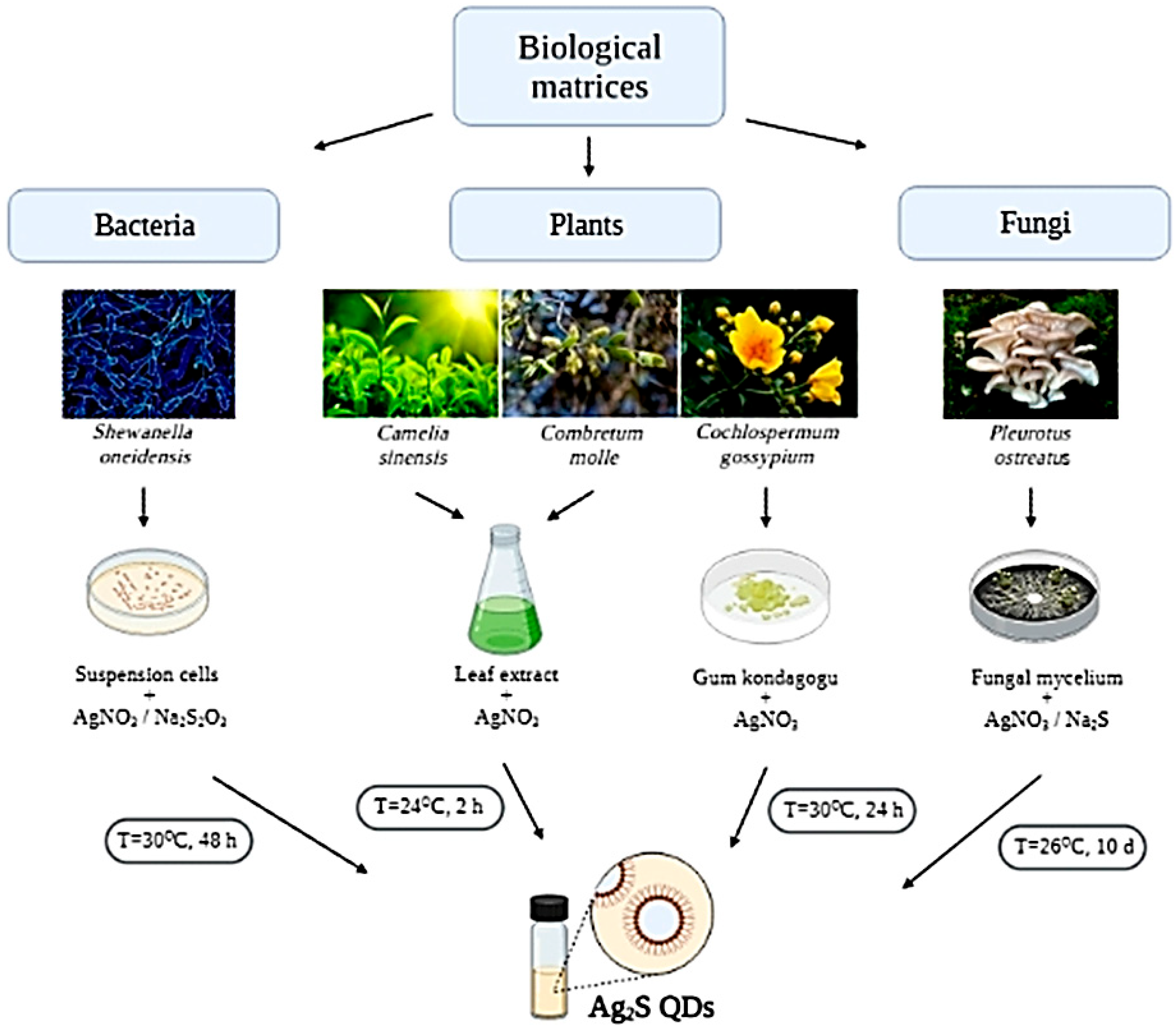
5. Ag2S QDs Biosynthesis
6. Ag2Se QDs Biosynthesis
7. Ag2Te QDs Biosynthesis
8. AgInS2 QDs Biosynthesis
9. Advantages of “Green” Synthesis of Ag-Based QDs
10. Toxicity of Ag-Based QDs
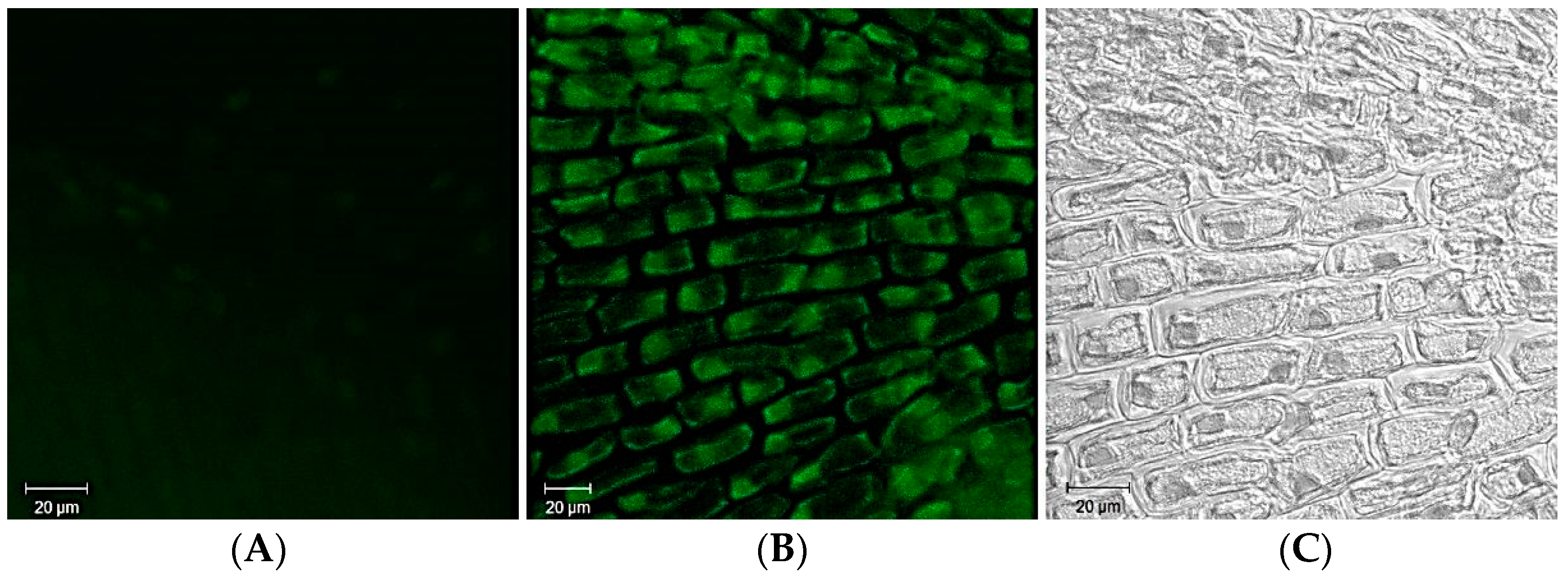
11. Bioimaging Applications of Ag-Based QDs
12. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wagner, A.M.; Knipe, J.M.; Orivec, G.; Peppas, N.A. Quantum dots in biomedical applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Mir, I.A.; Radhakrishanan, V.S.; Rawat, K.; Prasad, T.; Bohidar, H.B. Bandgap tunable AgInS based quantum dots for high contrast cell imaging with enhanced photodynamic and antifungal applications. Sci. Rep. 2018, 8, 9322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borovaya, M.N.; Burlaka, O.M.; Yemets, A.I.; Blume, Y.B. Biosynthesis of quantum dots and their potential applications in biology and biomedicine. In Nanoplasmonics, Nano-Optics, Nanocomposites, and Surface Studies; Fesenko, O., Yatsenko, L., Eds.; Springer Proceedings in Physics; Springer: Chem, Switzerland, 2015; Volume 167, pp. 339–362. [Google Scholar] [CrossRef]
- Carrillo-Carrión, C.; Simonet, B.M.; Valcárcel, M.; Lendl, B. Determination of pesticides by capillary chromatography and SERS detection using a novel silver-quantum dots “sponge” nanocomposite. J. Chromatogr. A 2012, 1225, 55–61. [Google Scholar] [CrossRef]
- Zhang, X.; Kong, X.; Lv, Z.; Zhou, S.; Du, X. Bifunctional quantum dot-decorated Ag@SiO2 nanostructures for simultaneous immunoassays of surface-enhanced Raman scattering (SERS) and surface-enhanced fluorescence (SEF). J. Mater. Chem. B 2013, 1, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Li, Y.; Wang, J.; Pu, Y.; Xue, W.; Liu, X. Green synthesis of graphene quantum dots and silver nanoparticles compounds with excellent surface enhanced Raman scattering performance. J. Alloys Compd. 2016, 663, 166–171. [Google Scholar] [CrossRef]
- Pereira, C.F.; Viegas, I.M.A.; Souza Sobrinha, I.G.; Pereira, G.; Pereira, G.A.L.; Krebs, P.; Mizaikoff, B. Surface-enhanced infrared absorption spectroscopy using silver selenide quantum dots. J. Mater. Chem. C 2020, 8, 10448–10455. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, C.; Lu, H.; Zou, T.; Singh, S.C.; Yu, Z.; Yao, C.; Zheng, X.; Xing, J.; Zou, Y.; et al. SERS study on the synergistic effects of electric field enhancement and charge transfer in an Ag2S quantum dots/plasmonic bowtie nanoantenna composite system. Photonics Res. 2020, 8, 548–563. [Google Scholar] [CrossRef]
- Song, Y.; Huang, H.C.; Lu, W.; Li, N.; Su, J.; Cheng, S.B.; Lai, Y.; Chen, J.; Zhan, J. Ag@WS2 quantum dots for Surface Enhanced Raman Spectroscopy: Enhanced charge transfer induced highly sensitive detection of thiram from honey and beverages. Food Chem. 2021, 344, 128570. [Google Scholar] [CrossRef]
- De Oliveira, E.G.; de Oliveira, H.P.; Gomes, A.S.L. Metal nanoparticles/carbon dots nanocomposites for SERS devices: Trends and perspectives. SN Appl. Sci. 2020, 2, 1491. [Google Scholar] [CrossRef]
- Borovaya, M.; Pirko, Y.; Krupodorova, T.; Naumenko, A.; Blume, Y.; Yemets, A. Biosynthesis of cadmium sulphide quantum dots by using Pleurotus ostreatus (Jacq.) P. Kumm. Biotechnol. Biotech. Equipm. 2015, 29, 1156–1163. [Google Scholar] [CrossRef]
- Borovaya, M.N.; Naumenko, A.P.; Matvieieva, N.A.; Blume, Y.B.; Yemets, A.I. Biosynthesis of luminescent CdS quantum dots using plant hairy root culture. Nanoscale Res. Lett. 2014, 9, 686. [Google Scholar] [CrossRef] [Green Version]
- Borovaya, M.N.; Burlaka, O.M.; Naumenko, A.P.; Blume, Y.B.; Yemets, A.I. Extracellular synthesis of luminescent CdS quantum dots using plant cell culture. Nanoscale Res. Lett. 2016, 11, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borovaya, M.; Naumenko, A.; Horiunova, I.; Plokhovska, S.; Blume, Y.; Yemets, A. “Green” synthesis of Ag2S nanoparticles, study of their properties and bioimaging applications. Appl. Nanosci. 2020, 10, 4931–4940. [Google Scholar] [CrossRef]
- Vus, K.; Tarabara, U.; Danylenko, I.; Pirko, Y.; Krupodorova, T.; Yemets, A.; Blume, Y.; Turchenko, V.; Klymchuk, D.; Smertenko, P.; et al. Silver nanoparticles as inhibitors of insulin amyloid formation: A fluorescence study. J. Mol. Liq. 2021, 342, 342–117508. [Google Scholar] [CrossRef]
- Cotta, M.A. Quantum dots and their applications: What lies ahead? ACS Appl. Nano Mater. 2020, 3, 4920–4924. [Google Scholar] [CrossRef]
- Ren, Q.; Ma, Y.; Zhang, S.; Ga, L.; Ai, J. One-step synthesis of water-soluble silver sulfide quantum dots and their application to bioimaging. ACS Omega 2021, 6, 6361–6367. [Google Scholar] [CrossRef]
- Abdel-Salam, M.; Omran, B.; Whitehead, K.; Baek, K.-H. Superior properties and biomedical applications of microorganism-derived fluorescent quantum dots. Molecules 2020, 25, 4486. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, L.A.; Liberto, G.D.; Tosoni, S.; Pacchion, G. Quantum confinement in group III-V semiconductor 2D nanostructures. Nanoscale 2020, 12, 17494–17501. [Google Scholar] [CrossRef]
- Zaini, M.S.; Ying, J.; Liew, C.; Kamarudin, M.A. Quantum confinement effect and photoenhancement of photoluminescence of PbS and PbS/MnS quantum dots. Appl. Sci. 2020, 10, 6282. [Google Scholar] [CrossRef]
- Cui, C.; Li, X.; Liu, J.; Hou, Y.; Zhao, Y.; Zhong, G. Synthesis and functions of Ag2S nanostructures. Nanoscale Res. Lett. 2015, 10, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buz, P.T.; Duman, F.D.; Erkisa, M.; Demirci, G.; Ari, F.; Ulukaya, E.; Acar, H.Y. Development of near-infrared region luminescent N-acetyl-L-cysteine-coated Ag2S quantum dots with differential therapeutic effect. Nanomedicine 2019, 14, 969–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadovnikov, S.I.; Gusev, A.I. Synthesis and characterization of novel stellate sea-urchin-like silver particles with extremely low density and superhydrophobicity. J. Mater. Chem. A 2017, 5, 20289–20297. [Google Scholar] [CrossRef] [Green Version]
- Purushothaman, B.; Song, J.M. Ag2S quantum dot theragnostics. Biomater. Sci. 2021, 9, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Jood, P.; Chetty, R.; Ohta, M. Structural stability enables high thermoelectric performance in room temperature Ag2Se. J. Mater. Chem. A 2020, 8, 13024–13037. [Google Scholar] [CrossRef]
- Ivanauska, R.; Milasiene, D. Fabrication of polyamide-Ag2Se composite films with controllable properties by an adsorption-diffusion method. J. Phys. Chem. Solids 2020, 145, 109533. [Google Scholar] [CrossRef]
- Ge, X.L.; Biao, H.; Zhang, Z.L.; Liu, X.; He, M.; Yu, Z.; Hu, B.; Cui, R.; Liang, X.J.; Pang, D.W. Glucose-functionalized near-infrared Ag2Se quantum dots with renal excretion ability for long-term in vivo tumor imaging. J. Mater. Chem. B 2019, 7, 5782–5788. [Google Scholar] [CrossRef]
- Zhu, C.N.; Jiang, P.; Zhang, Z.L.; Zhu, D.L.; Tian, Z.Q.; Pang, D.W. Ag2Se quantum dots with tunable emission in the second near-infrared window. ACS Appl. Mater. Interfaces 2013, 5, 1186–1189. [Google Scholar] [CrossRef]
- Tappan, B.A.; Zhu, B.; Cottingham, P.; Mecklenburg, M.; Scanlon, D.O.; Brutchey, R.L. Crystal structure of colloidally prepared metastable Ag2Se nanocrystals. Nano Lett. 2021, 21, 5881–5887. [Google Scholar] [CrossRef]
- Yang, M.; Gui, R.; Jina, H.; Wang, Z.; Zhang, F.; Xia, J.; Bi, S.; Xia, Y. Ag2Te quantum dots with compact surface coatings of multivalent polymers: Ambient one-pot aqueous synthesis and the second near-infrared bioimaging. Colloids Surf. B Biointerfaces 2015, 126, 115–120. [Google Scholar] [CrossRef]
- Jiang, L.; Zhu, Y.J. A general solvothermal route to the synthesis of CoTe, Ag2Te/Ag, and CdTe nanostructures with varied morphologies. Eur. J. Inorg. Chem. 2010, 8, 1238–1243. [Google Scholar] [CrossRef]
- Ouyang, T.; Zhang, X.; Ming, H. Thermal conductivity of ordered-disordered material: A case study of superionic Ag2Te. Nanotechnology 2015, 26, 025702. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.; Lin, W.H.; Tzeng, W.Y.; Le, P.H.; Luo, C.W.; Milenov, T.I. The optical properties of Ag2Te crystals from THz to UV. J. Alloys Compd. 2017, 725, 433–440. [Google Scholar] [CrossRef]
- Chang, Y.; Guo, J.; Tang, Y.Q.; Zhang, Y.X.; Feng, J.; Ge, Z.H. Facile synthesis of Ag2Te nanowires and thermoelectric properties of Ag2Te polycrystals sintered by spark plasma sintering. CrystEngComm 2019, 21, 1718–1727. [Google Scholar] [CrossRef]
- Lee, S.; Shin, H.S.; Song, J.Y.; Jung, M. Thermoelectric properties of a single crystalline Ag2Te nanowire. J. Nanomater. 2017, 2017, 4308968. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, K.; Pradhan, S.; Basel, S.; Clarke, M.; Brito, B.; Thapa, S.; Roy, P.; Borthakur, S.; Saikia, L.; Shankar, A.; et al. Tunable NIR-II emitting silver chalcogenide quantum dots using thio/selenourea precursors: Preparation of an MRI/NIR-II multimodal imaging agent. Dalton Trans. 2020, 49, 15425–15432. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.X.; Ma, J.J.; Wang, J.M.; Cai, W.G.; Zhang, Z.; Huang, B.; Sun, M.Y.; Cheng, Q.Y.; Zhang, Z.L.; Pang, D.W.; et al. Ag2Te quantum dots as contrast agents for near-Infrared fluorescence and computed tomography imaging. ACS Appl. Nano Mater. 2020, 3, 6071–6077. [Google Scholar] [CrossRef]
- Halder, G.; Ghosh, A.; Parvin, S.; Bhattacharyya, S. Cation exchange in Zn–Ag–In–Se core/alloyed shell quantum dots and their applications in photovoltaics and water photolysis. Chem. Mater. 2019, 31, 161–170. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, S.; Mhaisalkar, S.G.; Ramakrishna, S. Synthesis of AgInS2 nanocrystal ink and its photoelectrical application. Phys. Chem. Chem. Phys. 2012, 24, 8523–8529. [Google Scholar] [CrossRef]
- Hong, S.P.; Park, K.H.; Oh, J.H.; Yang, H.; Do, Y.R. Comparisons of the structural and optical properties of o-AgInS2, t-AgInS2, and c-AgIn5S8nanocrystals and their solid-solution nanocrystals with ZnS. J. Mater. Chem. 2012, 22, 18939–18949. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, Z.; Gao, S.; Goh, B.L.; Samsudin, B.; Wen, K.; Wu, Y.; Wu, C.; Su, X. Recent advances in non-toxic quantum dots and their biomedical applications. Progress Nat. Sci. Mater. Int. 2019, 29, 628–640. [Google Scholar] [CrossRef]
- Gromova, Y.; Sokolova, A.; Kurshanov, D.; Korsakov, I.; Osipova, V.; Cherevkov, S.; Dubavik, A.; Maslov, V.; Perova, T.; Gun’ko, Y.; et al. Investigation of AgInS2/ZnS quantum dots by magnetic circular dichroism spectroscopy. Materials 2019, 12, 3616. [Google Scholar] [CrossRef] [Green Version]
- Li, P.L.; Ghule, A.V.; Chang, J.J. Direct aqueous synthesis of quantum dots for high-performance AgInSe2 quantum-dot-sensitized solar cell. J. Power Source 2017, 354, 100–107. [Google Scholar] [CrossRef]
- Allen, P.M.; Bawendi, M.G. Ternary I−III−VI quantum dots luminescent in the red to near-infrared. J. Am. Chem. Soc. 2008, 130, 9240–9241. [Google Scholar] [CrossRef] [Green Version]
- Deng, D.; Qu, L.; Gu, Y. Near-infrared broadly emissive AgInSe2/ZnS quantum dots for biomedical optical imaging. J. Mater. Chem. C 2014, 2, 7077–7085. [Google Scholar] [CrossRef]
- Tappan, B.A.; Horton, M.K.; Brutchey, R.L. Ligand-mediated phase control in colloidal AgInSe2 nanocrystals. Chem. Mater. 2020, 32, 2935–2945. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, R.; Bao, F.; Han, Z.; Gu, Y.; Deng, D. Water-soluble Zn–Ag–In–Se quantum dots with bright and widely tunable emission for biomedical optical imaging. RSC Adv. 2015, 5, 88583–88589. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.-Y.; Wang, S.; Wang, X.; Wei, Q.; Jiang, X.; Wang, K.; Xu, J.; Ke, Q.; Zhang, Q.; et al. Quaternary two dimensional Zn–Ag–In–S nanosheets for highly efficient photocatalytic hydrogen generation. J. Mater. Chem. A 2018, 6, 11670–11675. [Google Scholar] [CrossRef]
- Borowik, A.; Butowska, K.; Konkel, K.; Banasiuk, R.; Derewonko, N.; Wyrzykowski, D.; Davydenko, M.; Cherepanov, V.; Styopkin, V.; Prylutskyy, Y.; et al. The impact of surface functionalization on the biophysical properties of silver nanoparticles. Nanomaterials 2019, 9, 973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Zhang, D.W.; Shi, W.F. A gamma-ray irradiation reduction route to prepare rod-like Ag2S nanocrystallines at room temperature. Mater. Lett. 2007, 61, 3232–3234. [Google Scholar] [CrossRef]
- Chen, M.H.; Gao, L. Synthesis of leaf-like Ag2S nanosheets by hydrothermal method in water alcohol homogenous medium. Mater. Lett. 2006, 60, 1059–1062. [Google Scholar] [CrossRef]
- Chaudhuri, R.G.; Paria, S. A novel method for the templated synthesis of Ag2S hollow nanospheres in aqueous surfactant media. J. Colloid Interface Sci. 2011, 369, 117–122. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. The Physics and Chemistry of Sol-Gel Processing; Academic Press Inc.: San Diego, CA, USA, 1990; pp. 391–392. [Google Scholar]
- Armelao, L.; Colombo, P.; Fabrizio, M.; Gross, S.; Tondello, E. Sol-gel synthesis and characterization of Ag2S nanocrystallites in silica thin film glasses. J. Mater. Chem. 1999, 9, 2893–2898. [Google Scholar] [CrossRef]
- Ahemad, M.J.; Yu, Y.T. Investigating the mechanism of uniform Ag-SiO2 core-shell nanostructures synthesis by a one-pot sol-gel method. J. Sol-Gel Sci. Technol. 2020, 96, 679–689. [Google Scholar] [CrossRef]
- Mousavi-Kamazani, M.; Salavati-Niasari, M. A simple microwave approach for synthesis and characterization of Ag2S-AgInS2 nanocomposites. Compos. B Eng. 2014, 56, 490–496. [Google Scholar] [CrossRef]
- Gholamrezaei, S.; Salavati-Niasari, M.; Ghanbari, D.; Bagheri, S. Hydrothermal preparation of silver telluride nanostructures and photo-catalytic investigation in degradation of toxic dyes. Sci. Rep. 2016, 6, 20060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Li, D.; Chen, Z.; Sun, M.; Li, W.; Lin, Q.; Fu, X. Microwave hydrothermal synthesis of AgInS2 with visible light photocatalytic activity. Mater. Res. Bull. 2011, 4, 975–982. [Google Scholar] [CrossRef]
- Rai, M.; Gade, A.; Yadav, A. Metal Nanoparticles in Microbiology, Biogenic Nanoparticles: An Introduction to What They Are, How They Are Synthesized and Their Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–14. [Google Scholar]
- Alomar, T.S.; AlMasoud, N.; Awad, M.A.; El-Tohamy, M.F.; Soliman, D.A. An eco-friendly plant-mediated synthesis of silver nanoparticles: Characterization, pharmaceutical and biomedical applications. Mater. Chem. Phys. 2020, 249, 123007. [Google Scholar] [CrossRef]
- Debabov, V.G.; Voeikova, T.A.; Shebanova, A.S.; Shaitan, K.V.; Emelyanova, L.K.; Novikova, L.M.; Kirpichnikov, M.P. Bacterial synthesis of silver sulfide nanoparticles. Nanotechnol. Russ. 2013, 8, 269–276. [Google Scholar] [CrossRef]
- Sibiya, P.N.; Moloto, M.J. Green synthesis of Ag2S nanoparticles: Effect of pH and capping agent on size and shape of NPs and their antibacterial activity. Dig. J. Nanomater. Biostruct. 2018, 13, 411–418. [Google Scholar]
- Ayodhya, D.; Veerabhadram, G. Green synthesis, characterization, photocatalytic, fluorescence and antimicrobial activities of Cochlospermum gossypium capped Ag2S nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 157, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Božanić, D.K.; Djoković, V.; Blanuša, J.; Nair, P.S.; Radhakrishnan, T. Preparation and properties of nano-sized Ag and Ag2S particles in biopolymer matrix. Eur. Phys. J. E 2007, 22, 51–59. [Google Scholar] [CrossRef]
- Sibiya, N.P.; Moloto, M.J. Shape control of silver selenide nanoparticles using green capping molecules. Green Process. Synth. 2016, 6, 183–188. [Google Scholar] [CrossRef]
- Oluwafemi, O.S.; May, B.M.M.; Parani, S.J.; Rajendran, V. Cell viability assessments of green synthesized water-soluble AgInS2/ZnS core/shell quantum dots against different cancer cell lines. J. Mater. Res. 2019, 34, 4037–4044. [Google Scholar] [CrossRef]
- Fahmi, M.Z.; Chang, J.Y. Forming double layer-encapsulated quantum dots for bio-imaging and cell targeting. Nanoscale 2013, 5, 1517–1528. [Google Scholar] [CrossRef]
- Plastun, I.L.; Zakharov, A.A.; Naumov, A.A. Features of silver sulfide nanoparticles bacterial synthesis: Molecular modeling. In Proceedings of the International Conference on Actual Problems of Electron Devices Engineering (APEDE), Saratov, Russia, 24–25 September 2020; IEEE: Saratov, Russia, 2020; pp. 318–322. [Google Scholar] [CrossRef]
- Zahedifar, M.; Shirani, M.; Akbari, A.; Seyedi, N. Green synthesis of Ag2S nanoparticles on cellulose/Fe3O4 nanocomposite template for catalytic degradation of organic dyes. Cellulose 2019, 26, 6797–6812. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M.; Aqarbeh, M.M.; Abdulaziz, F.M. Green synthesis, characterization of silver sulfide nanoparticles and antibacterial activity evaluation. Chem. Int. 2020, 6, 42–48. [Google Scholar] [CrossRef]
- Yang, L.; Xing, R.; Shen, Q.; Jiang, K.; Wang, J.; Ren, Q. Fabrication of protein-conjugated silver sulfide nanorods in the bovine serum albumin solution. J. Phys. Chem. B 2006, 110, 10534–105339. [Google Scholar] [CrossRef]
- Delgado-Beleño, Y.; Martinez-Nuñez, C.E.; Cortez-Valadez, M.; Flores-López, N.S. Optical properties of silver, silver sulfide and silver selenide nanoparticles and antibacterial applications. Mater. Res. Bull. 2018, 99, 385–392. [Google Scholar] [CrossRef]
- Mahlambi, P.N.; Moloto, M.J. Starch-capped silver selenide nanoparticles: Effect of capping agent concentration and extraction time on size. Asian J. Chem. 2016, 28, 1315–1320. [Google Scholar] [CrossRef]
- Sibiya, N.P.; Moloto, M.J. Effect of precursor concentration and pH on the shape and size of starch-capped silver selenide (Ag2Se) nanoparticles. Chalcogenide Lett. 2014, 11, 577–588. [Google Scholar]
- Liu, J.; Zheng, D.; Zhong, L.; Gong, A.; Wu, S.; Xie, Z. Biosynthesis of biocompatibility Ag2Se quantum dots in Saccharomyces cerevisiae and its application. Biochem. Biophys. Res. Commun. 2021, 544, 60–64. [Google Scholar] [CrossRef]
- Yuan, G.; Ka, W.; Haizeng, S.; Han, W.; Shancheng, Y.; Xu, X.; Shi, Y. Fabrication and electrical properties of silver telluride nanowires. J. Nanosci. Nanotechnol. 2020, 20, 2628–2632. [Google Scholar] [CrossRef]
- Qin, A.; Fang, Y.; Tao, P.; Zhang, J.; Su, C. Silver telluride nanotubes prepared by the hydrothermal method. Inorg. Chem. 2007, 46, 7403–7409. [Google Scholar] [CrossRef]
- Parween, K.; Banse, V.; Ledwani, L. Green synthesis of nanoparticles: Their advantages and disadvantages. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016; Volume 1724, p. 020048. [Google Scholar] [CrossRef]
- Suresh, K.A. Metallic Nanocrystallites and Their Interaction with Microbial Systems; Springer: Dordrecht, The Netherlands; Springer: New York, NY, USA; Springer: London, UK, 2012. [Google Scholar] [CrossRef]
- Arif, R.; Uddin, R. A review on recent developments in the biosynthesis of silver nanoparticles and its biomedical applications. Med. Devices Sens. 2020, 4, e10158. [Google Scholar] [CrossRef]
- Al-Shalabi, Z. Production of Cadmium Sulphide Quantum Dots in Tomato Hairy Root Cultures. Ph.D. Thesis, School of Biotechnology and Biomolecular Sciences (BABS), Kensington, NSW, Australia, 2010. [Google Scholar]
- Gholami, Z.; Dadmehr, M.; Jelodar, N.B.; Hosseini, M.; Oroojalian, F.; Parizi, A.P. One-pot biosynthesis of CdS quantum dots through in vitro regeneration of hairy roots of Rhaphanus sativus L. and their apoptosis effect on MCF-7 and AGS cancerous human cell lines. Mater. Res. Express 2020, 7, 015056. [Google Scholar] [CrossRef]
- Kobylinska, N.; Shakhovsky, A.; Khainakova, O.; Klymchuk, D.; Avdeeva, L.; Ratushnyak, Y.; Duplij, V.; Matvieieva, N. “Hairy” root extracts as source for ‘green’ synthesis of silver nanoparticles and medical applications. RSC Adv. 2020, 10, 39434–39446. [Google Scholar] [CrossRef]
- Al-Shalabi, Z.; Stevens-Kalceff, M.A.; Doran, P.M. Metal uptake and nanoparticle synthesis in hairy root cultures. Adv. Biochem. Eng. Biotechnol. 2013, 134, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Özkan, D.; Sevtap, V.; İbrahim, A.; Havva, H.; Acar, Y.; Basaran, N. An in vitro study on the cytotoxicity and genotoxicity of silver sulfide quantum dots coated with meso-2,3-dimercaptosuccinic Acid. J. Pharm. Sci. 2019, 16, 282–291. [Google Scholar] [CrossRef]
- Hocaoglu, I.; Demir, F.; Birer, O.; Kiraz, A.; Sevrin, C.; Grandfils, C.; Acar, H.Y. Emission tunable, cyto/hemocompatible, near-IR-emitting Ag2S quantum dots by aqueous decomposition of DMSA. Nanoscale 2014, 6, 11921–11930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, D.A.; Mendoza, L.; Vizuete, K.; Debut, A.; Arias, M.T.; Gavilanes, A.; Terencio, T.; Ávila, E.; Jeffryes, C.; Dahoumane, S.A. Sugar-mediated green synthesis of silver selenide semiconductor nanocrystals under ultrasound irradiation. Molecules 2020, 25, 5193. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hu, R.; Roy, I.; Lin, G.; Ye, L.; Reynolds, J.L.; Liu, J.; Liu, J.; Schwartz, S.A.; Zhang, X.; et al. Synthesis of luminescent near-infrared AgInS2 nanocrystals as optical probes for in vivo applications. Theranostics 2013, 3, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Chinnathambi, S.; Shirahata, N. Recent advances on fluorescent biomarkers of near-infrared quantum dots for in vitro and in vivo imaging. Sci. Technol. Adv. Mater. 2019, 20, 337–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, R.H.; Gao, X. Applications of Quantum Dots in Bioimaging and Bioassays. Mater. Matters 2019, 14, 49–53. [Google Scholar]
- Zhang, R.Y.; Hong, G.; Zhang, Y.; Chen, G.; Li, F.; Dai, H.; Wang, Q. Ag2S quantum dot: A bright and biocompatible fluorescent nanoprobe in the second near-infrared window. ACS Nano 2012, 6, 3695–3702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa-Espinoza, S.; Rodriguez-Gonzales, C.A.; Martel-Estrada, S.A.; Hernandez-Paz, J.F.; Olivas-Armendariz, I. Synthesis of Ag2S quantum dots and their biomedical applications. J. Non-Oxide Glass 2018, 10, 7–25. [Google Scholar]
- Tang, H.; Yang, S.T.; Yang, Y.F.; Ke, D.M.; Liu, J.H.; Chen, X.; Wang, H.; Liu, Y. Blood clearance, distribution, transformation, excretion, and toxicity of near-infrared quantum dots Ag2Se in mice. ACS Appl. Mater. Interfaces 2018, 8, 17859–17869. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Tang, H.; Chen, X.-Z.; Su, Q.; Xi, Z.-S.; Liu, Y.-Y.; Liu, Y.; Cao, A.; Wang, H. In vivo fate of Ag2Te quantum dot and comparison with other NIR-II silver chalcogenide quantum dots. J. Nanoparticle Res. 2020, 22, 287. [Google Scholar] [CrossRef]
- Song, J.; Ma, C.; Zhang, W.; Li, X.; Zhang, W.; Wu, R.; Cheng, X.; Ali, A.; Yang, M.; Zhu, L.; et al. Bandgap and structure engineering via cation exchange: From binary Ag2S to ternary AgInS2, quaternary AgZnInS alloy and AgZnInS/ZnS core/shell fluorescent nanocrystals for bioimaging. ACS Appl. Mater. Interfaces 2016, 8, 24826–24836. [Google Scholar] [CrossRef]
- Rhyner, M.N.; Smith, A.M.; Gao, X.; Mao, H.; Yang, L.; Nie, S. Quantum dots and multifunctional nanoparticles: New contrast agents for tumor imaging. Nanomedicine 2006, 1, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Rodríguez, A.; Shen, Y.I.; Gutierrez, Z.; Santos, H.D.A.; Vera, V.T.; Ximendes, E.; Villaverde, G.; Lifante, J.; Gerke, C.; Fernandez, N.; et al. 10-fold quantum yield improvement of Ag2S nanoparticles by fine compositional tuning. ACS Appl. Mater. Interfaces 2020, 12, 12500–12509. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Fang, Z.Y. Noble and Precious Metals—Properties, Nanoscale Effects and Applications; Seehra, M., Ed.; IntechOpen: London, UK, 2018; pp. 369–374. [Google Scholar] [CrossRef]
- Gu, Y.P.; Cui, R.; Zhang, Z.L.; Xie, Z.X.; Pang, D.W. Ultrasmall near-infrared Ag2Se quantum dots with tunable fluorescence for in vivo imaging. J. Am. Chem. Soc. 2012, 134, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Shamirian, A.; Appelbe, O.; Zhang, Q.; Ganesh, B.; Kron, S.J.; Snee, P.T. A toolkit for bioimaging using near-infrared AgInS2/ZnS quantum dots. J. Mater. Chem. B 2015, 41, 8188–8196. [Google Scholar] [CrossRef] [PubMed]

| Type of Silver-Based QDs | Band Gap (eV) | Crystal Structure | Phase Transition Temperature (°C) | References |
|---|---|---|---|---|
| Chalcogenides | ||||
| Ag2S | 0.9–1.1 | Monoclinic acanthite | Below 179 | [23] |
| Body-centered cubic argentite form | Above 180 | |||
| Face-centered cubic | Above 586 | |||
| Ag2Se | 0.02–0.22 | Orthorhombic structure | ~133 | [29] |
| Body-centered cubic form | Until 897 | |||
| Ag2Te | 0.67 | Monoclinic phase (β-form) | Transition at ~150 | [32] |
| Cubic phase (α-form superionic conductor) | ||||
| Ternary dichalcogenides | 1.8 | Cubic structure | >100 | [40] |
| AgInS2 | ||||
| AgInSe2 | 1.24–1.53 | Chalcopyrite phase | 300 | [46] |
| Metastable orthorhombic phase | 250 | |||
| Quaternary dichalcogenides | 1.7 | Hexagonal structure | <100 | [48] |
| ZnAgInS | ||||
| ZnAgInSe | 1.2 | Orthorhombic | 200–250 | [47] |
| Type of Quantum Dot | Chemical Synthesis Method | Average Diameter (nm) | Morphology | Photoluminescence (nm) | Crystal Lattice Structure | References |
|---|---|---|---|---|---|---|
| Ag2S | Single source precursor | 5–10 | Spherical | 543 | Orthorhombic or α-phase sulfur | [52] |
| Sol-gel synthesis | 30–60 | Thin films | - | - | [21] | |
| Hydrothermal method | 70–90 in length | Rice-shaped | - | Monoclinic | [51] | |
| Gamma-ray irradiation | 200–500 | Rod-like | - | Monoclinic | [50] | |
| Hydrothermal method | 1.45–5.20 | Spherical | 748–840 | Monoclinic | [22] | |
| Pyrolysis method | 10.2 ± 0.4 | - | 1058 | - | [21] | |
| Hot-injection method | 1.5–4.6 | Spherical | 690–1227 | |||
| Hydrothermal method | 2.6–3.7 | Spherical | 687–1096 | |||
| Microwave-assisted synthesis | 5.7 ± 0.93 | - | 1062 | |||
| Ag2Se | Co-precipitation method | 5–30 | Wire-type | 700–1330 | Orthorhombic | [28] |
| Solvothermal method | 3.4 | Spherical | ẞ-Ag2Se | |||
| Hydrothermal method | 60–80 in length | Rice-shaped | - | |||
| Hydrothermal method | 3.1–3.9 | Spherical | 1080–1330 | Orthorhombic | [28] | |
| Hydrothermal method | 2 | Spherical | - | Orthorhombic | [25] | |
| Ag2Te | Hydrothermal method | 200 | Wire-type | 995–1300 | Irregular dendrites | [56] |
| Solvothermal methods | 10 | Spherical | - | Monoclinic | [31] | |
| One-pot aqueous Synthesis | 3.8–4.7 | - | 995–1068 | Monoclinic | [30] | |
| Hydrothermal method | 2.4 ± 0.9 | Spherical | 1320 | Monoclinic | [37] | |
| AgInS2 | Hot-injection method | 3.7–4.3 | Spherical | - | - | [39] |
| Microwave synthesis | 20–80 | - | 520–650 | Tetragonal | ||
| ZnAgInSe | Synthesized in Aqueous phase | 3.5–4 | Spherical | 450–700 | Orthorhombic | [47] |
| Hydrothermal synthesis | 1.5–4.5 | Spherical | 450–700 | Cubic | [47] |
| Type of Quantum Dot | Living Organism/Derivatives/Biomolecules | Average Diameter (nm) | Morphology | Photoluminescence (nm) | Crystal Lattice Structure | References |
|---|---|---|---|---|---|---|
| Ag2S | Shewanella oneidensis MR-1 | 6–12 | Spherical | - | Monoclinic | [61] |
| Camellia sinensis | ~20 | Spherical | 387–402 | Monoclinic | [62] | |
| Comtretum molle | 360–365 | |||||
| Acacia mearnsii | 352–354 | |||||
| Chitosan | 343–350 | |||||
| Cochlospermum gossypium | 48–54 | Spherical and cubic | 500 | Cubic and individual spherical particles | [63] | |
| Pleurotus ostreatus (Jacq.) P. Kumm. (strain 551) | 10–17 | Spherical | 520 | - | [14] | |
| Sago starch | 9.5 ± 3.6 | Spherical | - | Monoclinic | [64] | |
| Ag2Se | Green tea | 30 | Spherical and rod | 240–330 390–550 | Orthorhombic | [65] |
| Glucose | 31 | Spherical and cubic | ||||
| Ascorbic acid | 96 | Spherical | ||||
| Chitosan | 8 | Spherical | ||||
| Glucose | 2.4 ± 0.5 | - | 561–705 | Orthorhombic | [27] | |
| AgInS2 AgInS2/ZnS | Shell precursors (ZnAc2 and thiourea) | 3.2–3.4 | Spherical | 667–677 | Tetragonal | [66,67] |
| Parameter | Dye | QDs |
|---|---|---|
| Absorption spectrum | Narrow | Broad and gradually increasing towards shorter wavelength |
| Emission spectrum | Broad | Narrow, symmetrical |
| Quantum yield (QY) | High-quality dyes and QDs have similar QYs | |
| Fluorescence lifetime | 5–20 nanoseconds | 50–200 nanoseconds |
| Photostability | Poor, rapid photobleaching | Highly stable |
| Type of QDs | Cell Line/Organism | Fluorescence (nm) | Route of Administration | Reference |
|---|---|---|---|---|
| Ag2S | Mouse fibroblast L929 cell line | 1100–1700 | Cells were fixed in 4% paraformaldehyde and treated with QDs (in vitro studies) | [91] |
| Human malignant glioma U87 MG cell line | ||||
| Human breast cancer MDA-MB-468 cell line (ATCC) | ||||
| Ag2Se | Male CD-1 (ICR) mice | 700–820 | Intravenous injection (in vivo studies) | [93] |
| Ag2Te | Male ICR mice | 900–1300 | Intravenous injection (in vivo studies) | [94] |
| AgInS2 | Radiation induced fibrosarcoma (RIF) cells | 800 | Intravenous injection (in vivo studies) | [88] |
| Human peripheral blood monocyte-derived macrophages (MDM) | ||||
| AgInS2/ZnS | Human hepatoma cell line (Hep G2) | 500–700 | QDs delivered into Hep G2 cells and specifically combined with antigens (in vitro studies) | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borovaya, M.; Horiunova, I.; Plokhovska, S.; Pushkarova, N.; Blume, Y.; Yemets, A. Synthesis, Properties and Bioimaging Applications of Silver-Based Quantum Dots. Int. J. Mol. Sci. 2021, 22, 12202. https://doi.org/10.3390/ijms222212202
Borovaya M, Horiunova I, Plokhovska S, Pushkarova N, Blume Y, Yemets A. Synthesis, Properties and Bioimaging Applications of Silver-Based Quantum Dots. International Journal of Molecular Sciences. 2021; 22(22):12202. https://doi.org/10.3390/ijms222212202
Chicago/Turabian StyleBorovaya, Mariya, Inna Horiunova, Svitlana Plokhovska, Nadia Pushkarova, Yaroslav Blume, and Alla Yemets. 2021. "Synthesis, Properties and Bioimaging Applications of Silver-Based Quantum Dots" International Journal of Molecular Sciences 22, no. 22: 12202. https://doi.org/10.3390/ijms222212202






