Diagnosis of Prostate Cancer and Prostatitis Using near Infra-Red Fluorescent AgInSe/ZnS Quantum Dots
Abstract
:1. Introduction
2. Results
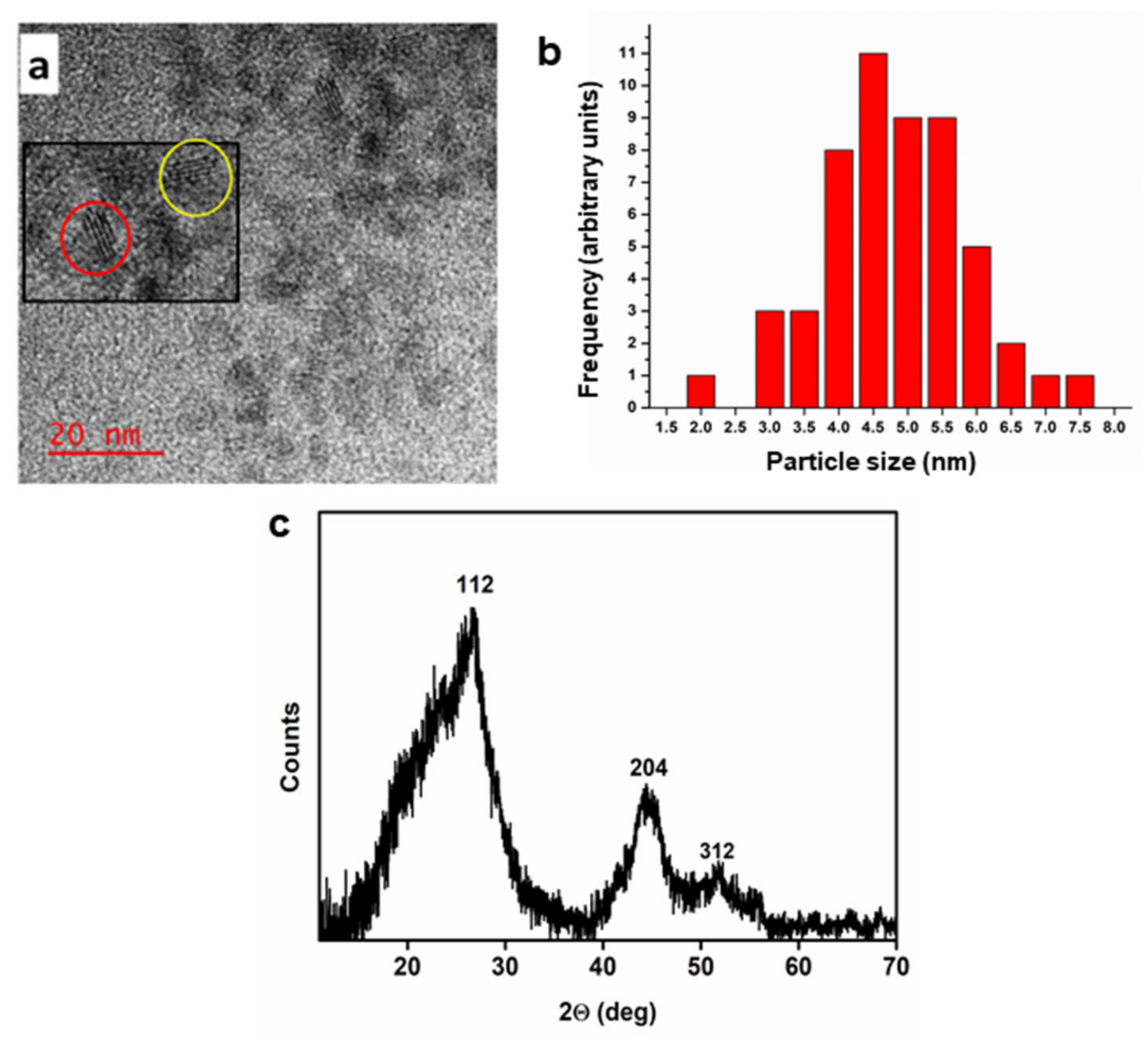
2.1. Physicochemical Characterization
2.2. Cytotoxicity
2.3. Biofilm Imaging
2.4. Cancer Cell Imaging
3. Materials and Methods
3.1. Materials
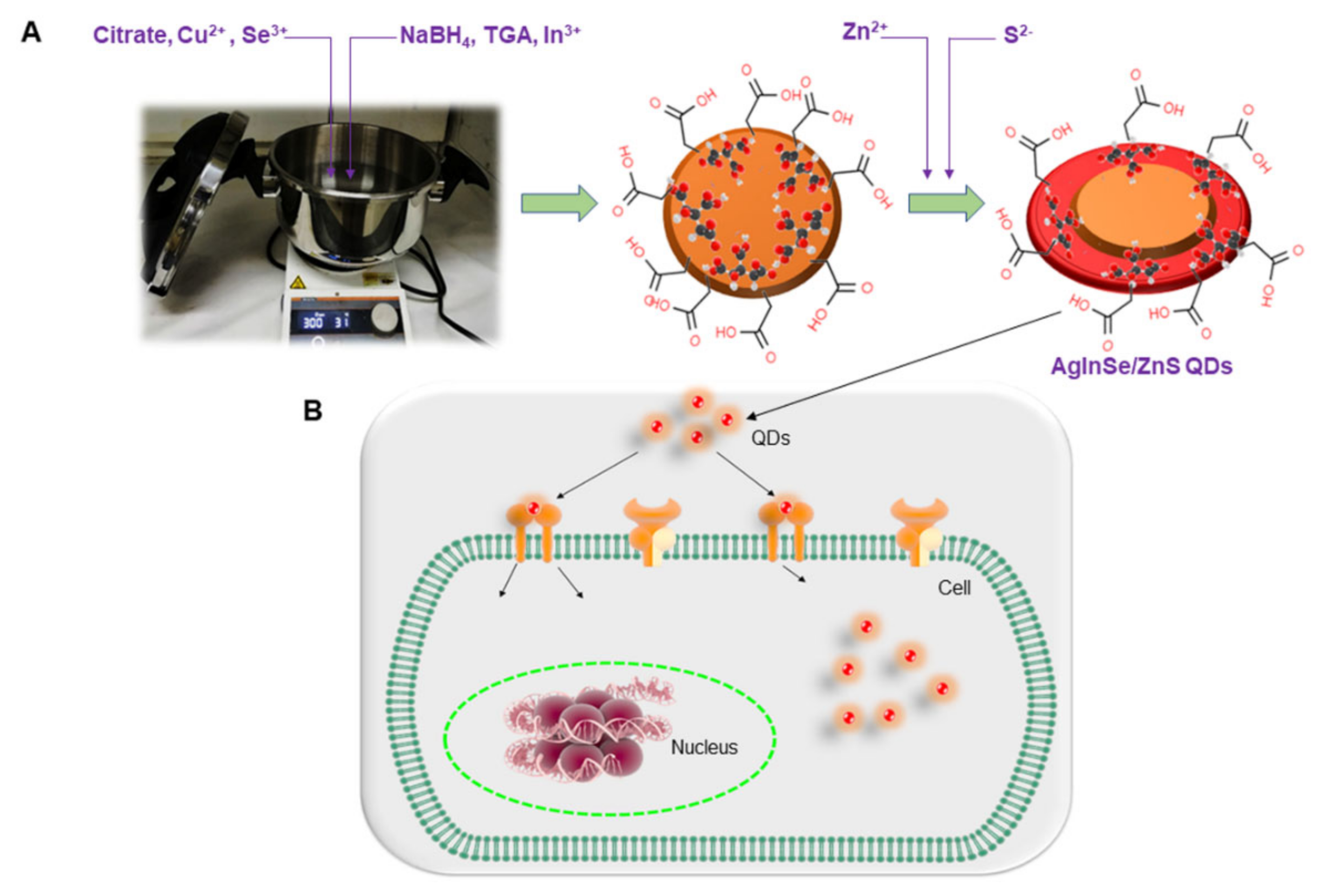
3.2. Synthesis of AgInSe/ZnS QDs
3.3. Characterization
3.4. Cytotoxicity
3.5. Biofilm Imaging
3.6. Prostate Cancer Cell Imaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dobell, C. The Discovery of the Intestinal Protozoa of Man. Proc. R. Soc. Med. 1920, 13, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Mahowald, M.A.; Ley, R.E.; Lozupone, C.A.; Hamady, M.; Martens, E.C.; Henrissat, B.; Coutinho, P.M.; Minx, P.; Latreille, P.; et al. Evolution of Symbiotic Bacteria in the Distal Human Intestine. PLoS Biol. 2007, 5, e156. [Google Scholar] [CrossRef] [PubMed]
- Cummins, J.; Tangney, M. Bacteria and tumours: Causative agents or opportunistic inhabitants? Infect. Agents Cancer 2013, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attiê, R.; Chinen, L.T.D.; Yoshioka, E.M.; Silva, M.C.F.; de Lima, V.C.C. Acute bacterial infection negatively impacts cancer specific survival of colorectal cancer patients. World J. Gastroenterol. 2014, 20, 13930–13935. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sfanos, K.S.; De Marzo, A.M. Prostate cancer and inflammation: The evidence. Histopathology 2012, 60, 199–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, F.J., Jr.; Barry, M.J.; Lu-Yao, G.; Wasson, J.; Roman, A.; Wennberg, J. Effect of radical prostatectomy for prostate cancer on patient quality of life: Results from a Medicare survey. Urology 1995, 45, 1007–1013. [Google Scholar] [CrossRef]
- Nelles, J.L.; Freedland, S.J.; Presti, J.C., Jr.; Terris, M.K.; Aronson, W.J.; Amling, C.L.; Kane, C.J. Impact of nerve sparing on surgical margins and biochemical recurrence: Results from the SEARCH database. Prostate Cancer Prostatic Dis. 2009, 12, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Ninan, N.; Simonkutty, P.M.; Thomas, S.; Grohens, Y. Chapter 13—Upconversion Nanoparticles. In Nanotechnology Applications for Tissue Engineering; Thomas, S., Grohens, Y., Ninan, N., Eds.; William Andrew Publishing: Oxford, UK, 2015; pp. 215–220. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Shirahata, N. Recent advances on fluorescent biomarkers of near-infrared quantum dots for in vitro and in vivo imaging. Sci. Technol. Adv. Mater. 2019, 20, 337–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsolekile, N.; Ncapayi, V.; Parani, S.; Sakho, E.H.M.; Matoetoe, M.C.; Songca, S.P.; Oluwafemi, O.S. Synthesis of fluorescent CuInS2/ZnS quantum dots—porphyrin conjugates for photodynamic therapy. MRS Commun. 2018, 8, 398–403. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum dots in biomedical applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Ninan, N.; Albrecht, H.; Blencowe, A. Chapter 5—Mammalian Cell-Based Assays for Studying Bio-Nano Interactions. In Characterization of Nanomaterials; Mohan Bhagyaraj, S., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 129–166. [Google Scholar] [CrossRef]
- Kang, X.; Yang, Y.; Huang, L.; Tao, Y.; Wang, L.; Pan, D. Large-Scale Synthesis of Water-Soluble CuInSe2/ZnS and AgInSe2/ZnS Core/Shell Quantum Dots. Green Chem. 2015, 17, 4482–4488. [Google Scholar] [CrossRef]
- Aswathy, R.G.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Near-infrared quantum dots for deep tissue imaging. Anal. Bioanal. Chem. 2010, 397, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xie, H.; Li, Q.C.; Sun, E.J.; Su, B.L. Adherence and interaction of cationic quantum dots on bacterial surfaces. J. Colloid Interface Sci. 2015, 450, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Aizik, G. Liposomes of Quantum Dots Configured for Passive and Active Delivery to Tumor Tissue. Nano Lett. 2019, 19, 5844–5852. [Google Scholar] [CrossRef] [PubMed]
- Rivel, T.; Ramseyer, C.; Yesylevskyy, S. The asymmetry of plasma membranes and their cholesterol content influence the uptake of cisplatin. Sci. Rep. 2019, 9, 5627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oluwafemi, O.S.; May, B.M.M.; Parani, S.; Tsolekile, N. Facile, large scale synthesis of water soluble AgInSe2/ZnSe quantum dots and its cell viability assessment on different cell lines. Mater. Sci. Eng. C 2020, 106, 110181. [Google Scholar] [CrossRef] [PubMed]
- Tsolekile, N.; Nahle, S.; Zikalala, N.; Parani, S.; Sakho, E.H.M.; Joubert, O.; Matoetoe, M.C.; Songca, S.P.; Oluwafemi, O.S. Cytotoxicity, fluorescence tagging and gene-expression study of CuInS/ZnS QDS—meso (hydroxyphenyl) porphyrin conjugate against human monocytic leukemia cells. Sci. Rep. 2020, 10, 4936. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ncapayi, V.; Ninan, N.; Lebepe, T.C.; Parani, S.; Girija, A.R.; Bright, R.; Vasilev, K.; Maluleke, R.; Tsolekile, N.; Kodama, T.; et al. Diagnosis of Prostate Cancer and Prostatitis Using near Infra-Red Fluorescent AgInSe/ZnS Quantum Dots. Int. J. Mol. Sci. 2021, 22, 12514. https://doi.org/10.3390/ijms222212514
Ncapayi V, Ninan N, Lebepe TC, Parani S, Girija AR, Bright R, Vasilev K, Maluleke R, Tsolekile N, Kodama T, et al. Diagnosis of Prostate Cancer and Prostatitis Using near Infra-Red Fluorescent AgInSe/ZnS Quantum Dots. International Journal of Molecular Sciences. 2021; 22(22):12514. https://doi.org/10.3390/ijms222212514
Chicago/Turabian StyleNcapayi, Vuyelwa, Neethu Ninan, Thabang C. Lebepe, Sundararajan Parani, Aswathy Ravindran Girija, Richard Bright, Krasimir Vasilev, Rodney Maluleke, Ncediwe Tsolekile, Tetsuya Kodama, and et al. 2021. "Diagnosis of Prostate Cancer and Prostatitis Using near Infra-Red Fluorescent AgInSe/ZnS Quantum Dots" International Journal of Molecular Sciences 22, no. 22: 12514. https://doi.org/10.3390/ijms222212514





