Alteration of Gene and miRNA Expression in Cervical Intraepithelial Neoplasia and Cervical Cancer
Abstract
:1. Introduction
2. Results
2.1. TCGA Methylation Data Analysis
2.2. TCGA Gene and miRNA Expression Analysis
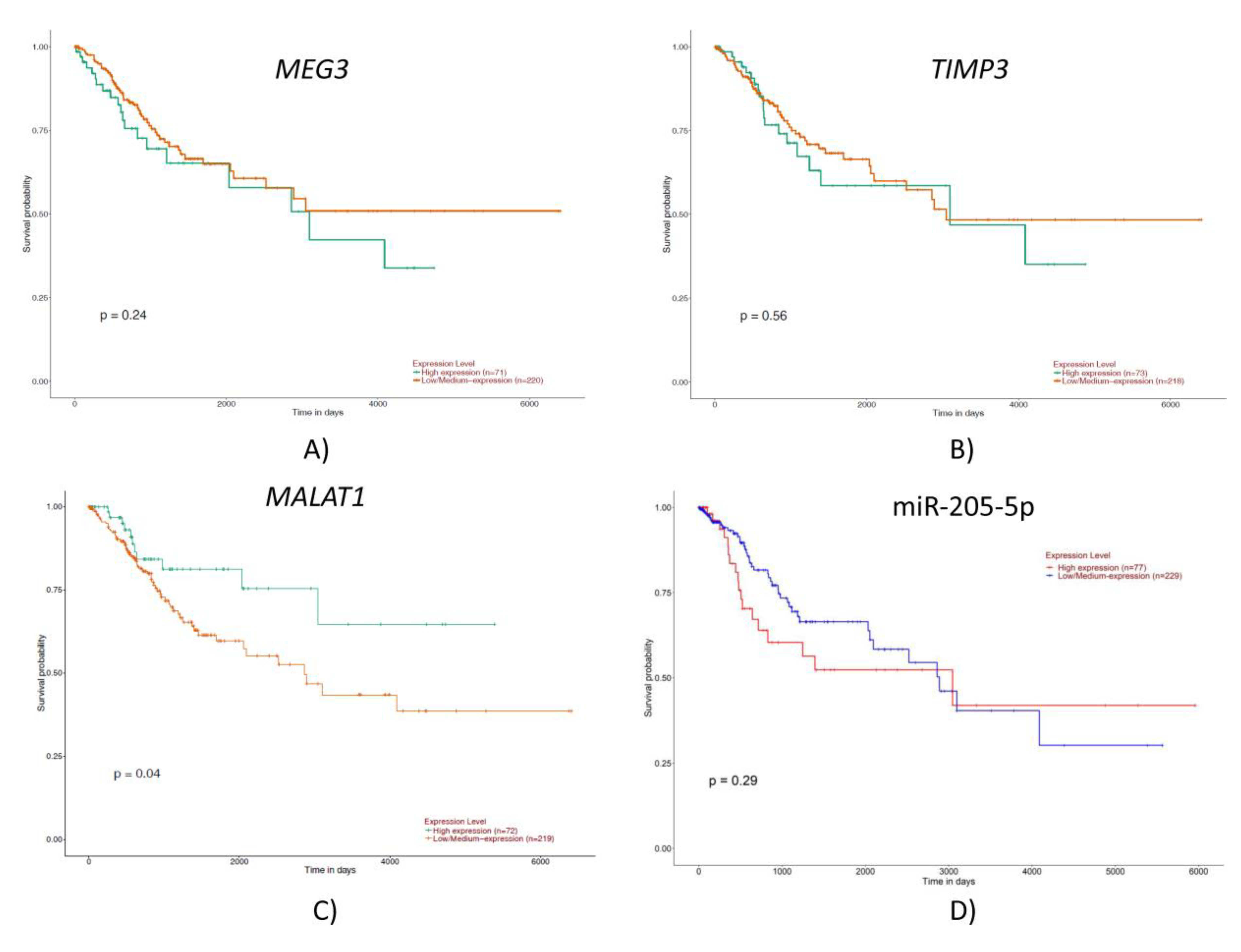
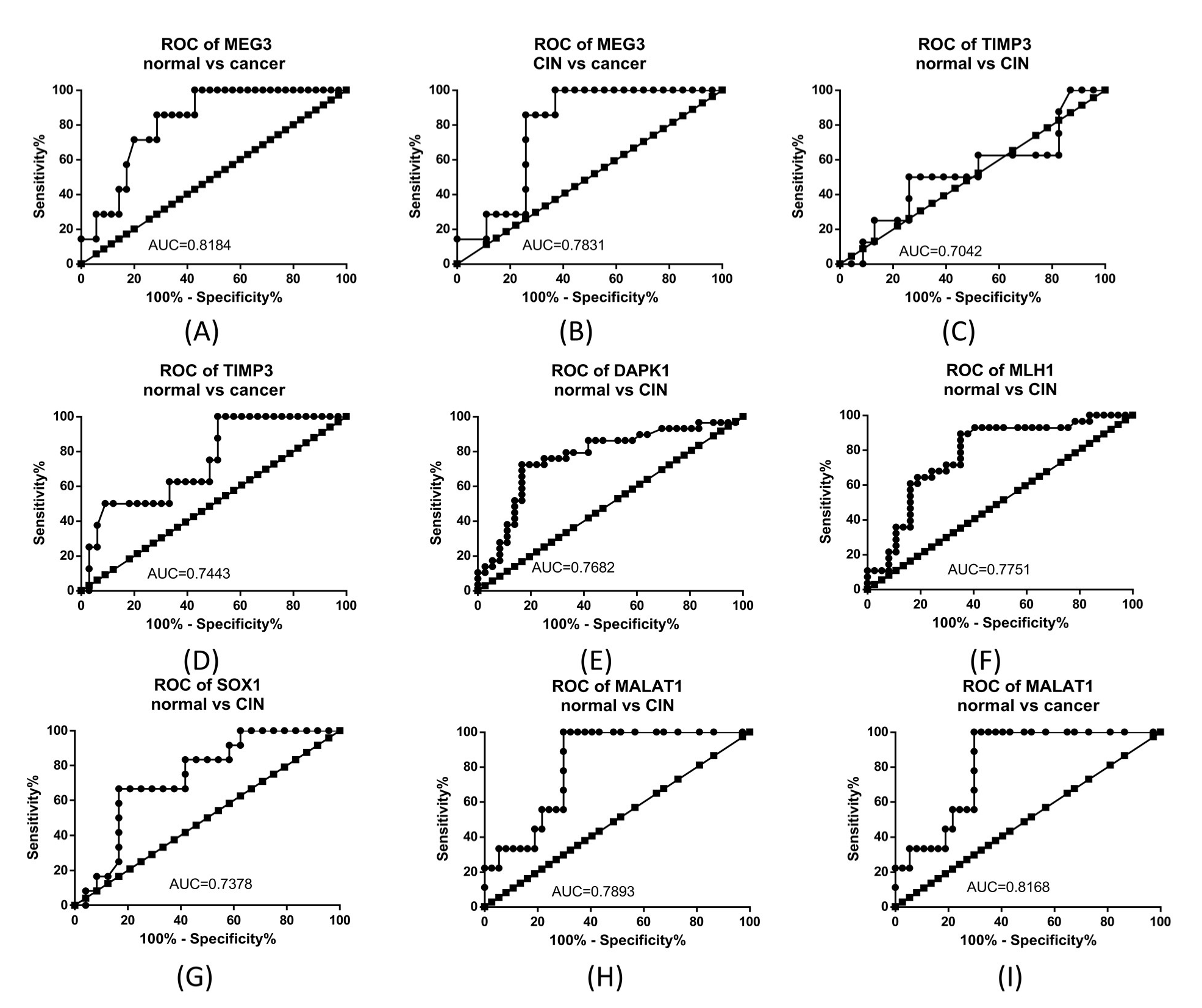
2.3. Gene Expression Analysis
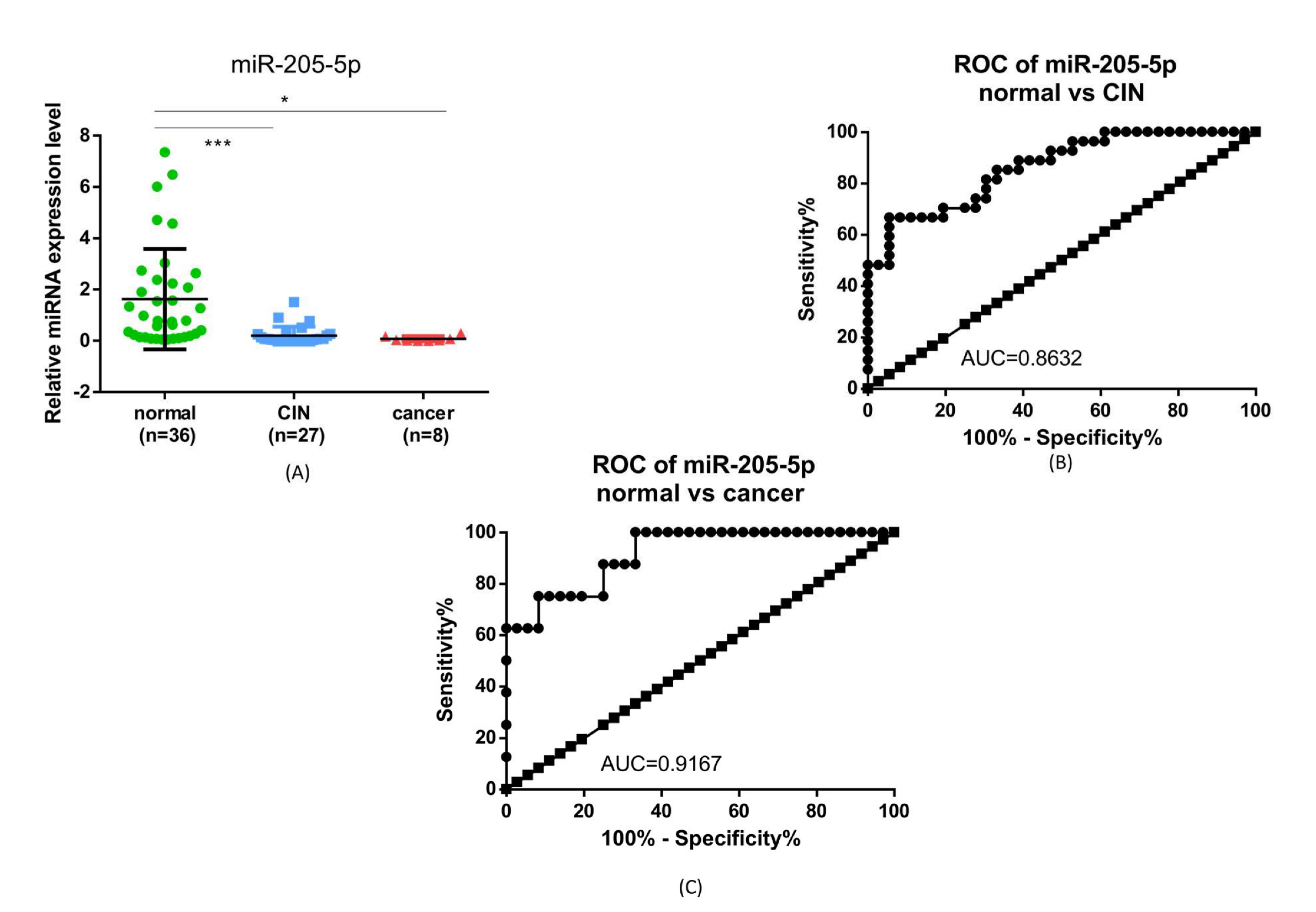
2.4. miR-205-5p Expression
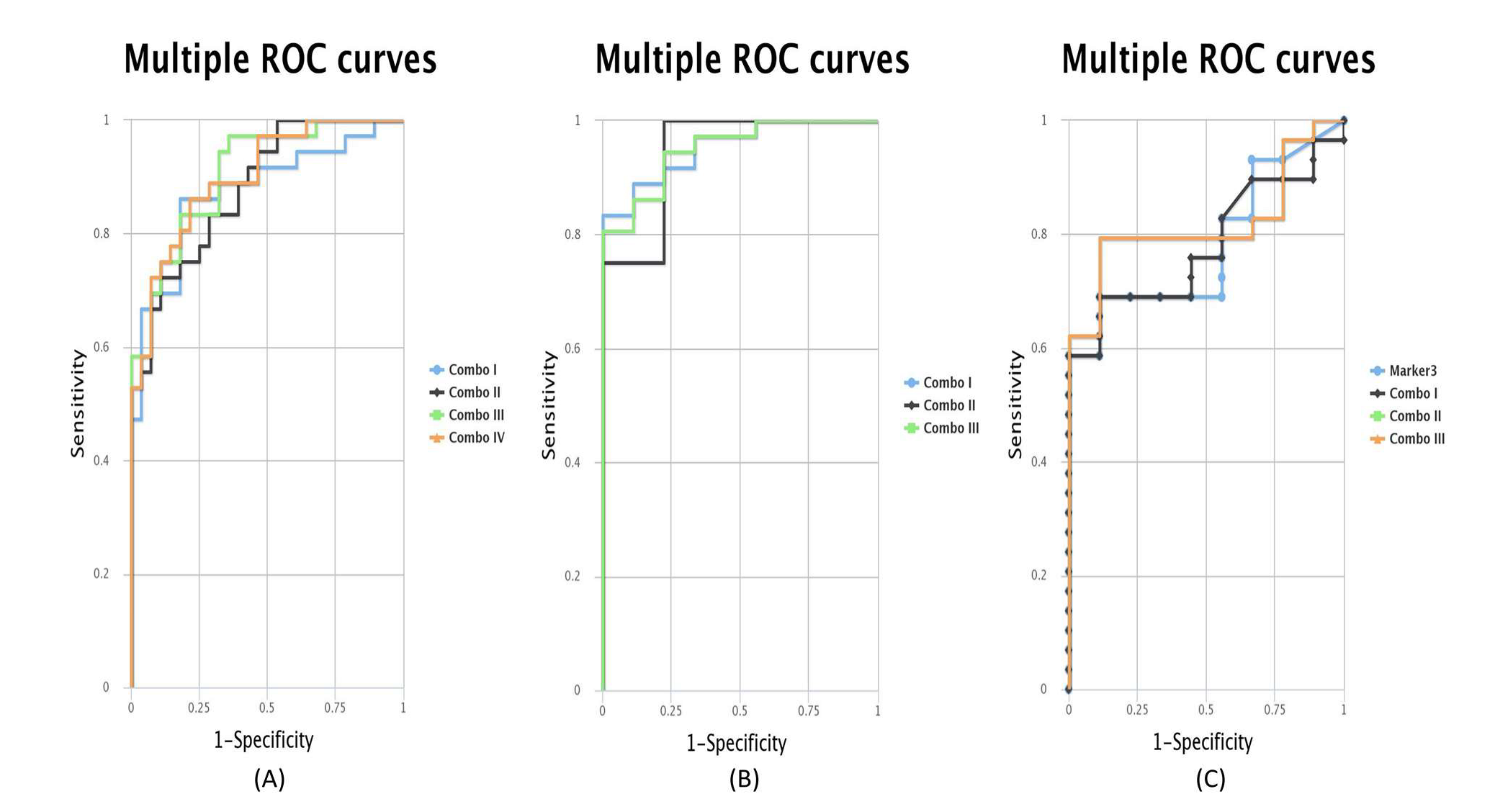
2.5. ROC Curves for the Combination of Candidate Targets
2.6. Correlation of the Expression Data
3. Discussion
4. Materials and Methods
4.1. Selection of Study Group
4.2. TCGA Methylation Data Analysis
4.3. TCGA Gene and miRNA Expression Analysis
4.4. RNA/DNA Extraction
4.5. Gene Expression Analysis
4.6. miR-205-5p Expression Analysis
4.7. ROC Curve Analysis Using CombiROC Sofrware
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Akinlotan, M.; Bolin, J.N.; Helduser, J.; Ojinnaka, C.; Lichorad, A.; McClellan, D. Cervical Cancer Screening Barriers and Risk Factor Knowledge Among Uninsured Women. J. Community Health 2017, 42, 770–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chelimo, C.; Wouldes, T.A.; Cameron, L.D.; Elwood, J.M. Risk factors for and prevention of human papillomaviruses (HPV), genital warts and cervical cancer. J. Infect. 2013, 66, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Curty, G.; de Carvalho, P.S.; Soares, M.A. The Role of the Cervicovaginal Microbiome on the Genesis and as a Biomarker of Premalignant Cervical Intraepithelial Neoplasia and Invasive Cervical Cancer. Int. J. Mol. Sci. 2019, 21, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plisko, O.; Zodzika, J.; Jermakova, I.; Pcolkina, K.; Prusakevica, A.; Liepniece-Karele, I.; Donders, G.G.G.; Rezeberga, D. Aerobic Vaginitis-Underestimated Risk Factor for Cervical Intraepithelial Neoplasia. Diagnostics (Basel, Switzerland) 2021, 11, 97. [Google Scholar] [CrossRef]
- Chang, H.K.; Seo, S.S.; Myong, J.P.; Yu, Y.L.; Byun, S.W. Incidence and costs of cervical intraepithelial neoplasia in the Korean population. J. Gynecol. Oncol. 2019, 30, e37. [Google Scholar] [CrossRef]
- Dudea-Simon, M.; Mihu, D.; Irimie, A.; Cojocneanu, R.; Korban, S.S.; Oprean, R.; Braicu, C.; Berindan-Neagoe, I. Identification of Core Genes Involved in the Progression of Cervical Cancer Using an Integrative mRNA Analysis. Int. J. Mol. Sci. 2020, 21, 7323. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Wang, Y.; Wu, X. The Role of Galectins in Cervical Cancer Biology and Progression. BioMed Res. Int. 2018, 2018, 2175927. [Google Scholar] [CrossRef]
- Liu, X.; Song, J.; Zhang, Y.; Wang, H.; Sun, H.; Feng, X.; Hou, M.; Chen, G.; Tang, Q.; Ji, M. ASF1B promotes cervical cancer progression through stabilization of CDK9. Cell Death Dis. 2020, 11, 705. [Google Scholar] [CrossRef]
- Chen, Y.F.; Shen, M.R. The Important Role of Ion Transport System in Cervical Cancer. Int. J. Mol. Sci. 2021, 23, 333. [Google Scholar] [CrossRef]
- Øvestad, I.T.; Engesæter, B.; Halle, M.K.; Akbari, S.; Bicskei, B.; Lapin, M.; Austdal, M.; Janssen, E.A.M.; Krakstad, C.; Lillesand, M.; et al. High-Grade Cervical Intraepithelial Neoplasia (CIN) Associates with Increased Proliferation and Attenuated Immune Signaling. Int. J. Mol. Sci. 2021, 23, 373. [Google Scholar] [CrossRef]
- De Nola, R.; Menga, A.; Castegna, A.; Loizzi, V.; Ranieri, G.; Cicinelli, E.; Cormio, G. The Crowded Crosstalk between Cancer Cells and Stromal Microenvironment in Gynecological Malignancies: Biological Pathways and Therapeutic Implication. Int. J. Mol. Sci. 2019, 20, 2401. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Steger, A.; Mahner, S.; Jeschke, U.; Heidegger, H. The Formation and Therapeutic Update of Tumor-Associated Macrophages in Cervical Cancer. Int. J. Mol. Sci. 2019, 20, 3310. [Google Scholar] [CrossRef] [Green Version]
- Discacciati, M.G.; da Silva, I.D.; Villa, L.L.; Reis, L.; Hayashi, P.; Costa, M.C.; Rabelo-Santos, S.H.; Zeferino, L.C. Prognostic value of DNA and mRNA e6/e7 of human papillomavirus in the evolution of cervical intraepithelial neoplasia grade 2. Biomark. Insights 2014, 9, 15–22. [Google Scholar] [CrossRef]
- Ostör, A.G. Natural history of cervical intraepithelial neoplasia: A critical review. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 1993, 12, 186–192. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Sui, L.; Wang, J.; Li, F. Phenyllactic acid promotes cell migration and invasion in cervical cancer via IKK/NF-κB-mediated MMP-9 activation. Cancer Cell Int. 2019, 19, 241. [Google Scholar] [CrossRef]
- Cao, L.; Sun, P.L.; He, Y.; Yao, M.; Gao, H. Immune stromal features in cervical squamous cell carcinoma are prognostic factors for distant metastasis: A retrospective study. Pathol. Res. Pract. 2020, 216, 152751. [Google Scholar] [CrossRef]
- Zhou, C.F.; Ma, J.; Huang, L.; Yi, H.Y.; Zhang, Y.M.; Wu, X.G.; Yan, R.M.; Liang, L.; Zhong, M.; Yu, Y.H.; et al. Cervical squamous cell carcinoma-secreted exosomal miR-221-3p promotes lymphangiogenesis and lymphatic metastasis by targeting VASH1. Oncogene 2019, 38, 1256–1268. [Google Scholar] [CrossRef] [Green Version]
- Dudea-Simon, M.; Dudea, S.M.; Burde, A.; Ciortea, R.; Malutan, A.; Mihu, D. Usefulness of real time elastography strain ratio in the assessment of cervical intraepithelial neoplasia and cervical cancer using a reference material. Med. Ultrason. 2020, 22, 145–151. [Google Scholar] [CrossRef]
- Rogeri, C.D.; Silveira, H.C.S.; Causin, R.L.; Villa, L.L.; Stein, M.D.; de Carvalho, A.C.; Arantes, L.; Scapulatempo-Neto, C.; Possati-Resende, J.C.; Antoniazzi, M.; et al. Methylation of the hsa-miR-124, SOX1, TERT, and LMX1A genes as biomarkers for precursor lesions in cervical cancer. Gynecol. Oncol. 2018, 150, 545–551. [Google Scholar] [CrossRef]
- Dudley, J.C.; Lin, M.T.; Le, D.T.; Eshleman, J.R. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 813–820. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Zhang, W.; Huang, W.; Hua, Z.; Li, S. LncRNA MALAT1 was regulated by HPV16 E7 independently of pRB in cervical cancer cells. J. Cancer 2021, 12, 6344–6355. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.H.; Wang, X. lncRNA MEG3 inhibit proliferation and metastasis of gastric cancer via p53 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3850–3856. [Google Scholar] [PubMed]
- Al-Rugeebah, A.; Alanazi, M.; Parine, N.R. MEG3: An Oncogenic Long Non-coding RNA in Different Cancers. Pathol. Oncol. Res. POR 2019, 25, 859–874. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Luo, Y.; Liang, B.; Ye, L.; Lu, G.; He, W. Potential applications of MEG3 in cancer diagnosis and prognosis. Oncotarget 2017, 8, 73282–73295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agodi, A.; Barchitta, M.; Quattrocchi, A.; Maugeri, A.; Vinciguerra, M. DAPK1 Promoter Methylation and Cervical Cancer Risk: A Systematic Review and a Meta-Analysis. PLoS ONE 2015, 10, e0135078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.B.; Cui, N.H.; Liu, X.N.; Ma, J.F.; Zhu, Q.H.; Guo, S.R.; Zhao, J.W.; Ming, L. Identification of DAPK1 Promoter Hypermethylation as a Biomarker for Intra-Epithelial Lesion and Cervical Cancer: A Meta-Analysis of Published Studies, TCGA, and GEO Datasets. Front. Genet. 2018, 9, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, F.; Liu, P.; Ma, C.F. A circulating serum miRNA panel as early detection biomarkers of cervical intraepithelial neoplasia. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4846–4851. [Google Scholar]
- Zhao, S.; Yao, D.; Chen, J.; Ding, N. Circulating miRNA-20a and miRNA-203 for screening lymph node metastasis in early stage cervical cancer. Genet. Test. Mol. Biomark. 2013, 17, 631–636. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, W.; Lv, Q.; Zhu, D. Overexpression of miR-21 promotes the proliferation and migration of cervical cancer cells via the inhibition of PTEN. Oncol. Rep. 2015, 33, 3108–3116. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Yao, D.S.; Chen, J.Y.; Ding, N. Aberrant expression of miR-20a and miR-203 in cervical cancer. Asian Pac. J. Cancer Prev. APJCP 2013, 14, 2289–2293. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, H.K.; Li, Y.; Hafner, M.; Banerjee, N.S.; Tang, S.; Briskin, D.; Meyers, C.; Chow, L.T.; Xie, X.; et al. MicroRNAs are biomarkers of oncogenic human papillomavirus infections. Proc. Natl. Acad. Sci. USA 2014, 111, 4262–4267. [Google Scholar] [CrossRef] [Green Version]
- Gocze, K.; Gombos, K.; Kovacs, K.; Juhasz, K.; Gocze, P.; Kiss, I. MicroRNA expressions in HPV-induced cervical dysplasia and cancer. Anticancer. Res. 2015, 35, 523–530. [Google Scholar]
- Laengsri, V.; Kerdpin, U.; Plabplueng, C.; Treeratanapiboon, L.; Nuchnoi, P. Cervical Cancer Markers: Epigenetics and microRNAs. Lab. Med. 2018, 49, 97–111. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Mazzara, S.; Rossi, R.L.; Grifantini, R.; Donizetti, S.; Abrignani, S.; Bombaci, M. CombiROC: An interactive web tool for selecting accurate marker combinations of omics data. Sci. Rep. 2017, 7, 45477. [Google Scholar] [CrossRef] [Green Version]



| Normal vs. CIN | Normal vs. Cancer | CIN vs. Cancer | ||||||
|---|---|---|---|---|---|---|---|---|
| Combination | Candidates | AUC | Combination | Candidates | AUC | Combination | Candidates | AUC |
| Combo I | MEG3 + MLH1 + miR-205-5p | 0.876 | Combo I | MEG3 + miR-205-5p | 0.954 | Combo I | MEG3 + TIMP3 | 0.782 |
| Combo II | MEG3 + TIMP3 + miR-205-5p | 0.881 | Combo II | TIMP3 + miR-205-5p | 0.944 | Combo II | MEG3 + miR-205-5p | 0.82 |
| Combo III | MLH1 + TIMP3 + miR-205-5p | 0.907 | Combo III | MEG3 + TIMP3 + miR-205-5p | 0.951 | Combo III | MEG3 + TIMP3 + miR-205-5p | 0.82 |
| Combo IV | MEG3 + MLH1 + TIMP3 + miR-205-5p | 0.9 | ||||||
| p Value | MEG3 | TIMP3 | DAPK1 | MLH1 | SOX1 | MALAT1 | miR-205-5p |
|---|---|---|---|---|---|---|---|
| MEG3 | 0.9832 | 0.0005 | 1.74 × 10−14 | 0.8237 | 9.11 × 10−5 | 2.89 × 10−5 | |
| TIMP3 | 0.9832 | 0.0012 | 0.8836 | 0.7740 | 0.0981 | 0.9408 | |
| DAPK1 | 0.0005 | 0.0012 | 5.49 × 10−10 | 0.4147 | 5.20 × 10−14 | 0.0033 | |
| MLH1 | 1.74 × 10−14 | 0.8836 | 5.49 × 10−10 | 0.6617 | 4.36 × 10−19 | 0.0199 | |
| SOX1 | 0.8237 | 0.7740 | 0.4147 | 0.6617 | 0.6888 | 0.7545 | |
| MALAT1 | 9.11 × 10−5 | 0.0981 | 5.20 × 10−14 | 4.36 × 10−19 | 0.6888 | 0.3439 | |
| miR-205-5p | 2.89 × 10−5 | 0.9408 | 0.0033 | 0.0199 | 0.7545 | 0.3439 |
| Demographics | CESC (n = 304) | Cervical Cancer (9) | CIN (30) | Normal (38) | |
|---|---|---|---|---|---|
| Age | Median, Range ♀ | 46, 20–88 | 43, 29–64 | 39, 22–63 | 44, 28–65 |
| HPV Status | Positive | 281 | 9 | 29 | 30 |
| Negative | 22 | 0 | 1 | 8 | |
| Indeterminate | 1 | 0 | |||
| Pathologic TNM | T1 | 140 | 5 | ||
| T2 | 71 | ||||
| T3 | 20 | ||||
| T4 | 10 | ||||
| Tis | 1 | ||||
| Tx | 17 | ||||
| T unknown | 45 | 4 | |||
| N0 | 133 | 5 | |||
| N1 | 60 | ||||
| Nx | 66 | ||||
| N unknown | 45 | 4 | |||
| M0 | 116 | 5 | |||
| M1 | 10 | ||||
| Mx | 128 | ||||
| M unknown | 50 | 4 | |||
| Clinical stage | I | 162 | 1 | ||
| II | 69 | 2 | |||
| III | 45 | 1 | |||
| IV | 21 | ||||
| Unknown | 7 | 5 | |||
| Birth control pill use | Current user | 15 | 1 | 4 | 4 |
| Former user | 53 | 3 | 12 | 15 | |
| Never used | 89 | 5 | 14 | 19 | |
| NA | 147 | ||||
| Histological type | Adenosquamous | 5 | |||
| Cervical squamous cell carcinoma | 252 | 9 | |||
| Endocervical adenocarcinoma of the usual type | 6 | ||||
| Endocervical type of adenocarcinoma | 21 | ||||
| Endometroid adenocarcinoma of endocervix | 3 | ||||
| Mucinous adenocarcinoma of endocervical type | 17 | ||||
| Tobacco smoking history | Lifelong non-smoker | 144 | 5 | 17 | 24 |
| Current smoker | 64 | 4 | 13 | 14 | |
| Reformed smoker > 15 years | 9 | ||||
| Reformed smoker ≤ 15 years | 40 | ||||
| Reformed smoker duration unknown | 4 | ||||
| NA | 43 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudea-Simon, M.; Mihu, D.; Pop, L.A.; Ciortea, R.; Malutan, A.M.; Diculescu, D.; Ciocan, C.A.; Cojocneanu, R.M.; Simon, V.; Bucuri, C.; et al. Alteration of Gene and miRNA Expression in Cervical Intraepithelial Neoplasia and Cervical Cancer. Int. J. Mol. Sci. 2022, 23, 6054. https://doi.org/10.3390/ijms23116054
Dudea-Simon M, Mihu D, Pop LA, Ciortea R, Malutan AM, Diculescu D, Ciocan CA, Cojocneanu RM, Simon V, Bucuri C, et al. Alteration of Gene and miRNA Expression in Cervical Intraepithelial Neoplasia and Cervical Cancer. International Journal of Molecular Sciences. 2022; 23(11):6054. https://doi.org/10.3390/ijms23116054
Chicago/Turabian StyleDudea-Simon, Marina, Dan Mihu, Laura Ancuta Pop, Razvan Ciortea, Andrei Mihai Malutan, Doru Diculescu, Cristina Alexandra Ciocan, Roxana Maria Cojocneanu, Vasile Simon, Carmen Bucuri, and et al. 2022. "Alteration of Gene and miRNA Expression in Cervical Intraepithelial Neoplasia and Cervical Cancer" International Journal of Molecular Sciences 23, no. 11: 6054. https://doi.org/10.3390/ijms23116054






