Expression and Biological Functions of miRNAs in Chronic Pain: A Review on Human Studies
Abstract
:1. Introduction
2. Results
2.1. miRNA Expression in Relation to Chronic Primary Pain
2.1.1. Chronic Widespread Pain
2.1.2. Complex Regional Pain Syndrome
2.1.3. Chronic Primary Headache or Orofacial Pain
2.1.4. Chronic Primary Visceral Pain
2.1.5. Chronic Primary Musculoskeletal Pain
| Type of Pain | miRNAs | miRNA Expression | Extraction Method | Detection Method | Study Design | Sample Size | Tissue | Reference |
|---|---|---|---|---|---|---|---|---|
| Chronic widespread pain | ||||||||
| Fibromyalgia | 103a-3p, 107, 130a-3p 125a-5p | ↓ ↑ | miRNeasy Mini kit (Qiagen, Germany) | qRT-PCR | Cross-sectional | 31 FM 16 HC | Blood | [24] |
| 21-5p, 23a-3p, 23b-3p 29a-3p, 99b-5p, 125b-5p, 145-5p, 195-5p, 223-3p | ↓ | miRNeasy Mini kit (Qiagen, Germany) | qRT-PCR | Cross-sectional | 10 FM 8 HC | Cerebrospinal fluid | [49] | |
| let-7a-5p, 30b-5p, 103a-3p, 107, 151a-5p, 142-3p, 374b-5p 320a-3p | ↓ ↑ | miRNeasy Mini kit (Qiagen, Germany) | qRT-PCR | Cross-sectional | 20 FM 20 HC | Serum | [50] | |
| 143-3p, 145-5p, 223-3p, 338-3p, 451a | ↓ | mirVana miRNA isolation kit | qRT-PCR | Cross-sectional | 11 FM 10 HC | PBMCs | [51] | |
| 1-3p, 23a-3p, 133a, 139-5p, 320b, 346 | ↓ | miRCURY RNA Isolation Kit-Biofluids | qRT-PCR | Cross-sectional | 14 FM 14 HC | Serum | [25] | |
| let-7d-5p, 103a-3p, 146a-5p | ↓ | RNeasy Micro kit (Qiagen, Germany) | qRT-PCR | Cross-sectional | 30 FM (12 reduced IENFD) 34 HC | Skin | [26] | |
| CRPS | let-7a-5p, let-7b-5p, let-7c, 25-5p, 126-3p, 181a-2-3p,320a-3p, 320b, 532-3p, 625-5p, 629-5p, 664a-3p, 939-5p, 1285-3p | ↓ | PAXgene blood miRNA kit (Qiagen, Valencia, CA, USA) | TLDA | Cross-sectional | 41 FM 20 HC | Whole blood | [28] |
| 33 miRNAs (before treatment) 43 miRNAs (after) | Dysregulated | PAXgene blood miRNA kit (Qiagen, Hilden, Germany) | TLDA | Cross-sectional | 7 Responders Ketamine, 6 PR Ketamine | Whole blood | [29] | |
| 34a-5p | ↑ | PAXgene blood RNA tubes (BD Biosciences, Franklin Lakes, NJ, USA) | qRT-PCR | Cross-sectional | 7 Responders Ketamine, 6 PR Ketamine | Whole blood | [30] | |
| Let-7a-5p, 19b-3p, 19b-1-5p, 29b-3p, 30a-5p, 34b-5p, 126-5p, 191-5p, 195-5p, 338-5p, 484, 518b, 542-3p, 551b-5p, 618, 1183, 1274b | Dysregulated | miRvana miRNA isolation kit (Life technologies) | TLDA | Retrospective case series | 3 RE-PE, 3 NR-PE | Serum | [31] | |
| Chronic primary headache or orofacial pain | ||||||||
| Trigeminal neuralgia | 132-3p, 146b-5p, 155-5p, 384 | ↑ | TRIzol reagent (Invitrogen, Carlsbad, CA, USA) | qRT-PCR | Cross-sectional | 28 TN 31 HC | Serum | [33] |
| Migraine | let-7g-5p, 126-3p, 155-5p | ↑ | miRNeasy Serum/ Plasma Kit (Qiagen, Germany) | qRT-PCR | Cross-sectional | 30 MP 30 HC | Plasma | [35] |
| 27b-3p let-7b-5p, 22-3p, 181a-5p | ↑ ↓ | mirCURY LNATM Universal cDNA syn- thesis kit (Exiqon) | qRT-PCR | Cross-sectional | 15 MP 13 HC | PBMC | [52] | |
| 34a-5p, 375 | ↑ | miRNeasy Serum/Plasma Kit (Qiagen, Venlo, The Netherlands) | qRT-PCR | Prospective parallel-group | 12 untreated- MPWA 12 HC | Saliva | [38] | |
| 12 untreated- MPWA 12 treated-MPWA | Saliva and serum | |||||||
| 30a-5p | ↑ in migraine | TRIzol reagent (TaKaRa, Otsu, Shiga, Japan) | qRT-PCR | Cross-sectional | Not reported | Serum | [53] | |
| 34a-5p, 382-5p | ↑ | Direct-zol RNA Mini prep plus (Zymo Research from Aurogene, Rome, Italy) | qRT-PCR | Cross-sectional observational controlled | 28 CM-MO 27 EM | PBMC | [39] | |
| 29c-5p, 34a-5p, 382-5p | ↑ | RNeasy® Plus 96 Kit (Qiagen, Hilden, Germany | qRT-PCR | Cohort | Cohort1: 8 MPA 8 MPPF | Serum | [54] | |
| 29c-5p, 382-5p | Cohort1: 8 MPPF 8 HC | |||||||
| 382-5p | miRNeasy® Serum/Plasma Kit (Qiagen, Hilden, Germany) | Cohort2: 12 MPPF 12 HC | ||||||
| Chronic primary visceral pain | ||||||||
| Irritable bowel syndrome | 199a-5p, 199b-5p | ↓ | Trizol (Invitrogen, Carlsbad, CA, USA) | qRT-PCR | Cross-sectional | 45 IBS-D 40 HC | Colon tissue | [41] |
| 24-3p | ↑ | miREasy Mini Kit Qiagen, Germany | qRT-PCR | Cross-sectional | 10 IBS-D 10 HC | Intestinal mucosa epithelial cells | [55] | |
| 29a-3p | ↑ | miRcute miRNA isolation kit (Tiangen Biotech Co., Ltd.) | qRT-PCR | Cross-sectional | 21 IBS-D 16 HC | Sigmoid colon mucosa | [43] | |
| 106b-5p, 532-5p 363-3p, 338-3p (IBS vs HC) 106b-5p 100-5p, 338-3p (IBS-C vs HC) 219-5p (IBS-D vs HC) | ↑ ↓ ↑ ↓ ↓ | Trizol (Invitrogen, Carlsbad, CA, USA) | nanoString nCounter Assay | Cross-sectional | 29 IBS (15 IBS-C, 14 IBS-D) 15 HC | Colon tissue | [44] | |
| 219-5p, 338-3p (IBS and IBS-C vs HC) | ↓ | qRT-PCR | ||||||
| Chronic primary musculoskeletal pain | 124-3p, 150-5p, 155-5p | ↑ | mirVana miRNA Isolation Kit (Ambion, Austin, TX, USA) | qRT-PCR | Prospective cohort | 34 LBP 20 HC | CD4+ cells | [46] |
2.2. miRNA Expression in Relation to Chronic Secondary Pain
2.2.1. Chronic Post-Surgical Pain or Post-Traumatic Pain
2.2.2. Chronic Neuropathic Pain
2.2.3. Chronic Secondary Visceral Pain
2.2.4. Chronic Secondary Musculoskeletal Pain
| Type of Pain | miRNA | miRNA Expression | Extraction Method | Detection Method | Study Design | Sample Size | Tissue | Reference |
|---|---|---|---|---|---|---|---|---|
| Post-surgical pain | 130b-3p, 145-5p, 146a-5p | ↓ (low-pain relief group vs high-pain relief group) | miRNeasy Serum/Plasma Advanced Kit (QIAGEN, Hilden, Germany) | qRT-PCR | Prospective (1 year) | 136 patients with a knee OA scheduled for total knee replacement, divided at follow-up in 22 low-pain relief group, and 114 high-pain relief group | Serum | [57] |
| Post-traumatic pain | 135a-5p, 3613-3p, 19b-3p, 502-3p 7-5p, 26b-3p, 185-5p | ↑ ↓ | PAXgene blood miRNA kit (Qiagen, Valencia, CA, USA) | qRT-PCR | Prospective (6 weeks) | 53 subjects with a motor vehicle collision, who developed (27) or not (26) persistent axial pain at follow-up | Whole blood | [59] |
| 320a-3p | ↓ (according to presence/severity of persistent axial and widespread musculoskeletal pain) | PAXgene blood miRNA kit (Qiagen) | Sequencing and qRT-PCR | Prospective (6 weeks) | 69 subjects with a motor vehicle collision, assessed for presence/severity of different axial and widespread musculoskeletal pain at follow-up | Whole blood | [60] | |
| 19b-3p | ↓ (women) ↑ (men) | PAXgene blood miRNA kit (Qiagen, Germantown, MD, USA) | Sequencing and qRT-PCR | Prospective (6 weeks) | 179 subjects with a motor vehicle collision, assessed for persistent posttraumatic widespread pain at follow-up | Whole blood | [61] | |
| 14 miRs | Deregulated | PAXgene blood microRNA kit (Qiagen, Germantown, MD, USA) | Sequencing and qRT-PCR | Prospective (1 year) | 43 subjects with a motor vehicle collision, with different neck pain outcomes during follow-up | Whole blood | [62] | |
| Chronic Neuropathic pain | 101-3p | ↓ | Trizol Reagent (Invitrogen, Carlsbad, CA, USA) | qRT-PCR | Cross-sectional | 32 CNP 10 HC | Plasma and sural nerve biopsies | [63] |
| 132-3p | ↑ | miRNEASY kit (Qiagen, Hilden, Germany) | qRT-PCR | Cross-sectional | 30 patients with peripheral neuropathy, 81 patients with painful or painless inflammatory or non-inflammatory neuropathies 30 HC | White blood cells and sural nerve biopsies | [90] | |
| 21-5p, 146a-5p, 155-5p | Deregulated | Not reported | qRT-PCR | Cross-sectional | 76 patients with peripheral neuropathies (of which: 24 with inflammatory neuropathy, 31 with non-inflammatory neuropathy, 21with neuropathy of unknown etiology, 39 with a painful neuropathy and 37 with painless neuropathy) 30 HC | White blood cells, sural nerve, and skin punch biopsies | [65] | |
| 190b, 571, 1276, 1303, 661, 943 | ↑ | TRIzol reagent (Invitrogen, San Diego, CA, USA) | TLDA and qRT-PCR | Cross-sectional | 41 acute Herpes Zoster patients, 35 HC | Serum | [66] | |
| 34c-5p, 107, 127–5p, 486–3p, 892b | ↑ | Trizol Reagent (Invitrogen, Carlsbad, CA, USA) | TLDA and qRT-PCR | Cross-sectional | 37 patients with acute Herpes Zoster, 29 patients with post-herpetic neuralgia | Serum | [91] | |
| 223-3p | ↑ | miRNeasy isolation kit (Qiagen) | qRT-PCR | Prospective (1 year) | 97 patients with lumbar radicular pain after disc herniation | Serum | [92] | |
| 21-5p | ↑ | Trizol Reagent (Invitrogen, Carlsbad, CA, USA) | qRT-PCR | Cross-sectional | 10 patients with lumbar disc herniation accompanied by nerve root pain, 10 patients with lumbar burst fractures (HC) | Nucleus pulposus tissues of intervertebral disc herniation | [93] | |
| Intervertebral disc degeneration | 17 miRNAs 56 miRNAs | ↑ ↓ | TRIzol® reagent (Invitrogen, Carlsbad, CA, USA) | Sequencing | Cross-sectional | 10 IDD 10 HC | Serum | [68] |
| 130b-3p, 200c-3p 10a-5p, 25-3p, 34a-5p, 182-5p | ↑ ↓ | miRNeasy mini kit (Qiagen, Valencia, CA, USA) | qRT-PCR | Cross-sectional | 3 IDD 3 HC | Disc | [94] | |
| 640 | ↑ | TRIzol (Invitrogen, 15596018) | qRT-PCR | Cross-sectional | 15 IDD 5 HC | NP and AF cells | [69] | |
| 185-5p | ↓ | miRNeasy Mini kit (Qiagen GmbH) | qRT-PCR | Cross-sectional | 10 IDD 10 HC | NP cells | [95] | |
| 17-5p | ↑ | miRNeasy serum plasma isolation kit (Qiagen, Hilden, Germany) | qRT-PCR | Cohort | 97 patients with lumbar radicular leg pain and disc herniation | Serum | [70] | |
| Chronic secondary visceral pain | ||||||||
| Bladder syndrome | 320-a-3p, 328-3p, 499b-5p, 500a-5p | ↑ | mirVana miRNA isolation kit (Ambion) | TLDA | Cross-sectional | 8 BPS 4 HC | Dome bladder biopsies | [72] |
| 320a-3p, 320b, 320c, 320d | ↓ | TRIzol reagent (Invitrogen, Carlsbad, CA, USA) | Sequencing | Cross-sectional | 8 IC cases, 5 normal bladder, 5 bladder cases | IC tissues | [73] | |
| Prostatitis | 21-5p, 103a-3p, 141-3p | ↑ | miRCURY RNA isolation kit (Exiqon, Woburn, MA, USA) | qRT-PCR | Prospective study | 21 IIIA CP/CPPS patients | Prostatic secretion | [75] |
| Endometriosis | 185-5p | ↓ | QIAamp miRNA Plasma/Serum Mini kit (QIAGEN, Germany) | qRT-PCR | Case-control | 25 patients 25 HC | Plasma | [77] |
| let-7a-5p, let-7b-5p, let-7d-5p, let-7f-5p, let-7g-5p, let-7i-5p, 199a-3p, 320a-3p, 320b, 320c, 320d, 320e, 328-3p, 331-3p | ↓ | Trizol reagent | qRT-PCR | Cross sectional | 19 OE patients 21 HC | Plasma | [96] | |
| 200c-3p | ↓ | TRIzol reagent (Invitrogen, Carlsbad, CA, USA) | qRT-PCR | Cross-sectional | 27 endometriosis patients, 12 uterine leiomyoma or hysterectomy in patients with grade II–III cervical intraepithelial neoplasia | Ectopic endometrial tissues | [76] | |
| 9-5p, 34b-3p, 34c-5p | ↓ | Trizol reagent (Invitrogen, Carlsbad, CA, USA) | qRT-PCR | Cross-sectional | 4 patients 3 HC | Endometrial biopsies | [97] | |
| Chronic secondary musculoskeletal pain | let-7d-5p, 98-5p 29c-3p, 205-5p, 222-3p, 320a-3p, 423-5p | ↑ (No vs Np) ↓ (No vs Np) | Exiqon miRCURY RNA isolation (Exiqon, Vedbaek, Denmark) | qRT-PCR | Cross-sectional | 100 patients (60 No, 40 Np) | Plasma | [79] |
| 210 | ↑ | Isothiocyanatephenol/chloroform extraction | qRT-PCR | Cross-sectional | 20 early-stage OA patients, 20 late-stage OA patients 10 HC | Synovial fluid from knee joints | [83] | |
| 146a-5p | ↑ | For serum: miRCURY RNA Isolation Kit–Biofluids (Exiqon, Denmark); for cartilage: miRVana miRNA isolation kit (Applied Biosysstems, Life Technologies, Carlsbad, CA, USA) | qRT-PCR | Cross-sectional | 28 OA patients 2 HC | Cartilage tissue and serum | [84] | |
| 34a-5p | ↑ | TRIzol reagent (Invitrogen, Carlsbad, CA, USA) | qRT-PCR | Cross-sectional | 15 OA patients 10 HC | Cartilage tissue and cultured primary chondrocytes | [85] | |
| 665 | ↓ | RNeasy Mini Kit (Qiagen, Valencia, CA, USA) | qRT-PCR | Cross-sectional | 30 OA patients 5 HC | Cartilage tissue and cultured primary chondrocytes | [88] | |
| let-7e-5p, 454-5p 885-5p | ↓ ↑ | miRNeasy kit (Qiagen, Inc., Valencia, CA, USA) | qRT-PCR | Prospective cohort (Bruneck study) | entire cohort (816 subjects) | Serum | [98] | |
| 16-5p, 29c-3p, 93-5p, 126-3p, 146a-5p, 184, 186-5p, 195-5p, 345-5p, 885-5p | ↑ | miRNeasy kit (Qiagen, Inc., Valencia, CA, USA) | qRT-PCR | Cross-sectional | 27 OA patients 27 HC | Plasma | [99] | |
| 9-5p, 138-5p, 146a-5p, 335-5p | ↑ | mirVana miRNA Isolation Kit (Exiqon, Vedbaek, Denmark) | qRT-PCR | Cross-sectional | 40 early and late OA patients 2 HC | Cartilage | [100] | |
| 146a-5p, 155-5p, 181a-5p, 223-3p | ↑ | Trizol reagent (Invitrogen, Carlsbad, CA, USA) | qRT-PCR | Cross-sectional | 36 OA patients 36 HC | PBMC | [100] | |
| 23a-3p, 30c-5p | ↑ | miRNeasy Serum/Plasma Kit (Qiagen, Germany) | qRT-PCR | Prospective (before and 6 months after high tibial osteotomy) | 22 patients with medial compartmental knee OA | Synovial fluid | [89] | |
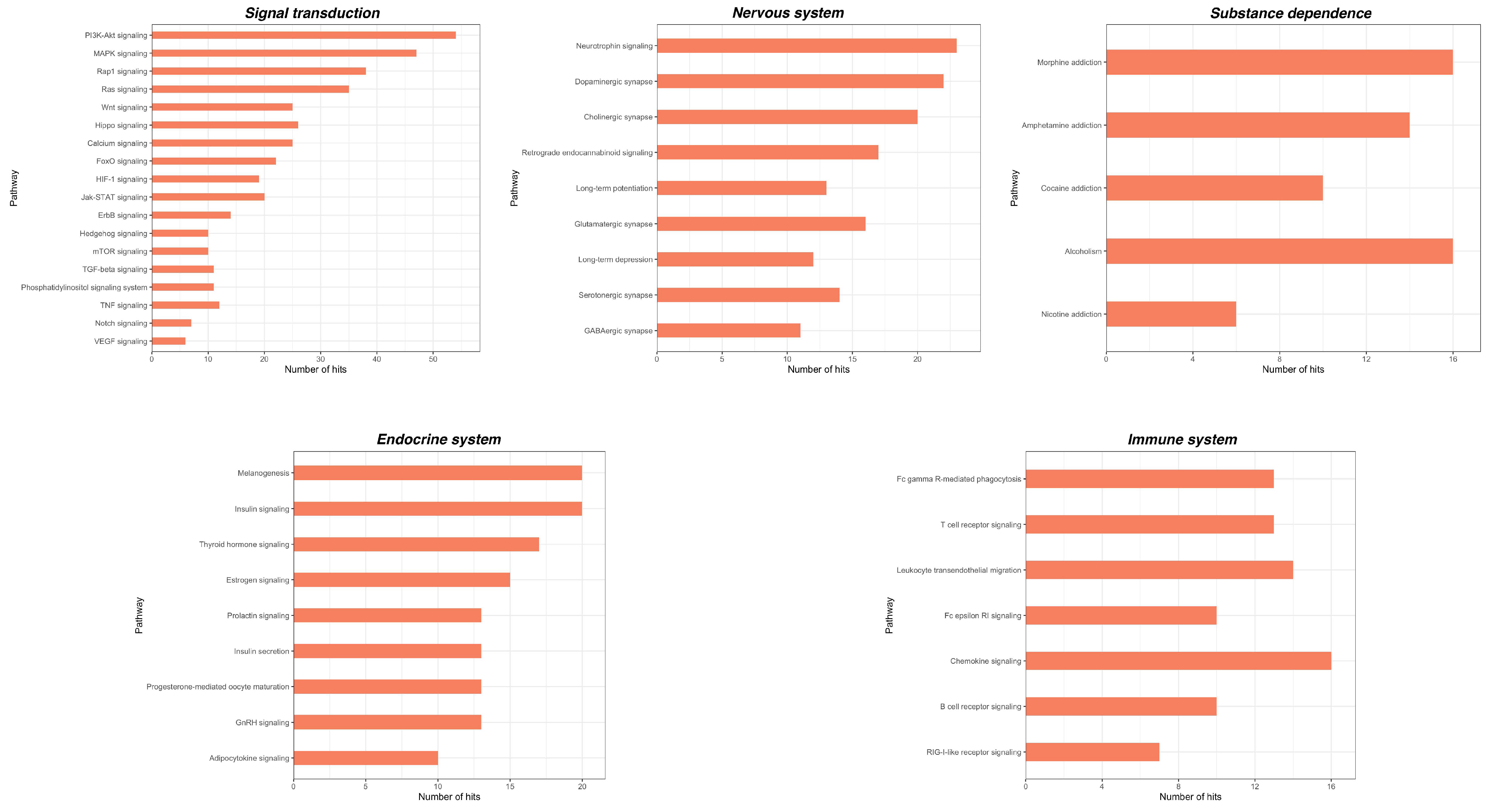
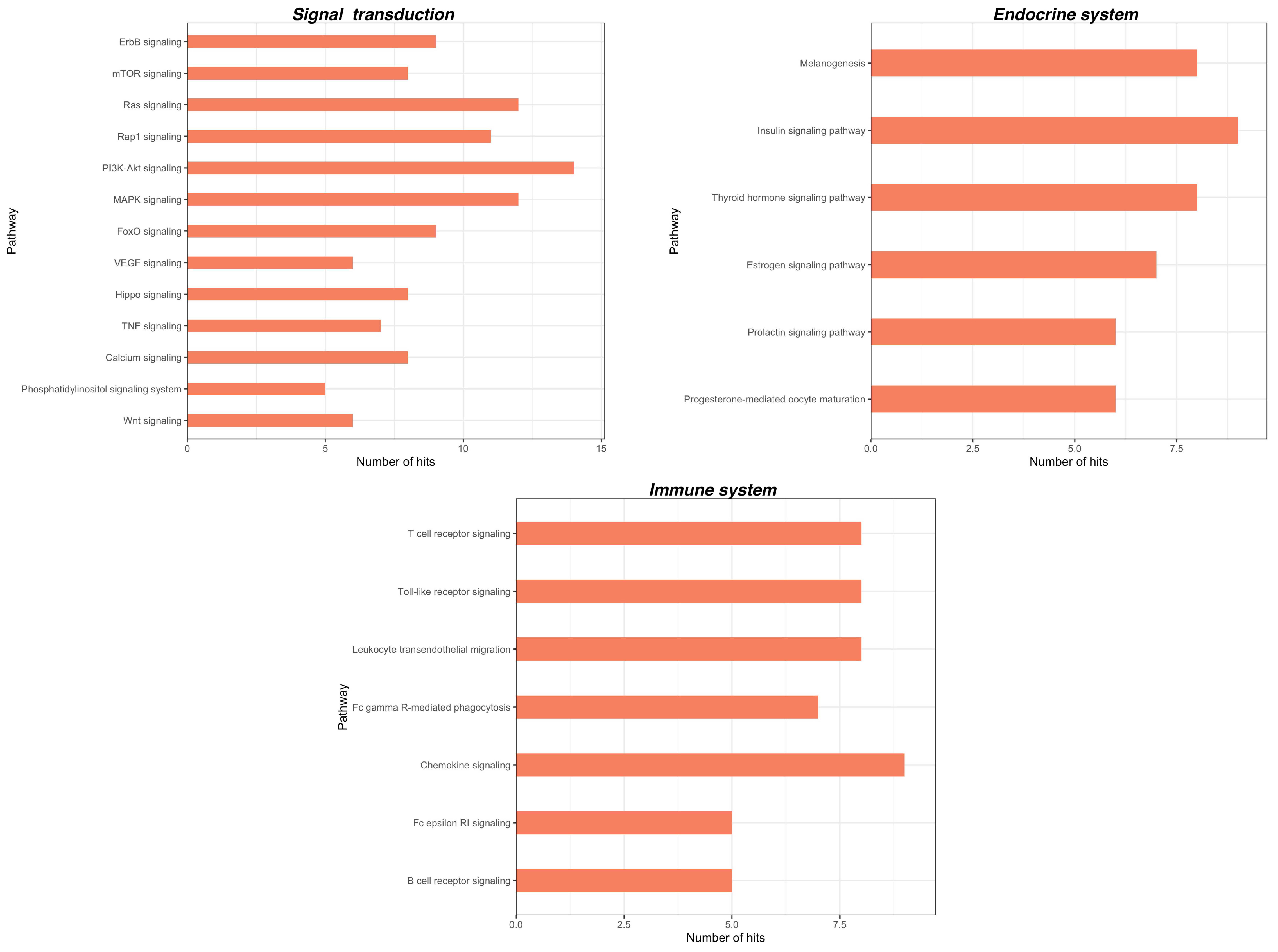
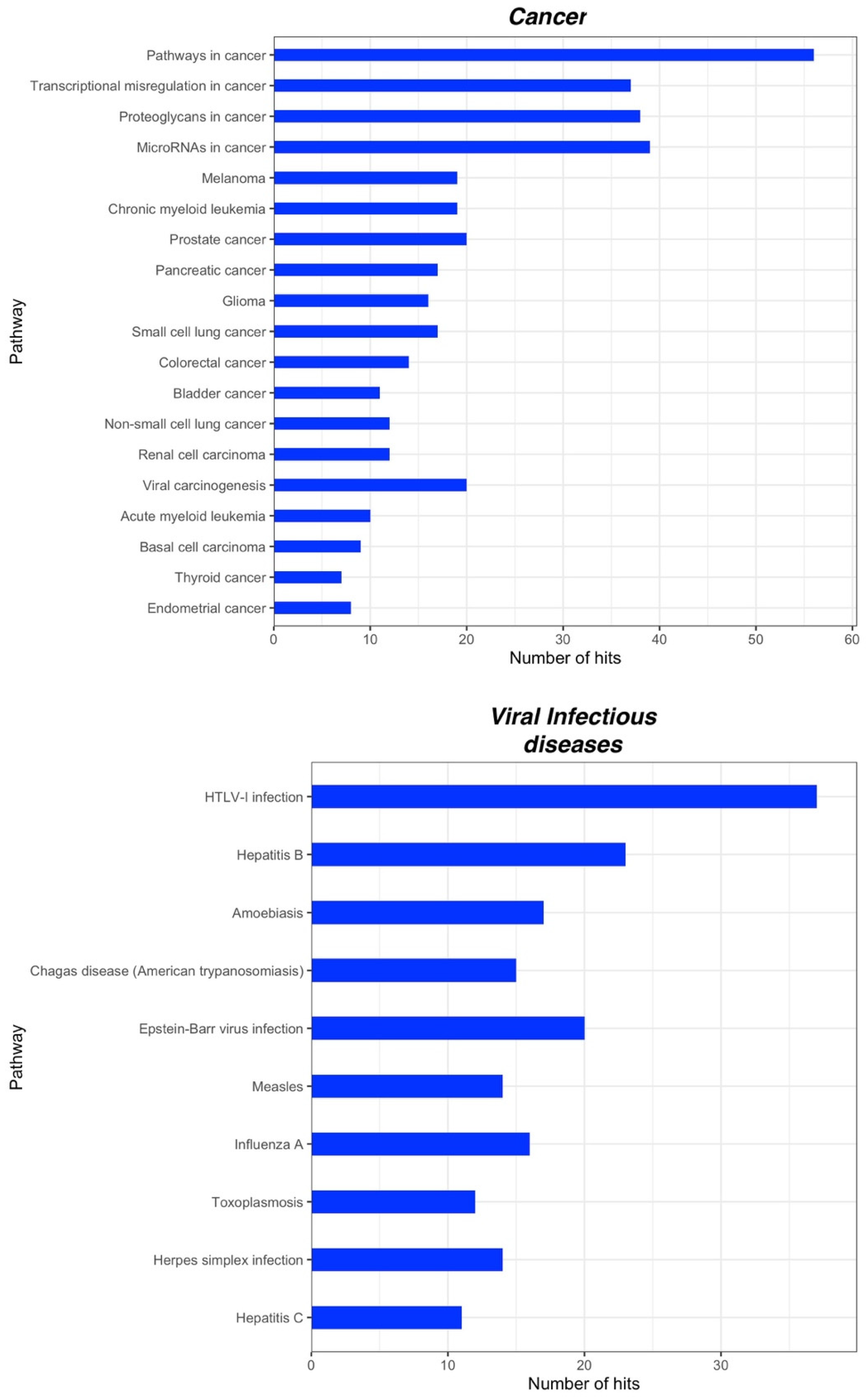
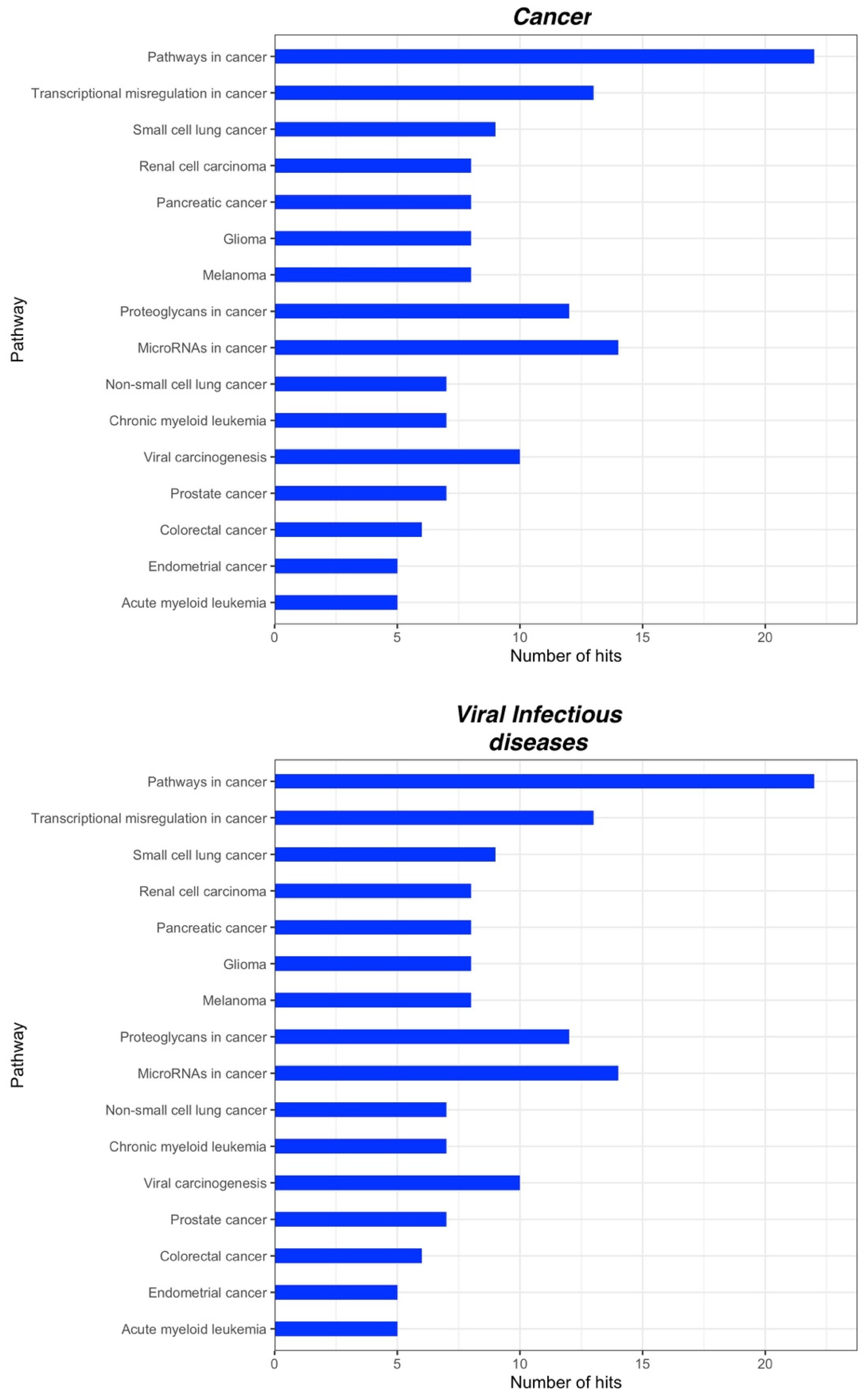
2.3. Bioinformatic Analysis of miRNA Gene Targets and Pathways
3. Discussion
4. Materials and Methods
4.1. Literature Search Strategy
4.2. Bioinformatic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287. [Google Scholar] [CrossRef]
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults—United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef]
- Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; Abegaz, K.H.; Abolhassani, H.; Aboyans, V.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Bernfort, L.; Gerdle, B.; Rahmqvist, M.; Husberg, M.; Levin, L. Severity of chronic pain in an elderly population in Sweden—Impact on costs and quality of life. Pain 2015, 156, 521–527. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, L.F.; Costa-Pereira, A.; Mendonça, L.; Dias, C.C.; Castro-Lopes, J. The economic impact of chronic pain: A nationwide population-based cost-of-illness study in Portugal. Eur. J. Health Econ. 2014, 17, 87–98. [Google Scholar] [CrossRef]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Balzani, E.; Fanelli, A.; Malafoglia, V.; Tenti, M.; Ilari, S.; Corraro, A.; Muscoli, C.; Raffaeli, W. A Review of the Clinical and Therapeutic Implications of Neuropathic Pain. Biomedicines 2021, 9, 1239. [Google Scholar] [CrossRef]
- Huang, Y.; Gu, M.; Tang, Y.; Sun, Z.; Luo, J.; Li, Z. Systematic review and meta-analysis of prognostic microRNA biomarkers for survival outcome in laryngeal squamous cell cancer. Cancer Cell Int. 2021, 21, 316. [Google Scholar] [CrossRef] [PubMed]
- Leo, C.G.; Mincarone, P.; Tumolo, M.R.; Panico, A.; Guido, M.; Zizza, A.; Guarino, R.; De Santis, G.; Sedile, R.; Sabina, S. MiRNA expression profiling in HIV pathogenesis, disease progression and response to treatment: A systematic review. Epigenomics 2021, 13, 1653–1671. [Google Scholar] [CrossRef] [PubMed]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tüfekci, K.U.; Meuwissen, R.L.J.; Genç, Ş. The Role of MicroRNAs in Biological Processes. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2014; Volume 1107, pp. 15–31. [Google Scholar] [CrossRef]
- Machado, M.T.; Navega, S.; Dias, F.; de Sousa, M.J.C.; Teixeira, A.L.; Medeiros, R. microRNAs for peripheral blood fraction identification: Origin, pathways and forensic relevance. Life Sci. 2015, 143, 98–104. [Google Scholar] [CrossRef]
- Hanson, E.K.; Lubenow, H.; Ballantyne, J. Identification of forensically relevant body fluids using a panel of differentially expressed microRNAs. Anal. Biochem. 2009, 387, 303–314. [Google Scholar] [CrossRef]
- Turchinovich, A.; Weiz, L.; Burwinkel, B. Extracellular miRNAs: The mystery of their origin and function. Trends Biochem. Sci. 2012, 37, 460–465. [Google Scholar] [CrossRef]
- Polli, A.; Godderis, L.; Ghosh, M.; Ickmans, K.; Nijs, J. Epigenetic and miRNA Expression Changes in People with Pain: A Systematic Review. J. Pain 2020, 21, 763–780. [Google Scholar] [CrossRef]
- Riolo, G.; Cantara, S.; Marzocchi, C.; Ricci, C. miRNA Targets: From Prediction Tools to Experimental Validation. Methods Protoc. 2020, 4, 1. [Google Scholar] [CrossRef]
- Dinakar, P.; Stillman, A.M. Pathogenesis of Pain. Semin. Pediatr. Neurol. 2016, 23, 201–208. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Nicholas, M.; Vlaeyen, J.W.; Rief, W.; Barke, A.; Aziz, Q.; Benoliel, R.; Cohen, M.; Evers, S.; Giamberardino, M.A.; Goebel, A.; et al. The IASP classification of chronic pain for ICD-11: Chronic primary pain. Pain 2019, 160, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, A.; Evdokimov, D.; Frank, J.; Sommer, C.; Üçeyler, N. MiR103a-3p and miR107 are related to adaptive coping in a cluster of fibromyalgia patients. PLoS ONE 2020, 15, e0239286. [Google Scholar] [CrossRef] [PubMed]
- Masotti, A.; Baldassarre, A.; Guzzo, M.P.; Iannuccelli, C.; Barbato, C.; Di Franco, M. Circulating microRNA Profiles as Liquid Biopsies for the Characterization and Diagnosis of Fibromyalgia Syndrome. Mol. Neurobiol. 2016, 54, 7129–7136. [Google Scholar] [CrossRef] [PubMed]
- Leinders, M.; Doppler, K.; Klein, T.; Deckart, M.; Rittner, H.; Sommer, C.; Üçeyler, N. Increased cutaneous miR-let-7d expression correlates with small nerve fiber pathology in patients with fibromyalgia syndrome. Pain 2016, 157, 2493–2503. [Google Scholar] [CrossRef]
- Philippou, A.; Halapas, A.; Maridaki, M.; Koutsilieris, M. Type I insulin-like growth factor receptor signaling in skeletal muscle regeneration and hypertrophy. J. Musculoskelet. Neuronal Interact. 2007, 7, 208–218. [Google Scholar]
- Orlova, I.A.; Alexander, G.M.; Qureshi, R.A.; Sacan, A.; Graziano, A.; Barrett, J.E.; Schwartzman, R.J.; Ajit, S.K. MicroRNA modulation in complex regional pain syndrome. J. Transl. Med. 2011, 9, 195. [Google Scholar] [CrossRef] [Green Version]
- Douglas, S.R.; Shenoda, B.B.; Qureshi, R.A.; Sacan, A.; Alexander, G.M.; Perreault, M.; Barrett, J.E.; Aradillas-Lopez, E.; Schwartzman, R.J.; Ajit, S.K. Analgesic Response to Intravenous Ketamine Is Linked to a Circulating microRNA Signature in Female Patients with Complex Regional Pain Syndrome. J. Pain 2015, 16, 814–824. [Google Scholar] [CrossRef]
- Shenoda, B.B.; Tian, Y.; Alexander, G.M.; Aradillas-Lopez, E.; Schwartzman, R.J.; Ajit, S.K. miR-34a-mediated regulation of XIST in female cells under inflammation. J. Pain Res. 2018, 11, 935–945. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, S.; Douglas, S.R.; Alexander, G.M.; Shenoda, B.B.; Barrett, J.E.; Aradillas, E.; Sacan, A.; Ajit, S.K. Exosome microRNA signatures in patients with complex regional pain syndrome undergoing plasma exchange. J. Transl. Med. 2019, 17, 81. [Google Scholar] [CrossRef]
- Yadav, Y.R.; Nishtha, Y.; Sonjjay, P.; Vijay, P.; Shailendra, R.; Yatin, K. Trigeminal neuralgia. Asian J. Neurosurg. 2017, 12, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, D.; Zhou, J.; Yan, Y.; Chen, L. Evaluation of circulating microRNA expression in patients with trigeminal neuralgia: An observational study. Medicine 2020, 99, e22972. [Google Scholar] [CrossRef] [PubMed]
- Pietrobon, D.; Moskowitz, M.A. Pathophysiology of Migraine. Annu. Rev. Physiol. 2013, 75, 365–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.-Y.; Chen, S.-P.; Liao, Y.-C.; Fuh, J.-L.; Wang, Y.-F.; Wang, S.-J. Elevated circulating endothelial-specific microRNAs in migraine patients: A pilot study. Cephalalgia 2017, 38, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Schober, A.; Zernecke, A. MicroRNAs in arterial remodelling, inflammation and atherosclerosis. Curr. Drug Targets 2010, 11, 950–956. [Google Scholar] [CrossRef]
- Urbich, C.; Kuehbacher, A.; Dimmeler, S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008, 79, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Gallelli, L.; Cione, E.; Peltrone, F.; Siviglia, S.; Verano, A.; Chirchiglia, D.; Zampogna, S.; Guidetti, V.; Sammartino, L.; Montana, A.; et al. Hsa-miR-34a-5p and hsa-miR-375 as Biomarkers for Monitoring the Effects of Drug Treatment for Migraine Pain in Children and Adolescents: A Pilot Study. J. Clin. Med. 2019, 8, 928. [Google Scholar] [CrossRef] [Green Version]
- Greco, R.; De Icco, R.; DeMartini, C.; Zanaboni, A.M.; Tumelero, E.; Sances, G.; Allena, M.; Tassorelli, C. Plasma levels of CGRP and expression of specific microRNAs in blood cells of episodic and chronic migraine subjects: Towards the identification of a panel of peripheral biomarkers of migraine? J. Headache Pain 2020, 21, 122. [Google Scholar] [CrossRef]
- Saha, L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 6759–6773. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, L.; Larson, S.; Basra, S.; Merwat, S.; Tan, A.; Croce, C.; Verne, G.N. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut 2015, 65, 797–805. [Google Scholar] [CrossRef]
- Dhaka, A.; Uzzell, V.; Dubin, A.E.; Mathur, J.; Petrus, M.J.; Bandell, M.; Patapoutian, A. TRPV1 Is Activated by Both Acidic and Basic pH. J. Neurosci. 2009, 29, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Xiao, X.; Shi, Y.; Wu, Y.; Huang, Y.; Li, D.; Xiong, F.; He, G.; Chai, Y.; Tang, H. Inhibition of miRNA-29a regulates intestinal barrier function in diarrhea-predominant irritable bowel syndrome by upregulating ZO-1 and CLDN1. Exp. Ther. Med. 2020, 20, 155. [Google Scholar] [CrossRef] [PubMed]
- Mahurkar-Joshi, S.; Rankin, C.R.; Videlock, E.J.; Soroosh, A.; Verma, A.; Khandadash, A.; Iliopoulos, D.; Pothoulakis, C.; Mayer, E.A.; Chang, L. The Colonic Mucosal MicroRNAs, MicroRNA-219a-5p, and MicroRNA-338-3p Are Downregulated in Irritable Bowel Syndrome and Are Associated with Barrier Function and MAPK Signaling. Gastroenterology 2021, 160, 2409–2422.e19. [Google Scholar] [CrossRef] [PubMed]
- Airaksinen, O.; Brox, J.I.; Cedraschi, C.; Hildebrandt, J.; Klaber-Moffett, J.; Kovacs, F.; Mannion, A.F.; Reis, S.; Staal, J.B.; Ursin, H.; et al. Chapter 4 European guidelines for the management of chronic nonspecific low back pain. Eur. Spine J. 2006, 15, s192–s300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luchting, B.; Heyn, J.; Hinske, L.C.; Azad, S.C. Expression of miRNA-124a in CD4 Cells Reflects Response to a Multidisciplinary Treatment Program in Patients with Chronic Low Back Pain. Spine 2017, 42, E226–E233. [Google Scholar] [CrossRef]
- Uchida, S.; Hara, K.; Kobayashi, A.; Funato, H.; Hobara, T.; Otsuki, K.; Yamagata, H.; McEwen, B.S.; Watanabe, Y. Early Life Stress Enhances Behavioral Vulnerability to Stress through the Activation of REST4-Mediated Gene Transcription in the Medial Prefrontal Cortex of Rodents. J. Neurosci. 2010, 30, 15007–15018. [Google Scholar] [CrossRef]
- Dwivedi, Y. Emerging role of microRNAs in major depressive disorder: Diagnosis and therapeutic implications. Dialog. Clin. Neurosci. 2014, 16, 43–61. [Google Scholar] [CrossRef]
- Bjersing, J.L.; Lundborg, C.; Bokarewa, M.I.; Mannerkorpi, K. Profile of Cerebrospinal microRNAs in Fibromyalgia. PLoS ONE 2013, 8, e78762. [Google Scholar] [CrossRef] [Green Version]
- Bjersing, J.L.; Bokarewa, M.I.; Mannerkorpi, K. Profile of circulating microRNAs in fibromyalgia and their relation to symptom severity: An exploratory study. Rheumatol. Int. 2014, 35, 635–642. [Google Scholar] [CrossRef]
- Cerdá-Olmedo, G.; Mena-Durán, A.V.; Monsalve, V.; Oltra, E. Identification of a MicroRNA Signature for the Diagnosis of Fibromyalgia. PLoS ONE 2015, 10, e0121903. [Google Scholar] [CrossRef] [Green Version]
- Tafuri, E.; Santovito, D.; De Nardis, V.; Marcantonio, P.; Paganelli, C.; Affaitati, G.; Bucci, M.; Mezzetti, A.; Giamberardino, M.A.; Cipollone, F. MicroRNA profiling in migraine without aura: Pilot study. Ann. Med. 2015, 47, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhu, Y.-Y. MiR-30a relieves migraine by degrading CALCA. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2022–2028. [Google Scholar] [PubMed]
- Andersen, H.H.; Duroux, M.; Gazerani, P. Serum MicroRNA Signatures in Migraineurs During Attacks and in Pain-Free Periods. Mol. Neurobiol. 2015, 53, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.-J.; Mao, W.-M.; Wang, Q.; Yang, G.-G.; Wu, W.-J.; Shao, S.-X. MicroRNA-24 inhibits serotonin reuptake transporter expression and aggravates irritable bowel syndrome. Biochem. Biophys. Res. Commun. 2016, 469, 288–293. [Google Scholar] [CrossRef]
- Brat, G.A.; Agniel, D.; Beam, A.; Yorkgitis, B.; Bicket, M.; Homer, M.; Fox, K.P.; Knecht, D.B.; McMahill-Walraven, C.N.; Palmer, N.; et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: Retrospective cohort study. BMJ 2018, 360, j5790. [Google Scholar] [CrossRef] [Green Version]
- Giordano, R.; Petersen, K.K.; Andersen, H.H.; Lichota, J.; Valeriani, M.; Simonsen, O.; Arendt-Nielsen, L. Preoperative serum circulating microRNAs as potential biomarkers for chronic postoperative pain after total knee replacement. Mol. Pain 2020, 16. [Google Scholar] [CrossRef]
- Vucetic, M.; Roganovic, J.; Freilich, M.; Shafer, D.; Milic, M.; Djukic, L.; Petrovic, N.; Markovic, E.; Markovic, A.; Brkovic, B. Bone microRNA-21 as surgical stress parameter is associated with third molar postoperative discomfort. Clin. Oral Investig. 2020, 25, 319–328. [Google Scholar] [CrossRef]
- Linnstaedt, S.D.; Walker, M.G.; Parker, J.S.; Yeh, E.; Sons, R.L.; Zimny, E.; Lewandowski, C.; Hendry, P.L.; Damiron, K.; Pearson, C.; et al. MicroRNA circulating in the early aftermath of motor vehicle collision predict persistent pain development and suggest a role for microRNA in sex-specific pain differences. Mol. Pain 2015, 11, 66. [Google Scholar] [CrossRef] [Green Version]
- Linnstaedt, S.D.; Riker, K.D.; Walker, M.G.; Nyland, J.E.; Zimny, E.; Lewandowski, C.; Hendry, P.L.; Damiron, K.; Pearson, C.; Velilla, M.-A.; et al. MicroRNA 320a Predicts Chronic Axial and Widespread Pain Development Following Motor Vehicle Collision in a Stress-Dependent Manner. J. Orthop. Sports Phys. Ther. 2016, 46, 911–919. [Google Scholar] [CrossRef]
- Linnstaedt, S.D.; Rueckeis, C.A.; Riker, K.D.; Pan, Y.; Wu, A.; Yu, S.; Wanstrath, B.; Gonzalez, M.; Harmon, E.; Green, P.; et al. MicroRNA-19b predicts widespread pain and posttraumatic stress symptom risk in a sex-dependent manner following trauma exposure. Pain 2019, 161, 47–60. [Google Scholar] [CrossRef]
- Elliott, J.M.; Rueckeis, C.A.; Pan, Y.; Parrish, T.B.; Walton, D.M.; Linnstaedt, S.D. microRNA let-7i-5p mediates the relationship between muscle fat infiltration and neck pain disability following motor vehicle collision: A preliminary study. Sci. Rep. 2021, 11, 3140. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xue, D.; Wang, X.; Ai, D.; Qin, P. MiR-101 relates to chronic peripheral neuropathic pain through targeting KPNB1 and regulating NF-κB signaling. Kaohsiung J. Med. Sci. 2019, 35, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Barczyk, K.; Makuch, W.; Rojewska, E.; Pilat, D.; Mika, J. Inhibition of intracellular signaling pathways NF-κB and MEK1/2 attenuates neuropathic pain development and enhances morphine analgesia. Pharmacol. Rep. 2014, 66, 845–851. [Google Scholar] [CrossRef]
- Leinders, M.; Üçeyler, N.; Thomann, A.; Sommer, C. Aberrant microRNA expression in patients with painful peripheral neuropathies. J. Neurol. Sci. 2017, 380, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, Y.; Zhang, Y.; He, N. Evaluation of microRNA Expression in Patients with Herpes Zoster. Viruses 2016, 8, 326. [Google Scholar] [CrossRef] [Green Version]
- Kalichman, L.; Hunter, D.J. The genetics of intervertebral disc degeneration.Familial predisposition and heritability estimation. Jt. Bone Spine 2008, 75, 383–387. [Google Scholar] [CrossRef]
- Cui, S.; Zhou, Z.; Liu, X.; Richards, R.G.; Alini, M.; Peng, S.; Liu, S.; Zou, X.; Li, Z.; Grad, S. Identification and Characterization of Serum microRNAs as Biomarkers for Human Disc Degeneration: An RNA Sequencing Analysis. Diagnostics 2020, 10, 1063. [Google Scholar] [CrossRef]
- Dong, W.; Liu, J.; Lv, Y.; Wang, F.; Liu, T.; Sun, S.; Liao, B.; Shu, Z.; Qian, J. miR-640 aggravates intervertebral disc degeneration via NF-κB and WNT signalling pathway. Cell Prolif. 2019, 52, e12664. [Google Scholar] [CrossRef] [Green Version]
- Hasvik, E.; Schjølberg, T.; Jacobsen, D.P.; Haugen, A.J.; Grøvle, L.; Schistad, E.I.; Gjerstad, J. Up-regulation of circulating microRNA-17 is associated with lumbar radicular pain following disc herniation. Arthritis Res. Ther. 2019, 21, 186. [Google Scholar] [CrossRef] [Green Version]
- van de Merwe, J.P.; Nordling, J.; Bouchelouche, P.; Bouchelouche, K.; Cervigni, M.; Daha, L.K.; Elneil, S.; Fall, M.; Hohlbrugger, G.; Irwin, P.; et al. Diagnostic Criteria, Classification, and Nomenclature for Painful Bladder Syndrome/Interstitial Cystitis: An ESSIC Proposal. Eur. Urol. 2008, 53, 60–67. [Google Scholar] [CrossRef]
- Freire, V.S.; Burkhard, F.C.; Kessler, T.M.; Kuhn, A.; Draeger, A.; Monastyrskaya, K. MicroRNAs May Mediate the Down-Regulation of Neurokinin-1 Receptor in Chronic Bladder Pain Syndrome. Am. J. Pathol. 2010, 176, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Fuse, M.; Goto, Y.; Kaga, K.; Kurozumi, A.; Yamada, Y.; Sugawara, S.; Okato, A.; Ichikawa, T.; Yamanishi, T.; et al. Molecular pathogenesis of interstitial cystitis based on microRNA expression signature: miR-320 family-regulated molecular pathways and targets. J. Hum. Genet. 2018, 63, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, A.J. Clinical Practice. Chronic Prostatitis and the Chronic Pelvic Pain Syndrome. N. Engl. J. Med. 2006, 355, 1690–1698. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Chen, S.; Zhang, J.; Wang, Y.; Jia, Z.; Zhang, X.; Han, X.; Guo, X.; Sun, X.; Shao, C.; et al. Expression profile of microRNAs in expressed prostatic secretion of healthy men and patients with IIIA chronic prostatitis/chronic pelvic pain syndrome. Oncotarget 2018, 9, 12186–12200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Z.; Chen, Y.; Zhao, Y.; Xu, C.; Zhang, A.; Zhang, Q.; Wang, D.; He, J.; Hua, W.; Duan, P. miR-200c suppresses endometriosis by targeting MALAT1 in vitro and in vivo. Stem Cell Res. Ther. 2017, 8, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razi, M.H.; Eftekhar, M.; Ghasemi, N.; Sheikhha, M.H.; Firoozabadi, A.D. Expression levels of circulatory mir-185-5p, vascular endothelial growth factor, and platelet-derived growth factor target genes in endometriosis. Int. J. Reprod. Biomed. 2020, 18, 347–358. [Google Scholar] [CrossRef]
- Perrot, S.; Cohen, M.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.D.; IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: Chronic secondary musculoskeletal pain. Pain 2019, 160, 77–82. [Google Scholar] [CrossRef]
- Dayer, C.F.; Luthi, F.; Le Carré, J.; Vuistiner, P.; Terrier, P.; Benaim, C.; Giacobino, J.-P.; Léger, B. Differences in the miRNA signatures of chronic musculoskeletal pain patients from neuropathic or nociceptive origins. PLoS ONE 2019, 14, e0219311. [Google Scholar] [CrossRef]
- Schaible, H.-G. Mechanisms of Chronic Pain in Osteoarthritis. Curr. Rheumatol. Rep. 2012, 14, 549–556. [Google Scholar] [CrossRef]
- Fu, K.; Robbins, S.; McDougall, J. Osteoarthritis: The genesis of pain. Rheumatology 2017, 57, iv43–iv50. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, S.; Wibberley, H.; Mapp, P.I.; Hill, R.; Wilson, D.; Walsh, D. Increased vascular penetration and nerve growth in the meniscus: A potential source of pain in osteoarthritis. Ann. Rheum. Dis. 2010, 70, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Su, W.; Xia, H.; Wang, Z.; Su, C.; Su, B. Synovial Fluid MicroRNA-210 as a Potential Biomarker for Early Prediction of Osteoarthritis. BioMed Res. Int. 2019, 2019, 7165406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrzypa, M.; Szala, D.; Gablo, N.; Czech, J.; Pajak, J.; Kopańska, M.; Trzeciak, M.; Gargasz, K.; Snela, S.; Zawlik, I. miRNA-146a-5p is upregulated in serum and cartilage samples of patients with osteoarthritis. Pol. J. Surg. 2018, 91, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Wang, J.; Zhang, Z.; Yang, J. LncRNA SNHG7/miR-34a-5p/SYVN1 axis plays a vital role in proliferation, apoptosis and autophagy in osteoarthritis. Biol. Res. 2020, 53, 9. [Google Scholar] [CrossRef] [PubMed]
- Brandenburger, T.; Johannsen, L.; Prassek, V.; Kuebart, A.; Raile, J.; Wohlfromm, S.; Köhrer, K.; Huhn, R.; Hollmann, M.W.; Hermanns, H. MiR-34a is differentially expressed in dorsal root ganglia in a rat model of chronic neuropathic pain. Neurosci. Lett. 2019, 708, 134365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-H.; Zhang, X.-Q.; Wang, W.-Y.; Xue, Q.-S.; Lu, H.; Huang, J.-L.; Gui, T.; Yu, B.-W. Increased Synaptophysin Is Involved in Inflammation-Induced Heat Hyperalgesia Mediated by Cyclin-Dependent Kinase 5 in Rats. PLoS ONE 2012, 7, e46666. [Google Scholar] [CrossRef]
- Wang, T.; Hao, Z.; Liu, C.; Yuan, L.; Li, L.; Yin, M.; Li, Q.; Qi, Z.; Wang, Z. LEF1 mediates osteoarthritis progression through circRNF121/miR-665/MYD88 axis via NF-κB signaling pathway. Cell Death Dis. 2020, 11, 598. [Google Scholar] [CrossRef]
- Kwak, Y.H.; Kwak, D.-K.; Kim, N.Y.; Kim, Y.J.; Lim, J.S.; Yoo, J.-H. Significant changes in synovial fluid microRNAs after high tibial osteotomy in medial compartmental knee osteoarthritis: Identification of potential prognostic biomarkers. PLoS ONE 2020, 15, e0227596. [Google Scholar] [CrossRef] [Green Version]
- Leinders, M.; Üçeyler, N.; Pritchard, R.; Sommer, C.; Sorkin, L. Increased miR-132-3p expression is associated with chronic neuropathic pain. Exp. Neurol. 2016, 283, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Li, X.; Tao, G.; Zhu, T.; Lin, J. Comparing serum microRNA levels of acute herpes zoster patients with those of postherpetic neuralgia patients. Medicine 2017, 96, e5997. [Google Scholar] [CrossRef]
- Moen, A.; Jacobsen, D.; Phuyal, S.; Legfeldt, A.; Haugen, F.; Røe, C.; Gjerstad, J. MicroRNA-223 demonstrated experimentally in exosome-like vesicles is associated with decreased risk of persistent pain after lumbar disc herniation. J. Transl. Med. 2017, 15, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Zhang, W.; Zhou, T.; Li, W.; Chen, Z.; Ji, C.; Zhang, C.; He, F. Mechanism of microRNA-21 regulating IL-6 inflammatory response and cell autophagy in intervertebral disc degeneration. Exp. Ther. Med. 2017, 14, 1441–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Yu, Q.; Li, H.; Guo, X.; He, X. Characterization of microRNA expression profiles in patients with intervertebral disc degeneration. Int. J. Mol. Med. 2013, 33, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.; Zhang, B.; Sun, C.; Duan, H.; Liu, W.; Mu, K.; Zhao, L.; Li, H.; Dong, Z.; Cui, Q. Circular RNA derived from TIMP2 functions as a competitive endogenous RNA and regulates intervertebral disc degeneration by targeting miR-185-5p and matrix metalloproteinase 2. Int. J. Mol. Med. 2020, 46, 621–632. [Google Scholar] [CrossRef]
- Gu, C.-L.; Zhang, Z.; Fan, W.-S.; Li, L.-A.; Ye, M.-X.; Zhang, Q.; Zhang, N.-N.; Li, Z.; Meng, Y.-G. Identification of MicroRNAs as Potential Biomarkers in Ovarian Endometriosis. Reprod. Sci. 2020, 27, 1715–1723. [Google Scholar] [CrossRef]
- Burney, R.; Hamilton, A.; Aghajanova, L.; Vo, K.; Nezhat, C.; Lessey, B.; Giudice, L. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol. Hum. Reprod. 2009, 15, 625–631. [Google Scholar] [CrossRef]
- Beyer, C.; Zampetaki, A.; Lin, N.-Y.; Kleyer, A.; Perricone, C.; Iagnocco, A.; Distler, A.; Langley, S.R.; Gelse, K.; Sesselmann, S.M.; et al. Signature of circulating microRNAs in osteoarthritis. Ann. Rheum. Dis. 2014, 74, e18. [Google Scholar] [CrossRef] [Green Version]
- Cuadra, V.M.B.; González-Huerta, N.C.; Romero-Cordoba, S.L.; Hidalgo-Miranda, A.; Miranda-Duarte, A. Altered Expression of Circulating MicroRNA in Plasma of Patients with Primary Osteoarthritis and In Silico Analysis of Their Pathways. PLoS ONE 2014, 9, e97690. [Google Scholar] [CrossRef]
- Okuhara, A.; Nakasa, T.; Shibuya, H.; Niimoto, T.; Adachi, N.; Deie, M.; Ochi, M. Changes in microRNA expression in peripheral mononuclear cells according to the progression of osteoarthritis. Mod. Rheumatol. 2011, 22, 446–457. [Google Scholar] [CrossRef]
- Mathé, E.; Nguyen, G.H.; Funamizu, N.; He, P.; Moake, M.; Croce, C.M.; Hussain, S.P. Inflammation regulates microRNA expression in cooperation with p53 and nitric oxide. Int. J. Cancer 2011, 131, 760–765. [Google Scholar] [CrossRef] [Green Version]
- Rukov, J.L.; Shomron, N. MicroRNA pharmacogenomics: Post-transcriptional regulation of drug response. Trends Mol. Med. 2011, 17, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Pehserl, A.-M.; Ress, A.L.; Stanzer, S.; Resel, M.; Karbiener, M.; Stadelmeyer, E.; Stiegelbauer, V.; Gerger, A.; Mayr, C.; Scheideler, M.; et al. Comprehensive Analysis of miRNome Alterations in Response to Sorafenib Treatment in Colorectal Cancer Cells. Int. J. Mol. Sci. 2016, 17, 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finnerty, J.R.; Wang, W.-X.; Hébert, S.; Wilfred, B.R.; Mao, G.; Nelson, P.T. The miR-15/107 Group of MicroRNA Genes: Evolutionary Biology, Cellular Functions, and Roles in Human Diseases. J. Mol. Biol. 2010, 402, 491–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Q.; Ma, Z.; Ding, Y.; Bedarida, T.; Chen, L.; Xie, Z.; Song, P.; Zou, M.-H. Circulating miR-103a-3p contributes to angiotensin II-induced renal inflammation and fibrosis via a SNRK/NF-κB/p65 regulatory axis. Nat. Commun. 2019, 10, 2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarnico, I.; Branca, C.; Lanzillotta, A.; Porrini, V.; Benarese, M.; Spano, P.F.; Pizzi, M. NF-κB and epigenetic mechanisms as integrative regulators of brain resilience to anoxic stress. Brain Res. 2012, 1476, 203–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, H.; Davies, A.M. Regulation of neural process growth, elaboration and structural plasticity by NF-κB. Trends Neurosci. 2011, 34, 316–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alivernini, S.; Gremese, E.; McSharry, C.; Tolusso, B.; Ferraccioli, G.; McInnes, I.B.; Kurowska-Stolarska, M. MicroRNA-155—At the Critical Interface of Innate and Adaptive Immunity in Arthritis. Front. Immunol. 2018, 8, 1932. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Huang, S.; Liu, X.; Zhang, Y.; Wei, S.; Hu, X. miR-155: An Important Role in Inflammation Response. J. Immunol. Res. 2022, 2022, 7437281. [Google Scholar] [CrossRef]
- Chamorro-Jorganes, A.; Araldi, E.; Suárez, Y. MicroRNAs as pharmacological targets in endothelial cell function and dysfunction. Pharmacol. Res. 2013, 75, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Zingale, V.D.; Gugliandolo, A.; Mazzon, E. MiR-155: An Important Regulator of Neuroinflammation. Int. J. Mol. Sci. 2021, 23, 90. [Google Scholar] [CrossRef]
- Ou, G.-Y.; Lin, W.-W.; Zhao, W.-J. Neuregulins in Neurodegenerative Diseases. Front. Aging Neurosci. 2021, 13, 662474. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, A.D.; Fonken, L.K.; Ayala, M.T.; Maier, S.F.; Watkins, L.R. Aging and miR-155 in mice influence survival and neuropathic pain after spinal cord injury. Brain Behav. Immun. 2021, 97, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Zhang, J.; Li, J.; Pang, X.; Wang, H. Inhibition of microRNA-155 Reduces Neuropathic Pain During Chemotherapeutic Bortezomib via Engagement of Neuroinflammation. Front. Oncol. 2020, 10, 416. [Google Scholar] [CrossRef]
- Tan, Y.; Yang, J.; Xiang, K.; Tan, Q.; Guo, Q. Suppression of MicroRNA-155 Attenuates Neuropathic Pain by Regulating SOCS1 Signalling Pathway. Neurochem. Res. 2014, 40, 550–560. [Google Scholar] [CrossRef]
- Roush, S.; Slack, F.J. The let-7 family of microRNAs. Trends Cell Biol. 2008, 18, 505–516. [Google Scholar] [CrossRef]
- Esquela-Kerscher, A.; Slack, F. Oncomirs—microRNAs with a role in cancer. Nat. Cancer 2006, 6, 259–269. [Google Scholar] [CrossRef]
- Cho, K.J.; Song, J.; Oh, Y.; Lee, J.E. MicroRNA-Let-7a regulates the function of microglia in inflammation. Mol. Cell. Neurosci. 2015, 68, 167–176. [Google Scholar] [CrossRef]
- He, Y.; Wang, Z.J. Let-7 microRNAs and Opioid Tolerance. Front. Genet. 2012, 3, 110. [Google Scholar] [CrossRef] [Green Version]
- Yun, Z.; Wang, Y.; Feng, W.; Zang, J.; Zhang, D.; Gao, Y. Overexpression of microRNA-185 alleviates intervertebral disc degeneration through inactivation of the Wnt/β-catenin signaling pathway and downregulation of Galectin-3. Mol. Pain 2020, 16, 1744806920902559. [Google Scholar] [CrossRef]
- Huang, A.; Ji, L.; Huang, Y.; Yu, Q.; Li, Y. miR-185-5p alleviates CCI-induced neuropathic pain by repressing NLRP3 inflammasome through dual targeting MyD88 and CXCR4. Int. Immunopharmacol. 2022, 104, 108508. [Google Scholar] [CrossRef]
- Cheng, Z.; Qiu, S.; Jiang, L.; Zhang, A.; Bao, W.; Liu, P.; Liu, J. MiR-320a is Downregulated in Patients with Myasthenia Gravis and Modulates Inflammatory Cytokines Production by Targeting Mitogen-activated Protein Kinase 1. J. Clin. Immunol. 2012, 33, 567–576. [Google Scholar] [CrossRef]
- Wu, J.; Sabirzhanov, B.; Stoica, B.A.; Lipinski, M.M.; Zhao, Z.; Zhao, S.; Ward, N.; Yang, D.; Faden, A.I. Ablation of the transcription factors E2F1-2 limits neuroinflammation and associated neurological deficits after contusive spinal cord injury. Cell Cycle 2015, 14, 3698–3712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopańska, M.; Szala, D.; Czech, J.; Gabło, N.; Gargasz, K.; Trzeciak, M.; Zawlik, I.; Snela, S. MiRNA expression in the cartilage of patients with osteoarthritis. J. Orthop. Surg. Res. 2017, 12, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Gibson, G.; Kim, J.-S.; Kroin, J.; Xu, S.; van Wijnen, A.J.; Im, H.-J. MicroRNA-146a is linked to pain-related pathophysiology of osteoarthritis. Gene 2011, 480, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakasa, T.; Miyaki, S.; Okubo, A.; Hashimoto, M.; Nishida, K.; Ochi, M.; Asahara, H. Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Care Res. 2008, 58, 1284–1292. [Google Scholar] [CrossRef] [Green Version]
- Ji, R.-R.; Chamessian, A.; Zhang, Y.-Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.; Michailidis, V.; Martin, L.J. Revealing brain mechanisms of mTOR-mediated translational regulation: Implications for chronic pain. Neurobiol. Pain 2018, 4, 27–34. [Google Scholar] [CrossRef]
- Totsch, S.K.; Sorge, R.E. Immune system involvement in specific pain conditions. Mol. Pain 2017, 13. [Google Scholar] [CrossRef] [Green Version]
- Malcangio, M. Role of the immune system in neuropathic pain. Scand. J. Pain 2019, 20, 33–37. [Google Scholar] [CrossRef]
- Khan, N.; Smith, M.T. Neurotrophins and Neuropathic Pain: Role in Pathobiology. Molecules 2015, 20, 10657–10688. [Google Scholar] [CrossRef]
- Wood, P.B. Role of central dopamine in pain and analgesia. Expert Rev. Neurother. 2008, 8, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Elman, I.; Borsook, D. Common Brain Mechanisms of Chronic Pain and Addiction. Neuron 2016, 89, 11–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBlanc, D.M.; McGinn, M.A.; Itoga, C.; Edwards, S. The affective dimension of pain as a risk factor for drug and alcohol addiction. Alcohol 2015, 49, 803–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Tong, H.; Li, T.; Wang, X.; Chen, Y. Research progress in molecular biology related quantitative methods of MicroRNA. Am. J. Transl. Res. 2020, 12, 3198–3211. [Google Scholar]
- Tam, S.; De Borja, R.; Tsao, M.; McPherson, J. Robust global microRNA expression profiling using next-generation sequencing technologies. Lab. Investig. 2014, 94, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Dweep, H.; Gretz, N.; Sticht, C. miRWalk Database for miRNA–Target Interactions. Methods Mol. Biol. 2014, 1182, 289–305. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V.; Da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A Web-Based Tool for the Analysis of Sets through Venn Diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Su, G.; Morris, J.H.; Demchak, B.; Bader, G.D. Biological network exploration with Cytoscape 3. Curr. Protoc. Bioinform. 2014, 47, 8.13.1–8.13.24. [Google Scholar] [CrossRef] [Green Version]
- Stöckel, D.; Kehl, T.; Trampert, P.; Schneider, L.; Backes, C.; Ludwig, N.; Gerasch, A.; Kaufmann, M.; Gessler, M.; Graf, N.; et al. Multi-omics enrichment analysis using the GeneTrail2 web service. Bioinformatics 2016, 32, 1502–1508. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 19 November 2020).

| Chronic Pain | Chronic Primary Pain | Chronic widespread pain Complex regional pain syndrome Chronic primary headache or orofacial pain Chronic primary visceral pain Chronic primary musculoskeletal pain |
| Chronic Secondary Pain | Chronic cancer-related pain Chronic postsurgical or posttraumatic pain Chronic neuropathic pain Chronic secondary headache or orofacial pain Chronic secondary visceral pain Chronic secondary musculoskeletal pain |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabina, S.; Panico, A.; Mincarone, P.; Leo, C.G.; Garbarino, S.; Grassi, T.; Bagordo, F.; De Donno, A.; Scoditti, E.; Tumolo, M.R. Expression and Biological Functions of miRNAs in Chronic Pain: A Review on Human Studies. Int. J. Mol. Sci. 2022, 23, 6016. https://doi.org/10.3390/ijms23116016
Sabina S, Panico A, Mincarone P, Leo CG, Garbarino S, Grassi T, Bagordo F, De Donno A, Scoditti E, Tumolo MR. Expression and Biological Functions of miRNAs in Chronic Pain: A Review on Human Studies. International Journal of Molecular Sciences. 2022; 23(11):6016. https://doi.org/10.3390/ijms23116016
Chicago/Turabian StyleSabina, Saverio, Alessandra Panico, Pierpaolo Mincarone, Carlo Giacomo Leo, Sergio Garbarino, Tiziana Grassi, Francesco Bagordo, Antonella De Donno, Egeria Scoditti, and Maria Rosaria Tumolo. 2022. "Expression and Biological Functions of miRNAs in Chronic Pain: A Review on Human Studies" International Journal of Molecular Sciences 23, no. 11: 6016. https://doi.org/10.3390/ijms23116016









