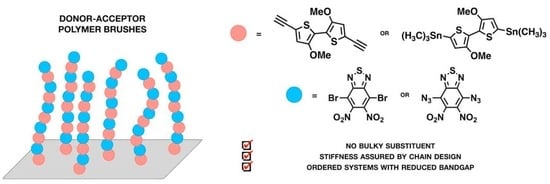
Precise Stepwise Synthesis of Donor-Acceptor Conjugated Polymer Brushes Grafted from Surfaces
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of Donor and Acceptor Moieties
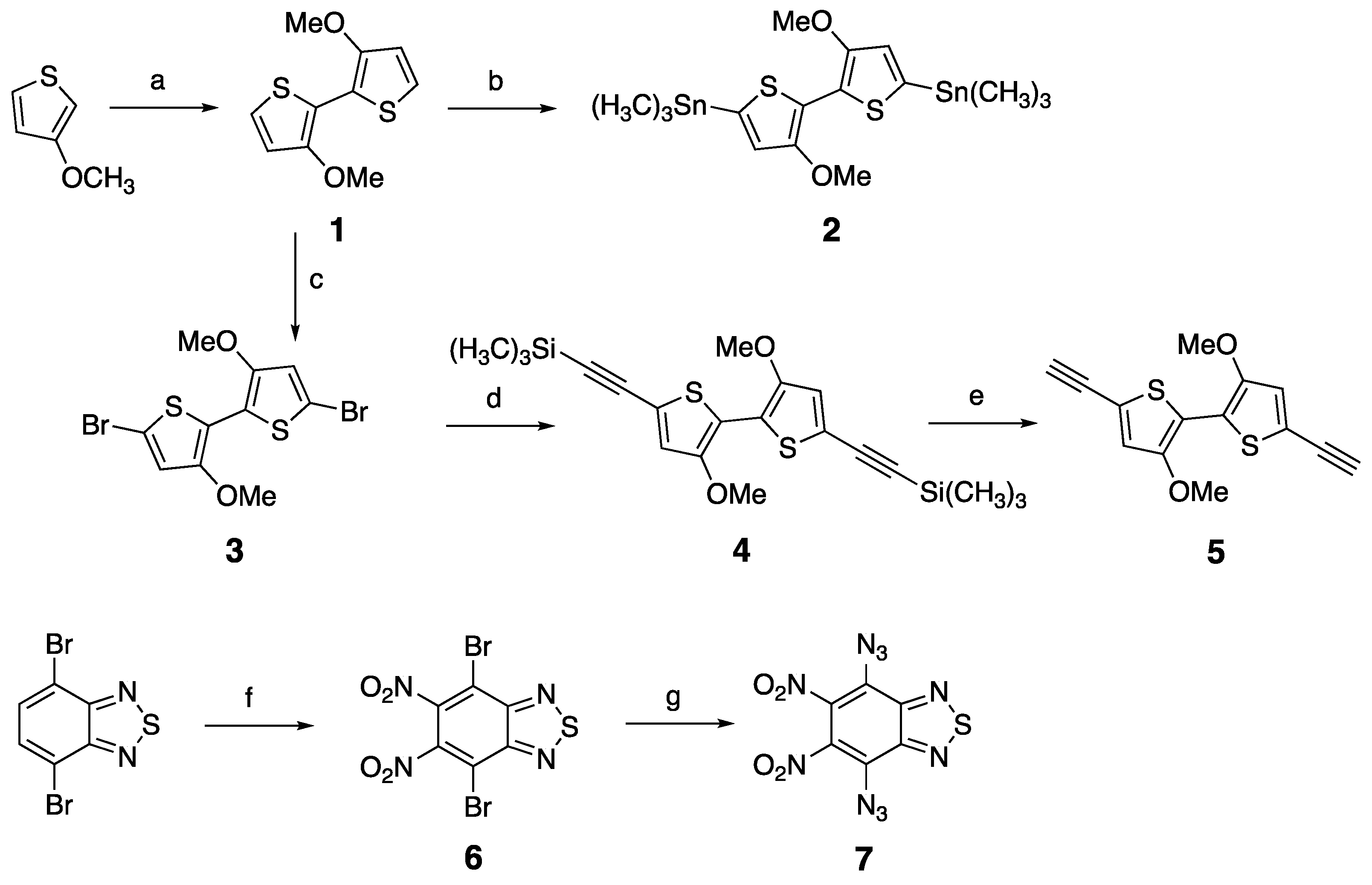
2.2. Synthesis of Monomers
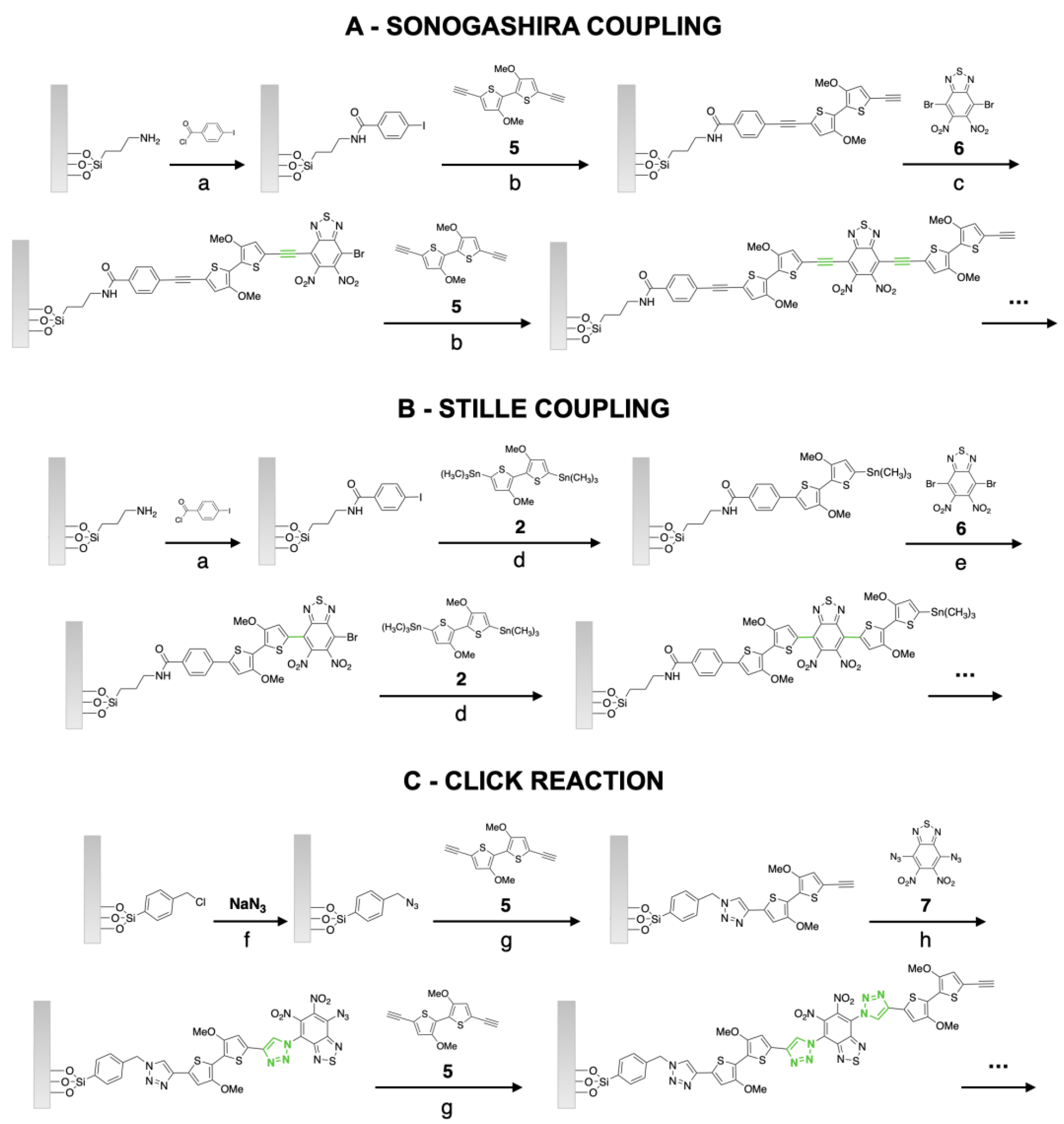
2.3. Synthesis of Grafted D-A Polymer Brushes by Sonogashira Coupling
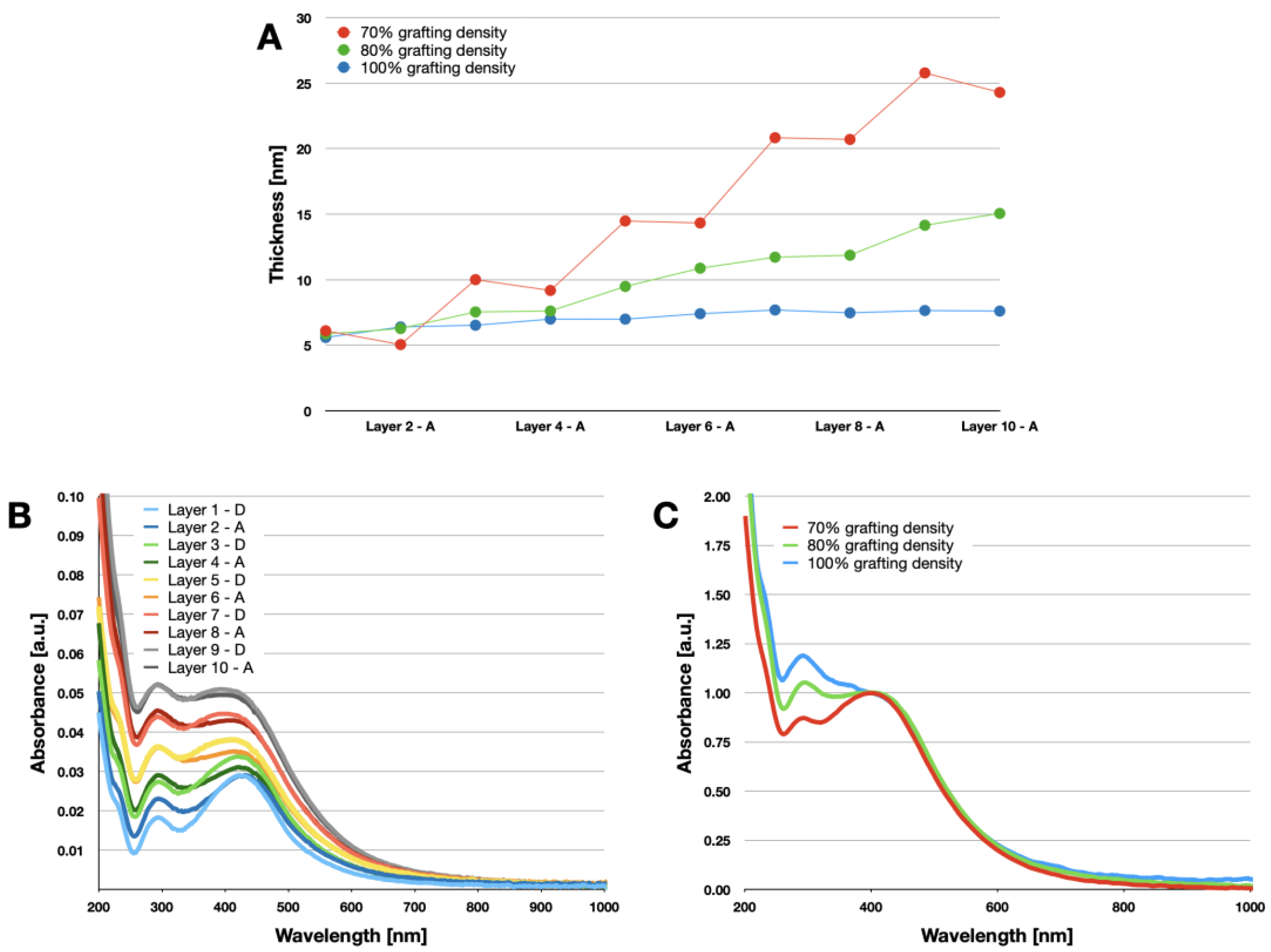
2.3.1. Varying the Grafting Density
2.3.2. Conductivity Measurements
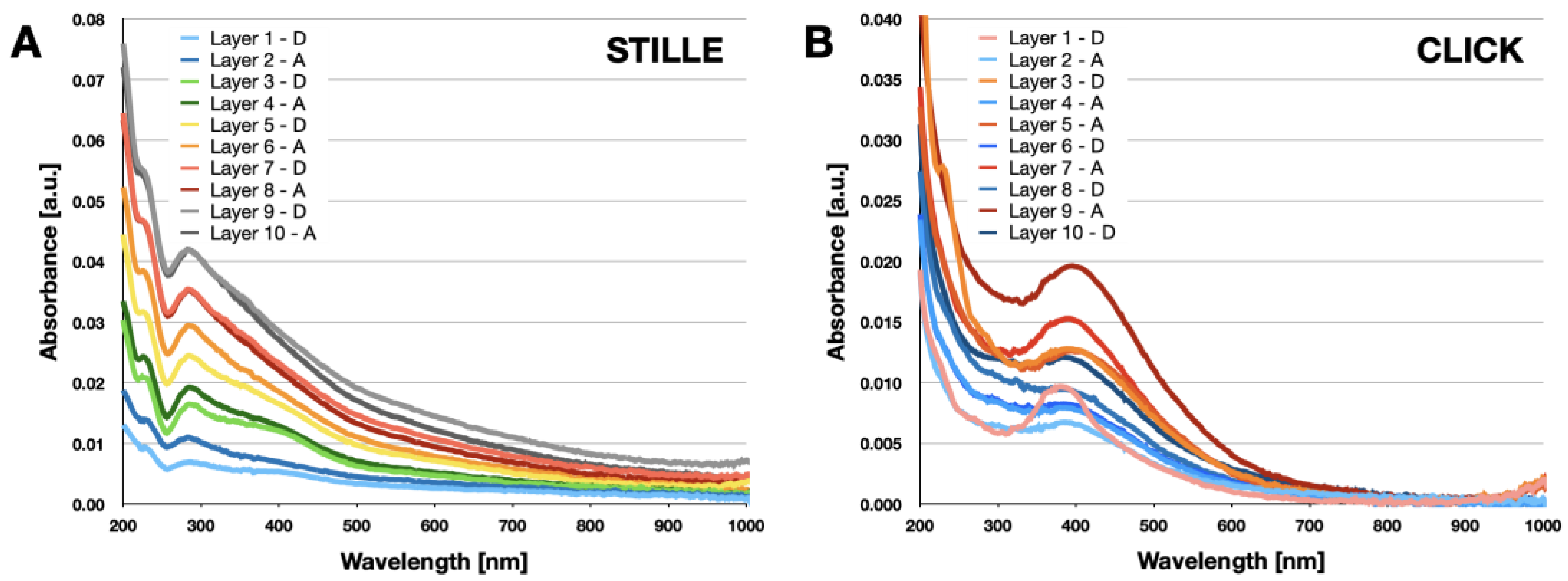
2.4. Synthesis of Grafted D-A Polymer Brushes by Stille Coupling
2.5. Synthesis of Grafted D-A Polymer Brushes by Click Reaction
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. DFT Calculation
3.4. Substrate Preparation
3.5. Preparation of the Initiator Monolayer for the Processes Based on Stille and Sonogashira Coupling
3.6. Preparation of the Initiator Monolayer for the Process Based on Huisgen Cycloaddition (Click Reaction)
3.7. Sonogashira Coupling
3.8. Stille Coupling
3.9. Click Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havinga, E.E.; ten Hoeve, W.; Wynberg, H. Alternate Donor-Acceptor Small-Band-Gap Semiconducting Polymers; Polysquaraines and Polycroconaines. Synth. Met. 1993, 55, 299–306. [Google Scholar] [CrossRef]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Abdullah, S.N.; Brza, M.A. Conducting Polymers for Optoelectronic Devices and Organic Solar Cells: A Review. Polymers 2020, 12, 2627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, C.; Pfattner, R.; Yan, H.; Jin, L.; Chen, S.; Molina-Lopez, F.; Lissel, F.; Liu, J.; Rabiah, N.I.; et al. A Highly Stretchable, Transparent, and Conductive Polymer. Sci. Adv. 2017, 3, e1602076. [Google Scholar] [CrossRef] [Green Version]
- Rao, J.; Yang, L.; Li, X.; Zhao, L.; Wang, S.; Tian, H.; Ding, J.; Wang, L. Sterically-Locked Donor–Acceptor Conjugated Polymers Showing Efficient Thermally Activated Delayed Fluorescence. Angew. Chem. Int. Ed. 2021, 60, 9635–9641. [Google Scholar] [CrossRef] [PubMed]
- Zucca, A.; Yamagishi, K.; Fujie, T.; Takeoka, S.; Mattoli, V.; Greco, F. Roll to Roll Processing of Ultraconformable Conducting Polymer Nanosheets. J. Mater. Chem. C 2015, 3, 6539–6548. [Google Scholar] [CrossRef]
- Someya, T.; Bao, Z.; Malliaras, G.G. The Rise of Plastic Bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef]
- Kwon, J.; Ganapathy, V.; Kim, Y.H.; Song, K.D.; Park, H.G.; Jun, Y.; Yoo, P.J.; Park, J.H. Nanopatterned Conductive Polymer Films as a Pt, TCO-Free Counter Electrode for Low-Cost Dye-Sensitized Solar Cells. Nanoscale 2013, 5, 7838–7843. [Google Scholar] [CrossRef]
- Ando, S.; Nishida, J.; Tada, H.; Inoue, Y.; Tokito, S.; Yamashita, Y. High Performance N-Type Organic Field-Effect Transistors Based on π-Electronic Systems with Trifluoromethylphenyl Groups. J. Am. Chem. Soc. 2005, 127, 5336–5337. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, W.Q.; Xiang, J.; Duan, X.M.; Zhan, X. A Low-Bandgap Conjugated Polymer Based on Squaraine with Strong Two-Photon Absorption. Macromolecules 2011, 44, 3759–3765. [Google Scholar] [CrossRef]
- Deckers, S.; Vandendriessche, S.; Cornelis, D.; Monnaie, F.; Koeckelberghs, G.; Asselberghs, I.; Verbiest, T.; van der Veen, M.A. Poly(3-Alkylthiophene)s Show Unexpected Second-Order Nonlinear Optical Response. Chem. Commun. 2014, 50, 2741–2743. [Google Scholar] [CrossRef] [Green Version]
- Keshtov, M.L.; Kuklin, S.A.; Khokhlov, A.R.; Xie, Z.; Alekseev, V.G.; Dahiya, H.; Singhal, R.; Sharma, G.D. New Medium Bandgap Donor D-A1-D-A2 Type Copolymers Based on Anthra [1,2-b: 4,3-b“:6,7-c”‘] Trithiophene-8,12-dione Groups for High-Efficient Non-Fullerene Polymer Solar Cells. Macromol. Rapid Commun. 2022, 43, 2100839. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, B.; Li, H.; Cheng, W.; Xue, L.; Chen, F.; Lu, H.; Tian, W. Molecular Engineering of Copolymers with Donor-Acceptor Structure for Bulk Heterojunction Photovoltaic Cells toward High Photovoltaic Performance. J. Phys. Chem. C 2011, 115, 2386–2397. [Google Scholar] [CrossRef]
- Mahesh, K.; Karpagam, S.; Pandian, K. How to Design Donor–Acceptor Based Heterocyclic Conjugated Polymers for Applications from Organic Electronics to Sensors. Top. Curr. Chem. 2019, 377, 12. [Google Scholar] [CrossRef] [PubMed]
- Yuen, J.D.; Fan, J.; Seifter, J.; Lim, B.; Hufschmid, R.; Heeger, A.J.; Wudl, F. High Performance Weak Donor-Acceptor Polymers in Thin Film Transistors: Effect of the Acceptor on Electronic Properties, Ambipolar Conductivity, Mobility, and Thermal Stability. J. Am. Chem. Soc. 2011, 133, 20799–20807. [Google Scholar] [CrossRef]
- Jiang, J.M.; Yuan, M.C.; Dinakaran, K.; Hariharan, A.; Wei, K.H. Crystalline Donor-Acceptor Conjugated Polymers for Bulk Heterojunction Photovoltaics. J. Mater. Chem. A 2013, 1, 4415–4422. [Google Scholar] [CrossRef]
- Mahesh, K.; Karpagam, S.; Putnin, T.; Le, H.; Bui, T.T.; Ounnunkad, K.; Goubard, F. Role of Cyano Substituents on Thiophene Vinylene Benzothiadiazole Conjugated Polymers and Application as Hole Transporting Materials in Perovskite Solar Cells. J. Photochem. Photobiol. A Chem. 2019, 371, 238–247. [Google Scholar] [CrossRef]
- Huo, L.; Hou, J.; Zhang, S.; Chen, H.Y.; Yang, Y. A Poh Benzo[1,2-6:4,5-B′]Dithiophene Derivative with Deep HOMO Level and Its Application in High-Performance Polymer Solar Cells. Angew. Chem. Int. Ed. 2010, 49, 1500–1503. [Google Scholar] [CrossRef]
- Yano, H.; Kudo, K.; Marumo, K.; Okuzaki, H. Fully Soluble Self-Doped Poly(3,4-Ethylenedioxythiophene) with an Electrical Conductivity Greater than 1000 S Cm −1. Sci. Adv. 2019, 5, eaav9492. [Google Scholar] [CrossRef] [Green Version]
- Namsheer, K.; Rout, C.S. Conducting Polymers: A Comprehensive Review on Recent Advances in Synthesis, Properties and Applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Whiting, G.L.; Snaith, H.J.; Khodabakhsh, S.; Andreasen, J.W.; Breiby, D.W.; Nielsen, M.M.; Greenham, N.C.; Friend, R.H.; Huck, W.T.S. Enhancement of Charge-Transport Characteristics in Polymeric Films Using Polymer Brushes. Nano Lett. 2006, 6, 573–578. [Google Scholar] [CrossRef]
- Mukherjee, R.; Sharma, A. Instability, Self-Organization and Pattern Formation in Thin Soft Films. Soft Matter 2015, 11, 8717–8740. [Google Scholar] [CrossRef] [PubMed]
- Wolski, K.; Szuwarzyński, M.; Kopeć, M.; Zapotoczny, S. Ordered Photo- and Electroactive Thin Polymer Layers. Eur. Polym. J. 2015, 65, 155–170. [Google Scholar] [CrossRef]
- Szuwarzyński, M.; Wolski, K.; Zapotoczny, S. Enhanced Stability of Conductive Polyacetylene in Ladder-like Surface-Grafted Brushes. Polym. Chem. 2016, 7, 5664–5670. [Google Scholar] [CrossRef]
- Eder, T.; Vogelsang, J.; Bange, S.; Remmerssen, K.; Schmitz, D.; Jester, S.; Keller, T.J.; Höger, S.; Lupton, J.M. Interplay Between J- and H-Type Coupling in Aggregates of Π-Conjugated Polymers: A Single-Molecule Perspective. Angew. Chem. 2019, 131, 19074–19078. [Google Scholar] [CrossRef] [Green Version]
- Besford, Q.A.; Yong, H.; Merlitz, H.; Christofferson, A.J.; Sommer, J.U.; Uhlmann, P.; Fery, A. FRET-Integrated Polymer Brushes for Spatially Resolved Sensing of Changes in Polymer Conformation. Angew. Chem. Int. Ed. 2021, 60, 16600–16606. [Google Scholar] [CrossRef]
- Tria, M.C.; Liao, K.S.; Alley, N.; Curran, S.; Advincula, R. Electrochemically Crosslinked Surface-Grafted PVK Polymer Brushes as a Hole Transport Layer for Organic Photovoltaics. J. Mater. Chem. 2011, 21, 10261–10264. [Google Scholar] [CrossRef]
- Page, Z.A.; Narupai, B.; Pester, C.W.; Bou Zerdan, R.; Sokolov, A.; Laitar, D.S.; Mukhopadhyay, S.; Sprague, S.; McGrath, A.J.; Kramer, J.W.; et al. Novel Strategy for Photopatterning Emissive Polymer Brushes for Organic Light Emitting Diode Applications. ACS Cent. Sci. 2017, 3, 654–661. [Google Scholar] [CrossRef] [Green Version]
- Lishchuk, A.; Csányi, E.; Darroch, B.; Wilson, C.; Nabok, A.; Leggett, G.J. Active control of strong plasmon–exciton coupling in biomimetic pigment–polymer antenna complexes grown by surface-initiated polymerisation from gold nanostructures. Chem. Sci. 2022, 13, 2405–2417. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, N.; Chen, H.; Bai, L.; Xu, H.; Wang, W.; Yang, H.; Wei, D.; Yang, L.; Cheng, Z. Preparation of a Novel Sandwich-Type Electrochemical Immunosensor for AFP Detection Based on an ATRP and Click Chemistry Technique. Polym. Chem. 2020, 11, 900–908. [Google Scholar] [CrossRef]
- Zhang, L.; Bei, H.P.; Piao, Y.; Wang, Y.; Yang, M.; Zhao, X. Polymer-Brush-Grafted Mesoporous Silica Nanoparticles for Triggered Drug Delivery. ChemPhysChem 2018, 19, 1956–1964. [Google Scholar] [CrossRef] [Green Version]
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.A. Surface-Initiated Controlled Radical Polymerization: State-of-the-Art, Opportunities, and Challenges in Surface and Interface Engineering with Polymer Brushes. Chem. Rev. 2017, 117, 1105–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zdyrko, B.; Luzinov, I. Polymer Brushes by the “Grafting to” Method. Macromol. Rapid Commun. 2011, 32, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Wolski, K.; Gruszkiewicz, A.; Wytrwal-Sarna, M.; Bernasik, A.; Zapotoczny, S. The Grafting Density and Thickness of Polythiophene-Based Brushes Determine the Orientation, Conjugation Length and Stability of the Grafted Chains. Polym. Chem. 2017, 8, 6250–6262. [Google Scholar] [CrossRef]
- Wolski, K.; Gruszkiewicz, A.; Zapotoczny, S. Conductive Polythiophene-Based Brushes Grafted from an ITO Surface via a Self-Templating Approach. Polym. Chem. 2015, 6, 7487–7632. [Google Scholar] [CrossRef]
- Vonwald, I.A.; Moog, M.M.; Lajoie, T.W.; Yablonski, J.D.; Delongchamp, D.M.; Locklin, J.; Tsui, F.; You, W. Morphology, Structure, and Enhanced Intramolecular Conduction in Ultralong Conjugated Polymer Brushes. J. Phys. Chem. C 2018, 122, 7586–7596. [Google Scholar] [CrossRef]
- Phuong Le, A.; Huang, T.M.; Chen, P.T.; Yang, A.C.M. Synthesis and Optoelectronic Behavior of Conjugated Polymer Poly(3-Hexylthiophene) Grafted on Multiwalled Carbon Nanotubes. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 581–590. [Google Scholar] [CrossRef]
- Słowikowska, M.; Wójcik, A.J.; Wolski, K.; Hatalak, A.; Zapotoczny, S. Light-Promoted Synthesis of Surface-Grafted Polymers Bearing Pyridine Groups by Metal-Free ATRP in Microliter Volumes. Polymer 2021, 234, 124244. [Google Scholar] [CrossRef]
- Ren, S.; Dawson, R.; Adams, D.J.; Cooper, A.I. Low Band-Gap Benzothiadiazole Conjugated Microporous Polymers. Polym. Chem. 2013, 4, 5585–5590. [Google Scholar] [CrossRef]
- Michinobu, T. Click Synthesis of Donor-Acceptor-Type Aromatic Polymers. Pure Appl. Chem. 2010, 82, 1001–1009. [Google Scholar] [CrossRef]
- Senkovskyy, V.; Khanduyeva, N.; Komber, H.; Oertel, U.; Stamm, M.; Kuckling, D.; Kiriy, A. Conductive Polymer Brushes of Regioregular Head-to-Tail Poly(3-Alkylthiophenes) via Catalyst-Transfer Surface-Initiated Polycondensation. J. Am. Chem. Soc. 2007, 129, 6626–6632. [Google Scholar] [CrossRef]
- Tkachov, R.; Senkovskyy, V.; Horecha, M.; Oertel, U.; Stamm, M.; Kiriy, A. Surface-Initiated Kumada Catalyst-Transfer Polycondensation of Poly(9,9-Dioctylfluorene) from Organosilica Particles: Chain-Confinement Promoted β-Phase Formation. Chem. Commun. 2010, 46, 1425–1427. [Google Scholar] [CrossRef] [PubMed]
- Gapin, A.; Idriss, H.; Blanc, S.; Billon, L.; Delville, M.H.; Bousquet, A.; Lartigau-Dagron, C. Low Band-Gap Polymer Brushes: Influence of the End-Group on the Morphology of Core-Shell Nanoparticles. React. Funct. Polym. 2020, 155, 104700. [Google Scholar] [CrossRef]
- Murugan, P.; Ananthakrishnan, S.J.; Somanathan, N.; Samanta, D.; Mandal, A.B. Nanoscale Functionalization of Surfaces by Graft-through Sonogashira Polymerization. RSC Adv. 2015, 5, 4121–4125. [Google Scholar] [CrossRef]
- Hwang, E.; Lusker, K.L.; Garno, J.C.; Losovyj, Y.; Nesterov, E.E. Semiconducting Polymer Thin Films by Surface-Confined Stepwise Click Polymerization. Chem. Commun. 2011, 47, 11990–11992. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.G.; Feng, F.; Ohnishi, Y.Y.; Abboud, K.A.; Hirata, S.; Schanze, K.S.; Reynolds, J.R. It Takes More than an Imine: The Role of the Central Atom on the Electron-Accepting Ability of Benzotriazole and Benzothiadiazole Oligomers. J. Am. Chem. Soc. 2012, 134, 2599–2612. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Pan, X.; Meng, X.; Liu, Y.; Wei, D.; Ma, W.; Huo, L.; Sun, X.; Lee, T.H.; Huang, M.; et al. Alkyl Side-Chain Engineering in Wide-Bandgap Copolymers Leading to Power Conversion Efficiencies over 10%. Adv. Mater. 2017, 29, 1604251. [Google Scholar] [CrossRef]
- Granadino-Roldán, J.M.; Garzón, A.; Moral, M.; García, G.; Peña-Ruiz, T.; Paz Fernández-Liencres, M.; Navarro, A.; Fernández-Gómez, M. Theoretical Estimation of the Optical Bandgap in a Series of Poly(Aryl-Ethynylene)s: A DFT Study. J. Chem. Phys. 2014, 140, 044908. [Google Scholar] [CrossRef]
- Rochat, S.; Swager, T.M. Water-Soluble Cationic Conjugated Polymers: Response to Electron-Rich Bioanalytes. J. Am. Chem. Soc. 2013, 135, 17703–17706. [Google Scholar] [CrossRef] [Green Version]
- Weinstock, L.M.; Davis, P.; Handelsman, B.; Tull, R. A General Synthetic System for 1,2,5-Thiadiazoles. J. Org. Chem. 1967, 32, 2823–2829. [Google Scholar] [CrossRef]
- Yasuda, T.; Imase, T.; Nakamura, Y.; Yamamoto, T. New Alternative Donor-Acceptor Arranged Poly(Aryleneethynylene)s and Their Related Compounds Composed of Five-Membered Electron-Accepting 1,3,4-Thiadiazole, 1,2,4-Triazole, or 3,4-Dinitrothiophene Units: Synthesis, Packing Structure, and Optical Properties. Macromolecules 2005, 38, 4687–4697. [Google Scholar] [CrossRef]
- Bunz, U.H.F. Poly(Aryleneethynylene)s: Syntheses, Properties, Structures, and Applications. Chem. Rev. 2000, 100, 1605–1644. [Google Scholar] [CrossRef]
- Karolewski, A.; Neubig, A.; Thelakkat, M.; Kümmel, S. Optical Absorption in Donor-Acceptor Polymers-Alternating vs. Random. Phys. Chem. Chem. Phys. 2013, 15, 20016–20025. [Google Scholar] [CrossRef] [PubMed]
- Saengdee, P.; Promptmas, C.; Thanapitak, S.; Srisuwan, A.; Pankiew, A.; Thornyanadacha, N.; Chaisriratanakul, W.; Chaowicharat, E.; Jeamsaksiri, W. Optimization of 3-Aminopropyltriethoxysilane Functionalization on Silicon Nitride Surface for Biomolecule Immobilization. Talanta 2020, 207, 120305. [Google Scholar] [CrossRef]
- Zengin, A.; Yildirim, E.; Caykara, T. RAFT-Mediated Synthesis and Temperature-Induced Responsive Properties of Poly(2-(2-Methoxyethoxy)Ethyl Methacrylate) Brushes. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 954–962. [Google Scholar] [CrossRef]
- Kitamura, C.; Saito, K.; Ouchi, M.; Yoneda, A.; Yamashita, Y. Synthesis and Crystal Structure of 4,7-Bis(2-Thienylethynyl)-2,1,3-Benzothiadiazole. J. Chem. Res. 2002, 10, 511–513. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannitti, A.; Thorley, K.J.; Nielsen, C.B.; Li, J.; Donahue, M.J.; Malliaras, G.G.; Rivnay, J.; McCulloch, I. Redox-Stability of Alkoxy-BDT Copolymers and their Use for Organic Bioelectronic Devices. Adv. Funct. Mater. 2018, 28, 1706325. [Google Scholar] [CrossRef]
- Hassan Omar, O.; la Gatta, S.; Tangorra, R.R.; Milano, F.; Ragni, R.; Operamolla, A.; Argazzi, R.; Chiorboli, C.; Agostiano, A.; Trotta, M.; et al. Synthetic Antenna Functioning As Light Harvester in the Whole Visible Region for Enhanced Hybrid Photosynthetic Reaction Centers. Bioconjug. Chem. 2016, 27, 1614–1623. [Google Scholar] [CrossRef] [PubMed]



| Moiety | HOMO (eV) | LUMO (eV) |
|---|---|---|
| M06-2X | ||
| 2,2′-bithiophene | −6.87387 | −2.76604 |
| 3,3′-dimethoxy-2,2′-bithiophene | −6.36066 | 0.34776 |
| 3,3′-dimethylo-2,2′-bithiophene | −7.04285 | 0.21334 |
| benzo[c][1,2,5]thiadiazole | −7.96260 | −1.37037 |
| 5,6-dinitrobenzo[c][1,2,5]thiadiazole | −9.41623 | −2.76604 |
| MP4 | ||
| 2,2′-bithiophene | −7.84341 | 1.62126 |
| 3,3′-dimethoxy-2,2′-bithiophene | −7.47987 | 1.66425 |
| 3,3′-dimethylo-2,2′-bithiophene | −8.06954 | 1.71922 |
| benzo[c][1,2,5]thiadiazole | −9.00779 | 0.66369 |
| 5,6-dinitrobenzo[c][1,2,5]thiadiazole | −10.67530 | −0.76546 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grobelny, A.; Grobelny, A.; Zapotoczny, S. Precise Stepwise Synthesis of Donor-Acceptor Conjugated Polymer Brushes Grafted from Surfaces. Int. J. Mol. Sci. 2022, 23, 6162. https://doi.org/10.3390/ijms23116162
Grobelny A, Grobelny A, Zapotoczny S. Precise Stepwise Synthesis of Donor-Acceptor Conjugated Polymer Brushes Grafted from Surfaces. International Journal of Molecular Sciences. 2022; 23(11):6162. https://doi.org/10.3390/ijms23116162
Chicago/Turabian StyleGrobelny, Anna, Artur Grobelny, and Szczepan Zapotoczny. 2022. "Precise Stepwise Synthesis of Donor-Acceptor Conjugated Polymer Brushes Grafted from Surfaces" International Journal of Molecular Sciences 23, no. 11: 6162. https://doi.org/10.3390/ijms23116162