Metabolites Analysis of Anti-Myocardial Ischemia Active Components of Saussurea involucrata Based on Gut Microbiota—Drug Interaction
Abstract
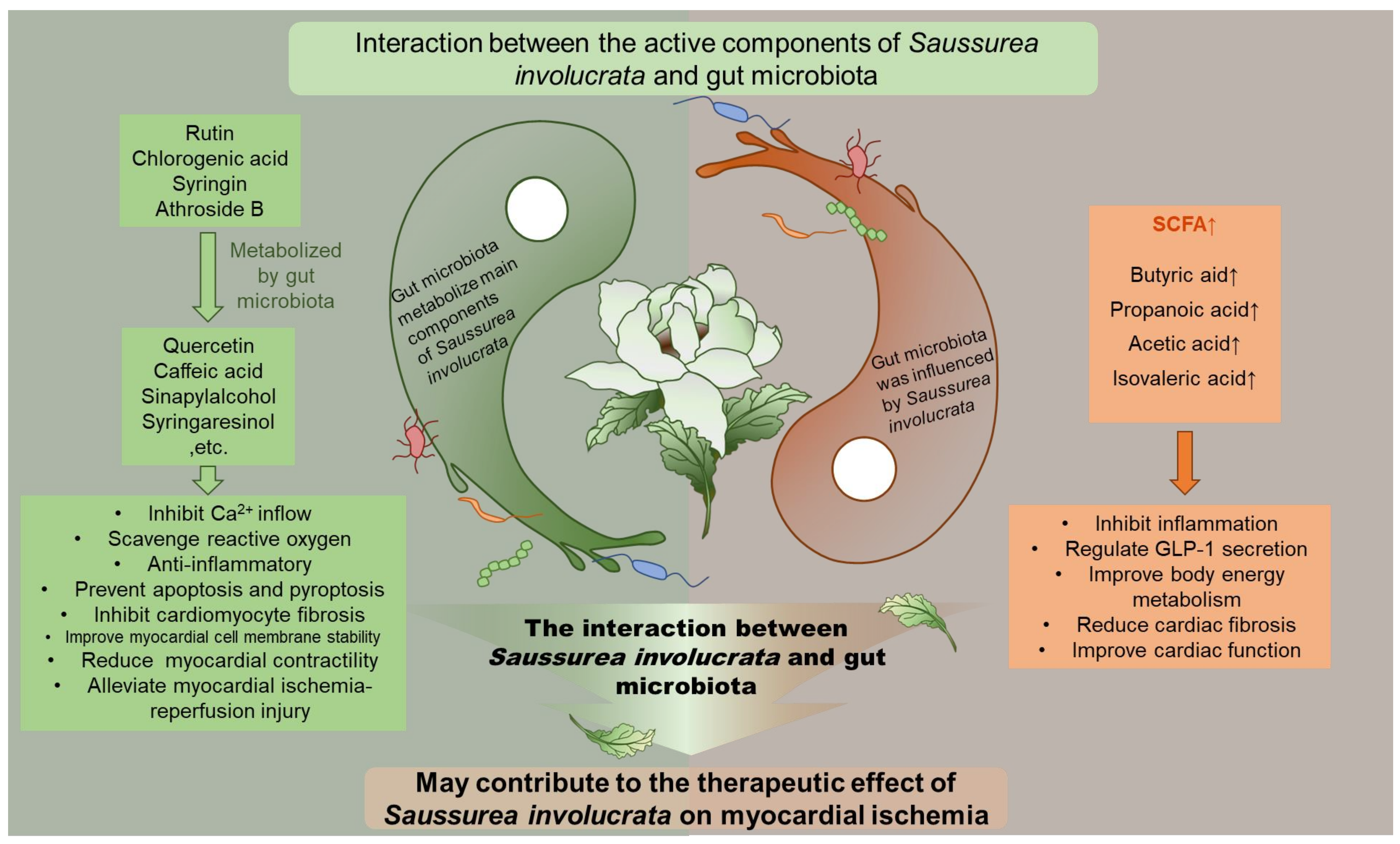
:1. Introduction
2. Results
2.1. Four Main Components Were Found in Fingerprint of Saussurea involucrata
2.2. Main Components of Saussurea involucrata Were Significantly Metabolized by the Gut Microbiota
2.3. Identification of Gut Microbiota Metabolites of Main Components (Saussurea involucrata) by HPLC/MSn-IT-TOF
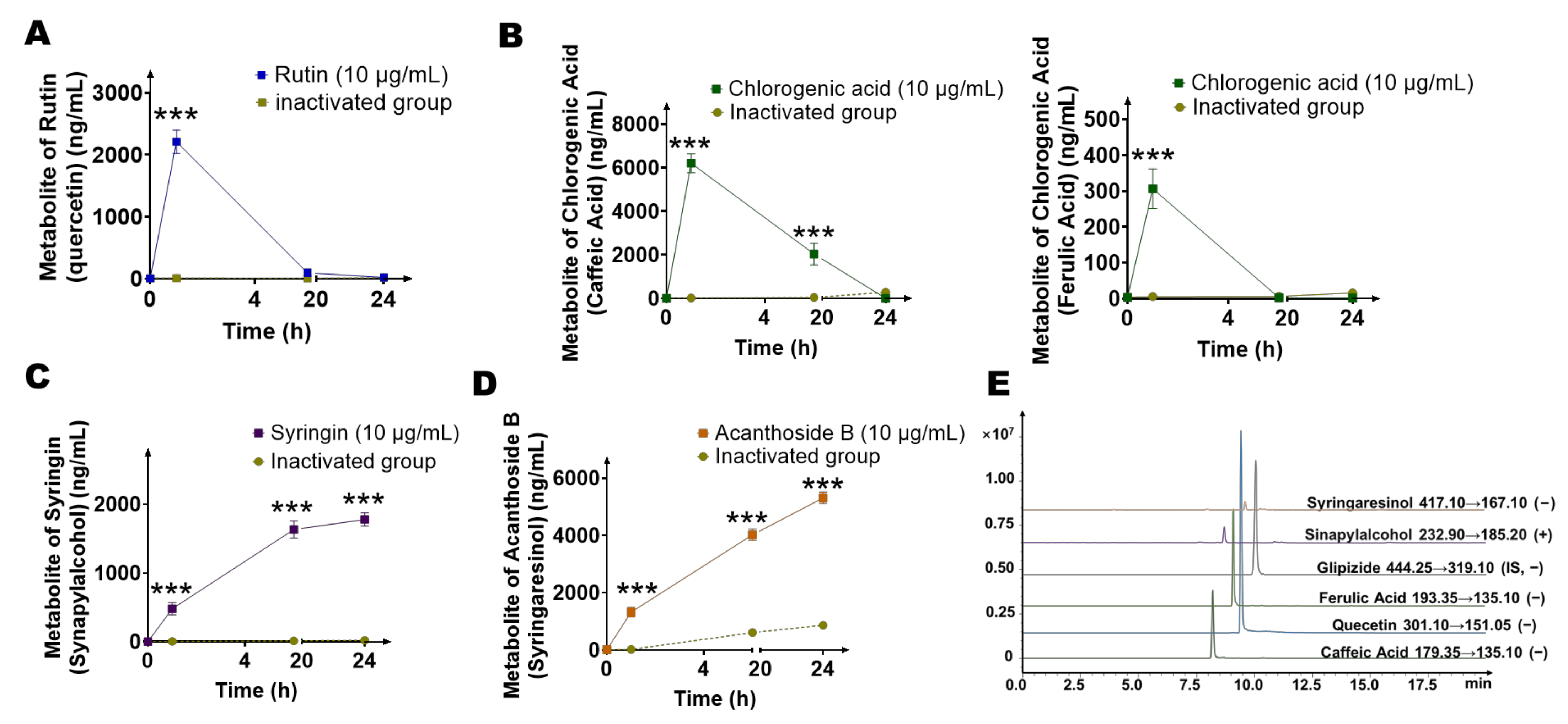
2.4. Quantification of Main Metabolites (Quercetin, Caffeic Acid, Ferulic Acid, Sinapylalcohol, and Syringaresinol) of Gut Microbiota by LC-MS/MS
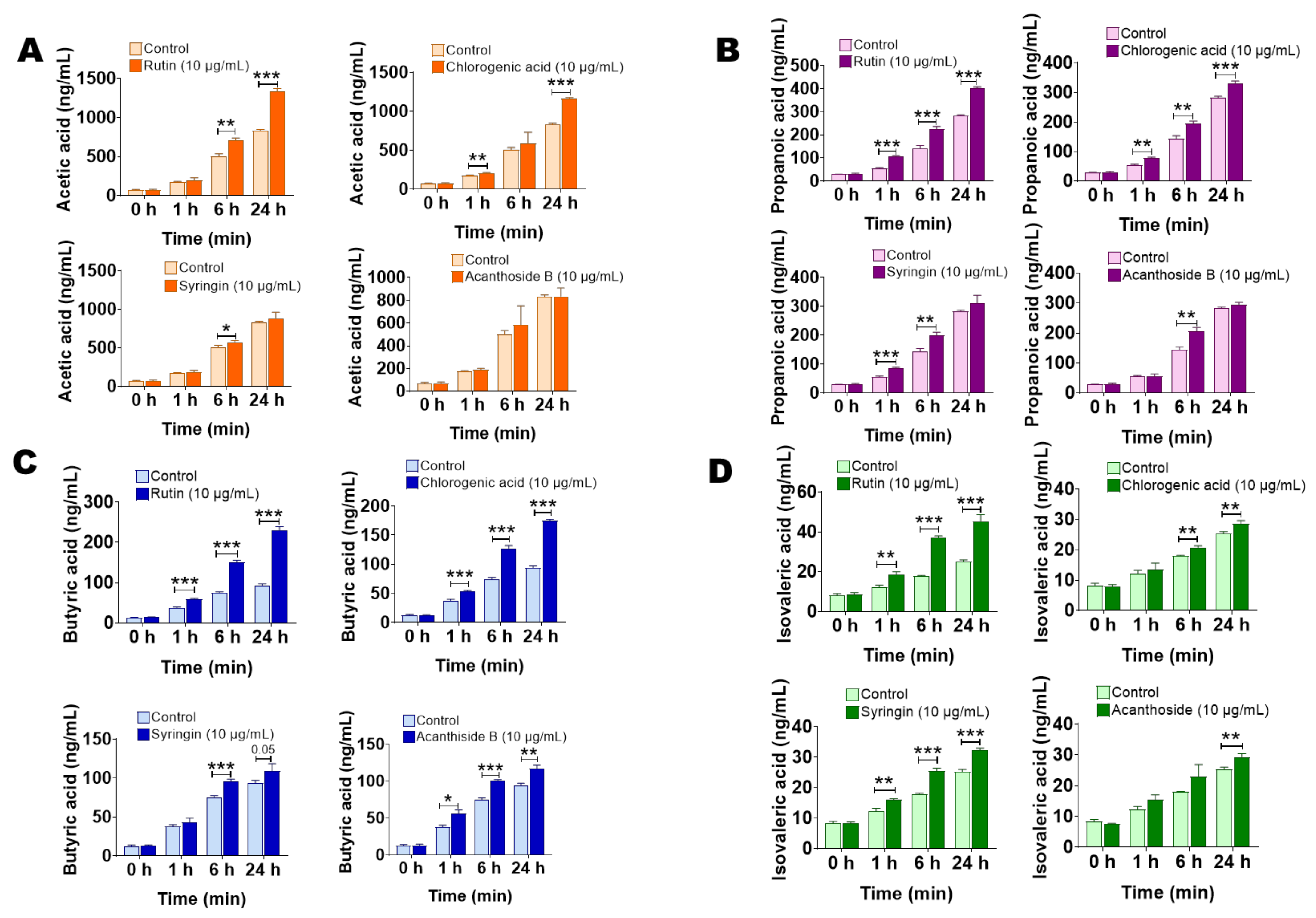
2.5. The Main Components of Saussurea involucrata Induce Short Chain Fatty Acid Production of Gut Microbiota
3. Discussion
4. Materials and Methods
4.1. Instruments and Reagents
4.2. Animals
4.3. Fingerprints of Saussurea involucrata by HPLC and LC-MSn-IT-TOF
4.4. Determination of Four Main Components of Saussurea involucrata by LC-MS/MS
4.5. Identification of the Metabolites (Main Components of Saussurea involucrata) by LC/MSn-IT-TOF
4.6. Determination of Short Chain Fatty Acid In Vitro
4.7. In Vitro Incubation of Four Main Components of Saussurea involucrata with Gut Microbiota
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pagliaro, B.R.; Cannata, F.; Stefanini, G.G.; Bolognese, L. Myocardial ischemia and coronary disease in heart failure. Heart Fail. Rev. 2020, 25, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yu, Y.; Luo, X.; Wang, J.; Lan, X.; Liu, P.; Feng, Y.; Jian, W. Myocardial Ischemia-Reperfusion Injury: Therapeutics from a Mitochondria-Centric Perspective. Cardiology 2021, 146, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Tibaut, M.; Mekis, D.; Petrovic, D. Pathophysiology of Myocardial Infarction and Acute Management Strategies. Cardiovasc. Hematol. Agents Med. Chem. 2017, 14, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Huang, J.; Yang, Y.; Qi, B.; Han, G.; Zheng, Y.; He, H.; Chan, K.; Tsim, K.W.; Dong, T.T. Saussureae Involucratae Herba (Snow Lotus): Review of Chemical Compositions and Pharmacological Properties. Front. Pharmacol. 2020, 10, 1549. [Google Scholar] [CrossRef]
- Zhang, Q.; He, L.; Jiang, Q.; Zhu, H.; Kong, D.; Zhang, H.; Cheng, Z.; Deng, H.; Zheng, Y.; Ying, X. Systems Pharmacology-Based Dissection of Anti-Cancer Mechanism of Traditional Chinese Herb Saussurea involucrata. Front. Pharmacol. 2021, 12, 678203. [Google Scholar] [CrossRef]
- Han, X.; Su, D.; Xian, X.; Zhou, M.; Li, X.; Huang, J.; Wang, J.; Gao, H. Inhibitory effects of Saussurea involucrata (Kar. et Kir.) Sch.-Bip. on adjuvant arthritis in rats. J. Ethnopharmacol. 2016, 194, 228–235. [Google Scholar] [CrossRef]
- Yao, L.; Zhao, Q.; Xiao, J.; Sun, J.; Yuan, X.; Zhao, B.; Su, H.; Niu, S. Composition and antioxidant activity of the polysaccharides from cultivated Saussurea involucrata. Int. J. Biol. Macromol. 2012, 50, 849–853. [Google Scholar] [CrossRef]
- Lee, J.C.; Kao, J.Y.; Kuo, D.H.; Liao, C.F.; Huang, C.H.; Fan, L.L.; Way, T.D. Antifatigue and Antioxidant Activity of Alcoholic Extract from Saussurea involucrata. J. Tradit. Complement. Med. 2011, 1, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Su, K.Y.; Yu, C.Y.; Chen, Y.P.; Hua, K.F.; Chen, Y.L. 3,4-Dihydroxytoluene, a metabolite of rutin, inhibits inflammatory responses in lipopolysaccharide-activated macrophages by reducing the activation of NF-κB signaling. BMC Complement. Altern. Med. 2014, 14, 21. [Google Scholar] [CrossRef] [Green Version]
- Annapurna, A.; Reddy, C.S.; Akondi, R.B.; Rao, S.R. Cardioprotective actions of two bioflavonoids, quercetin and rutin, in experimental myocardial infarction in both normal and streptozotocin-induced type I diabetic rats. J. Pharm. Pharmacol. 2009, 61, 1365–1374. [Google Scholar] [CrossRef]
- Li, G.R.; Wang, H.B.; Qin, G.W.; Jin, M.W.; Tang, Q.; Sun, H.Y.; Du, X.L.; Deng, X.L.; Zhang, X.H.; Chen, J.B.; et al. Acacetin, a natural flavone, selectively inhibits human atrial repolarization potassium currents and prevents atrial fibrillation in dogs. Circulation 2008, 117, 2449–2457. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Mudagal, M.P.; Goli, D. Cardioprotective effect of tetrahydrocurcumin and rutin on lipid peroxides and antioxidants in experimentally induced myocardial infarction in rats. Pharmazie 2009, 64, 132–136. [Google Scholar] [PubMed]
- Korkmaz, A.; Kolankaya, D. Inhibiting inducible nitric oxide synthase with rutin reduces renal ischemia/reperfusion injury. Can. J. Surg. 2013, 56, 6–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Yang, L.; Wu, H.J.; Chen, K.H.; Lin, F.; Li, G.; Sun, H.Y.; Xiao, G.S.; Wang, Y.; Li, G.R. Water-soluble acacetin prodrug confers significant cardioprotection against ischemia/reperfusion injury. Sci. Rep. 2016, 6, 36435. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Saha, S.; Khanna, S. Therapies to modulate gut microbiota: Past, present and future. World J. Gastroenterol. 2020, 26, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Illiano, P.; Brambilla, R.; Parolini, C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.T.; Huang, H.L.; Li, Y.Q.; Xu, H.M.; Zhou, Y.J. Therapeutic advances in non-alcoholic fatty liver disease: A microbiota-centered view. World J. Gastroenterol. 2020, 26, 1901–1911. [Google Scholar] [CrossRef]
- Al Bander, Z.; Nitert, M.D.; Mousa, A.; Naderpoor, N. The Gut Microbiota and Inflammation: An Overview. Int. J. Environ. Res. Public Health 2020, 17, 7618. [Google Scholar] [CrossRef]
- Liu, T.; Song, X.; Khan, S.; Li, Y.; Guo, Z.; Li, C.; Wang, S.; Dong, W.; Liu, W.; Wang, B.; et al. The gut microbiota at the intersection of bile acids and intestinal carcinogenesis: An old story, yet mesmerizing. Int. J. Cancer 2020, 146, 1780–1790. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Q.; Ma, S.R.; Zhao, Z.X.; Pan, L.B.; Cong, L.; Han, P.; Peng, R.; Yu, H.; Lin, Y.; et al. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal Transduct. Target. Ther. 2021, 6, 77. [Google Scholar] [CrossRef]
- Collins, S.L.; Patterson, A.D. The gut microbiome: An orchestrator of xenobiotic metabolism. Acta Pharm. Sin. B 2020, 10, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Ma, S.; Zang, C.; Bao, X.; Wang, Y.; Zhang, D. Gut microbiota mediates the absorption of FLZ, a new drug for Parkinson’s disease treatment. Acta Pharm. Sin. B 2021, 11, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, Y.; Huang, W.; Jin, M.; Gao, Z. The influence of the gut microbiota on the bioavailability of oral drugs. Acta Pharm. Sin. B 2021, 11, 1789–1812. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Han, P.; Ma, S.; Peng, R.; Wang, C.; Kong, W.; Cong, L.; Fu, J.; Zhang, Z.; Yu, H.; et al. Abnormal metabolism of gut microbiota reveals the possible molecular mechanism of nephropathy induced by hyperuricemia. Acta Pharm. Sin. B 2020, 10, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, J.; Jia, W. The influence of gut microbiota on drug metabolism and toxicity. Expert Opin. Drug Metab. Toxicol. 2016, 12, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Dong, G.M.; Yu, H.; Pan, L.B.; Ma, S.R.; Xu, H.; Zhang, Z.W.; Han, P.; Fu, J.; Yang, X.Y.; Keranmu, A.; et al. Biotransformation of Timosaponin BII into Seven Characteristic Metabolites by the Gut Microbiota. Molecules 2021, 26, 3861. [Google Scholar] [CrossRef]
- Wilson, I.D.; Nicholson, J.K. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl. Res. 2017, 179, 204–222. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.B.; Zhao, Z.X.; Peng, R.; Pan, L.B.; Fu, J.; Ma, S.R.; Han, P.; Cong, L.; Zhang, Z.W.; Sun, L.X.; et al. Gut Microbiota-Based Pharmacokinetics and the Antidepressant Mechanism of Paeoniflorin. Front. Pharmacol. 2019, 10, 268. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, S.P.; Wang, Y.Q.; Shi, L.; Zhang, Z.K.; Dong, F.; Li, H.R.; Zhang, J.Y.; Man, Y.Q. Comparative metabolism study on chlorogenic acid, cryptochlorogenic acid and neochlorogenic acid using UHPLC-Q-TOF MS coupled with network pharmacology. Chin. J. Nat. Med. 2021, 19, 212–224. [Google Scholar] [CrossRef]
- Chen, R.; Liu, X.; Zou, J.; Yang, L.; Dai, J. Qualitative and quantitative analysis of phenylpropanoids in cell culture, regenerated plantlets and herbs of Saussurea involucrata. J. Pharm. Biomed. Anal. 2013, 74, 39–46. [Google Scholar] [CrossRef]
- Du, P.; Lei, M.; Liu, Y.; Yang, S. Simultaneous Determination and Pharmacokinetic Study of Six Components in Rat Plasma by HPLC-MS/MS after Oral Administration of Acanthopanax sessiliflorus Fruit Extract. Int. J. Mol. Sci. 2016, 18, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Ren, X.; Wen, C.; Xu, Y.; Chen, Y. Separation and characterization of unknown impurities in rutin tablets using trap-free two-dimensional liquid chromatography coupled with ion trap/time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, e8739. [Google Scholar] [CrossRef] [PubMed]
- Díaz-de-Cerio, E.; Aguilera-Saez, L.M.; Gómez-Caravaca, A.M.; Verardo, V.; Fernández-Gutiérrez, A.; Fernández, I.; Arráez-Román, D. Characterization of bioactive compounds of Annona cherimola L. leaves using a combined approach based on HPLC-ESI-TOF-MS and NMR. Anal. Bioanal. Chem. 2018, 410, 3607–3619. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Y.; Li, Y.D.; Cui, Y.K.; Wu, C.; Hong, Y.X.; Li, G.; Wu, Y.; Jie, L.J.; Wang, Y.; Li, G.R. The Natural Flavone Acacetin Confers Cardiomyocyte Protection Against Hypoxia/Reoxygenation Injury via AMPK-Mediated Activation of Nrf2 Signaling Pathway. Front. Pharmacol. 2018, 9, 497. [Google Scholar] [CrossRef]
- Krishna, K.M.; Annapurna, A.; Gopal, G.S.; Chalam, C.R.; Madan, K.; Kumar, V.K.; Prakash, G.J. Partial reversal by rutin and quercetin of impaired cardiac function in streptozotocin-induced diabetic rats. Can. J. Physiol. Pharmacol. 2005, 83, 343–355. [Google Scholar] [CrossRef]
- Wang, D.; Tian, L.; Lv, H.; Pang, Z.; Li, D.; Yao, Z.; Wang, S. Chlorogenic acid prevents acute myocardial infarction in rats by reducing inflammatory damage and oxidative stress. Biomed. Pharmacother. 2020, 132, 110773. [Google Scholar] [CrossRef]
- Yao, J.; Li, Y.; Jin, Y.; Chen, Y.; Tian, L.; He, W. Synergistic cardioptotection by tilianin and syringin in diabetic cardiomyopathy involves interaction of TLR4/NF-κB/NLRP3 and PGC1a/SIRT3 pathways. Int. Immunopharmacol. 2021, 96, 107728. [Google Scholar] [CrossRef]
- Karthivashan, G.; Kweon, M.H.; Park, S.Y.; Kim, J.S.; Kim, D.H.; Ganesan, P.; Choi, D.K. Cognitive-enhancing and ameliorative effects of acanthoside B in a scopolamine-induced amnesic mouse model through regulation of oxidative/inflammatory/cholinergic systems and activation of the TrkB/CREB/BDNF pathway. Food Chem. Toxicol. 2019, 129, 444–457. [Google Scholar] [CrossRef]
- Michlmayr, H.; Kneifel, W. β-Glucosidase activities of lactic acid bacteria: Mechanisms, impact on fermented food and human health. FEMS Microbiol. Lett. 2014, 352, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.H.; Hyun, Y.J.; Shim, J.; Hong, S.W.; Kim, D.H. Metabolism of rutin and poncirin by human intestinal microbiota and cloning of their metabolizing α-L-rhamnosidase from Bifidobacterium dentium. J. Microbiol. Biotechnol. 2015, 25, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Riva, A.; Kolimár, D.; Spittler, A.; Wisgrill, L.; Herbold, C.W.; Abrankó, L.; Berry, D. Conversion of Rutin, a Prevalent Dietary Flavonol, by the Human Gut Microbiota. Front. Microbiol. 2020, 11, 585428. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, Y.; Liu, M.; Han, X.; Zhang, J.; Zhang, X.; Chu, L. Protective effect of quercetin against myocardial ischemia as a Ca(2+) channel inhibitor: Involvement of inhibiting contractility and Ca(2+) influx via L-type Ca(2+) channels. Arch. Pharm. Res. 2020, 43, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Zhang, Z.Y.; Wang, R.X. Protective Mechanisms of Quercetin Against Myocardial Ischemia Reperfusion Injury. Front. Physiol. 2020, 11, 956. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumaran, K.; Stanely Mainzen Prince, P. Protective effects of caffeic acid on lactate dehydrogenase isoenzymes, electrocardiogram, adenosine triphosphatases, and hematology on isoproterenol-induced myocardial infarcted rats. J. Biochem. Mol. Toxicol. 2011, 25, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qi, K.; Gong, Y.; Long, X.; Zhu, S.; Lu, F.; Lin, K.; Xu, J. Ferulic Acid Alleviates Myocardial Ischemia Reperfusion Injury Via Upregulating AMPKα2 Expression-Mediated Ferroptosis Depression. J. Cardiovasc. Pharmacol. 2021, 79, 489–500. [Google Scholar] [CrossRef]
- Choi, J.; Shin, K.M.; Park, H.J.; Jung, H.J.; Kim, H.J.; Lee, Y.S.; Rew, J.H.; Lee, K.T. Anti-inflammatory and antinociceptive effects of sinapyl alcohol and its glucoside syringin. Planta Med. 2004, 70, 1027–1032. [Google Scholar] [CrossRef]
- Li, G.; Yang, L.; Feng, L.; Yang, J.; Li, Y.; An, J.; Li, D.; Xu, Y.; Gao, Y.; Li, J.; et al. Syringaresinol Protects against Type 1 Diabetic Cardiomyopathy by Alleviating Inflammation Responses, Cardiac Fibrosis, and Oxidative Stress. Mol. Nutr. Food Res. 2020, 64, e2000231. [Google Scholar] [CrossRef]
- Wei, A.; Liu, J.; Li, D.; Lu, Y.; Yang, L.; Zhuo, Y.; Tian, W.; Cong, H. Syringaresinol attenuates sepsis-induced cardiac dysfunction by inhibiting inflammation and pyroptosis in mice. Eur. J. Pharmacol. 2021, 913, 174644. [Google Scholar] [CrossRef]
- Cait, A.; Hughes, M.R.; Antignano, F.; Cait, J.; Dimitriu, P.A.; Maas, K.R.; Reynolds, L.A.; Hacker, L.; Mohr, J.; Finlay, B.B.; et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal. Immunol. 2018, 11, 785–795. [Google Scholar] [CrossRef]
- Fernández, J.; Redondo-Blanco, S.; Gutiérrez-del-Río, I.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Colon microbiota fermentation of dietary prebiotics towards short-chain fatty acids and their roles as anti-inflammatory and antitumour agents: A review. J. Funct. Foods 2016, 25, 511–522. [Google Scholar] [CrossRef]
- Blachier, F.; Mariotti, F.; Huneau, J.F.; Tomé, D. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 2007, 33, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Muñoz-González, I.; Cueva, C.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. A survey of modulation of gut microbiota by dietary polyphenols. BioMed Res. Int. 2015, 2015, 850902. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Ao, H.; Peng, C. Gut Microbiota, Short-Chain Fatty Acids, and Herbal Medicines. Front. Pharmacol. 2018, 9, 1354. [Google Scholar] [CrossRef]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Gao, R.; Zhang, Y.; Pan, D.; Zhu, Y.; Zhang, X.; Yang, R.; Jiang, R.; Xu, Y.; Qin, H. Dysbiosis signatures of gut microbiota in coronary artery disease. Physiol. Genom. 2018, 50, 893–903. [Google Scholar] [CrossRef]
- Jie, Z.; Xia, H.; Zhong, S.L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [Green Version]
- Stoddart, L.A.; Smith, N.J.; Milligan, G. International Union of Pharmacology. LXXI. Free fatty acid receptors FFA1, -2, and -3: Pharmacology and pathophysiological functions. Pharmacol. Rev. 2008, 60, 405–417. [Google Scholar] [CrossRef] [Green Version]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, F.Z.; Nelson, E.; Chu, P.Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Tilg, H.; Kaser, A. Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Investig. 2011, 121, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Hong, S.; Yang, P.; Sun, Y.; Wang, Y.; Zhang, P.; Jiang, W.; Gu, Y. Discovery of an ene-reductase for initiating flavone and flavonol catabolism in gut bacteria. Nat. Commun. 2021, 12, 790. [Google Scholar] [CrossRef] [PubMed]
- Brune, A.; Schink, B. Phloroglucinol pathway in the strictly anaerobic Pelobacter acidigallici: Fermentation of trihydroxybenzenes to acetate via triacetic acid. Arch. Microbiol. 1992, 157, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Schoefer, L.; Mohan, R.; Schwiertz, A.; Braune, A.; Blaut, M. Anaerobic degradation of flavonoids by Clostridium orbiscindens. Appl. Environ. Microbiol. 2003, 69, 5849–5854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westfall, S.; Pasinetti, G.M. The Gut Microbiota Links Dietary Polyphenols With Management of Psychiatric Mood Disorders. Front. Neurosci. 2019, 13, 1196. [Google Scholar] [CrossRef] [Green Version]
- Ho, L.; Ferruzzi, M.G.; Janle, E.M.; Wang, J.; Gong, B.; Chen, T.Y.; Lobo, J.; Cooper, B.; Wu, Q.L.; Talcott, S.T.; et al. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 2013, 27, 769–781. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Wang, L.; Zhang, J.; Pan, T.; Yu, Y.; Lu, J.; Zhou, P.; Yang, H.; Li, P. Activated PKB/GSK-3β synergizes with PKC-δ signaling in attenuating myocardial ischemia/reperfusion injury via potentiation of NRF2 activity: Therapeutic efficacy of dihydrotanshinone-I. Acta Pharm. Sin. B 2021, 11, 71–88. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Ding, S.L.; Wang, Z.; Zhu, X.W.; Zhou, Z.L.; Zhang, W.W.; Yang, X.D.; Ge, J.B. Prmt1 upregulated by Hdc deficiency aggravates acute myocardial infarction via NETosis. Acta Pharm. Sin. B 2022, 12, 1840–1855. [Google Scholar] [CrossRef]
- Li, R.; Xie, L.; Li, L.; Chen, X.; Yao, T.; Tian, Y.; Li, Q.; Wang, K.; Huang, C.; Li, C.; et al. The gut microbial metabolite, 3,4-dihydroxyphenylpropionic acid, alleviates hepatic ischemia/reperfusion injury via mitigation of macrophage pro-inflammatory activity in mice. Acta Pharm. Sin. B 2022, 12, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, L.; Zheng, Q.; Hao, H.; Ye, H.; Li, P.; Yang, H. Protocatechuic aldehyde protects cardiomycoytes against ischemic injury via regulation of nuclear pyruvate kinase M2. Acta Pharm. Sin. B 2021, 11, 3553–3566. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, W.; Zhang, C.; Chen, L.; Xu, R.; Li, N.; Wang, F.; Han, L.; Yang, M.; Zhang, D. Energy metabolism disorders and potential therapeutic drugs in heart failure. Acta Pharm. Sin. B 2021, 11, 1098–1116. [Google Scholar] [CrossRef]
- Feng, R.; Zhao, Z.X.; Ma, S.R.; Guo, F.; Wang, Y.; Jiang, J.D. Gut Microbiota-Regulated Pharmacokinetics of Berberine and Active Metabolites in Beagle Dogs After Oral Administration. Front. Pharmacol. 2018, 9, 214. [Google Scholar] [CrossRef]
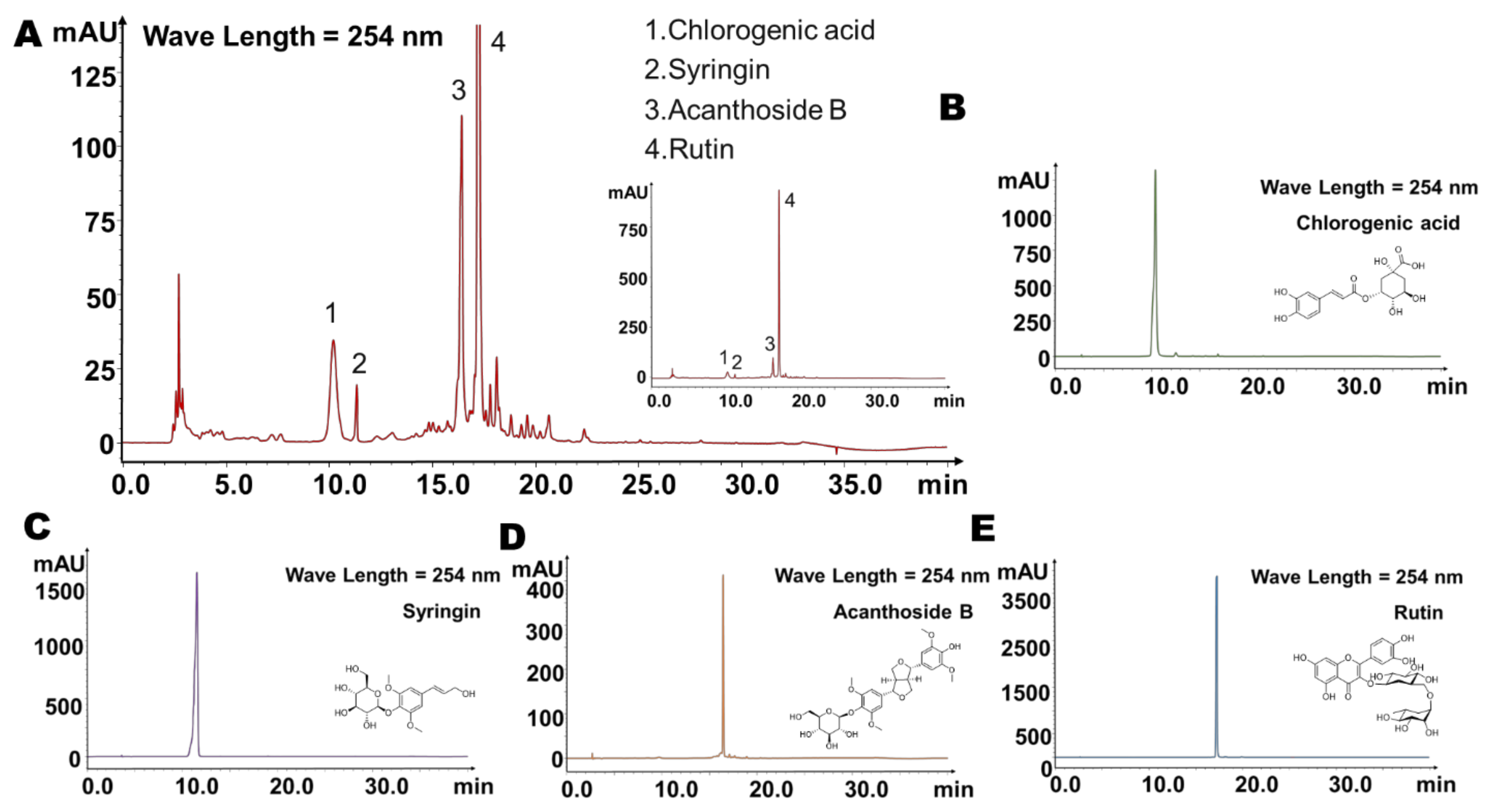
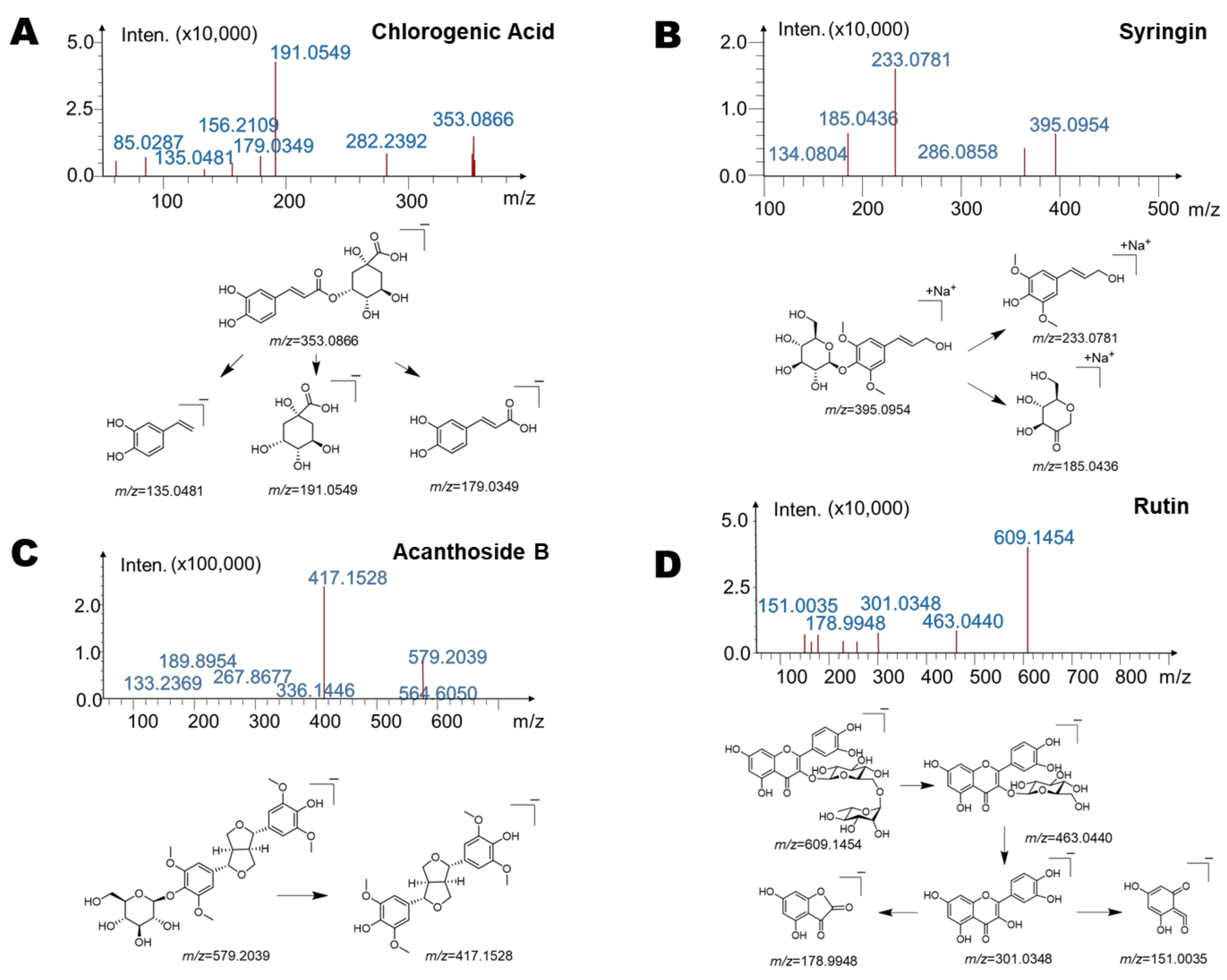


| Components | Retention Time (Min) | Predicted Molecular Weight | Molecular Formula | Fragment Characteristics | |
|---|---|---|---|---|---|
| MS | MS/MS | ||||
| Chlorogenic acid | 10.2 | 354.31 | C16H18O9 | 353 [M − H]− | 135, 191, 179 |
| Syringin | 11.3 | 372.37 | C17H24O9 | 395 [M + Na]+ | 233, 185 |
| Acanthoside B | 16.4 | 580.59 | C28H36O13 | 579 [M − H]− | 417 |
| Rutin | 17.3 | 610.52 | C27H30O16 | 609 [M − H]− | 463, 301, 178, 151 |
| Components | Retention Time (Min) | Reaction | Predicted Molecular Weight | Molecular Formula | Fragment Characteristics | |
|---|---|---|---|---|---|---|
| MS | MS/MS | |||||
| M1 | 19.8 | —Rutinose | 302.24 | C15H10O7 | 301 [M − H]− | 193, 178, 151, 107 |
| M2 | 13.1 | —Glucuronic Acid | 180.15 | C9H8O4 | 179 [M − H]− | 135 |
| M3 | 16.8 | —Glucuronic Acid, Methylation | 194.18 | C10H10O4 | 193 [M − H]− | 149, 135 |
| M4 | 15.3 | —Glucose | 210.23 | C11H14O4 | 233 [M + Na]+ | 185, 161 |
| M5 | 18.4 | —Glucose | 418.44 | C22H26O8 | 417 [M − H]− | 371, 327, 167 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Fu, J.; Guo, H.-H.; Pan, L.-B.; Xu, H.; Zhang, Z.-W.; Hu, J.-C.; Yang, X.-Y.; Zhang, H.-J.; Bu, M.-M.; et al. Metabolites Analysis of Anti-Myocardial Ischemia Active Components of Saussurea involucrata Based on Gut Microbiota—Drug Interaction. Int. J. Mol. Sci. 2022, 23, 7457. https://doi.org/10.3390/ijms23137457
Yu H, Fu J, Guo H-H, Pan L-B, Xu H, Zhang Z-W, Hu J-C, Yang X-Y, Zhang H-J, Bu M-M, et al. Metabolites Analysis of Anti-Myocardial Ischemia Active Components of Saussurea involucrata Based on Gut Microbiota—Drug Interaction. International Journal of Molecular Sciences. 2022; 23(13):7457. https://doi.org/10.3390/ijms23137457
Chicago/Turabian StyleYu, Hang, Jie Fu, Hui-Hui Guo, Li-Bin Pan, Hui Xu, Zheng-Wei Zhang, Jia-Chun Hu, Xin-Yu Yang, Hao-Jian Zhang, Meng-Meng Bu, and et al. 2022. "Metabolites Analysis of Anti-Myocardial Ischemia Active Components of Saussurea involucrata Based on Gut Microbiota—Drug Interaction" International Journal of Molecular Sciences 23, no. 13: 7457. https://doi.org/10.3390/ijms23137457





