Dialdehyde Starch Nanocrystals as a Novel Cross-Linker for Biomaterials Able to Interact with Human Serum Proteins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Starch Nanocrystals
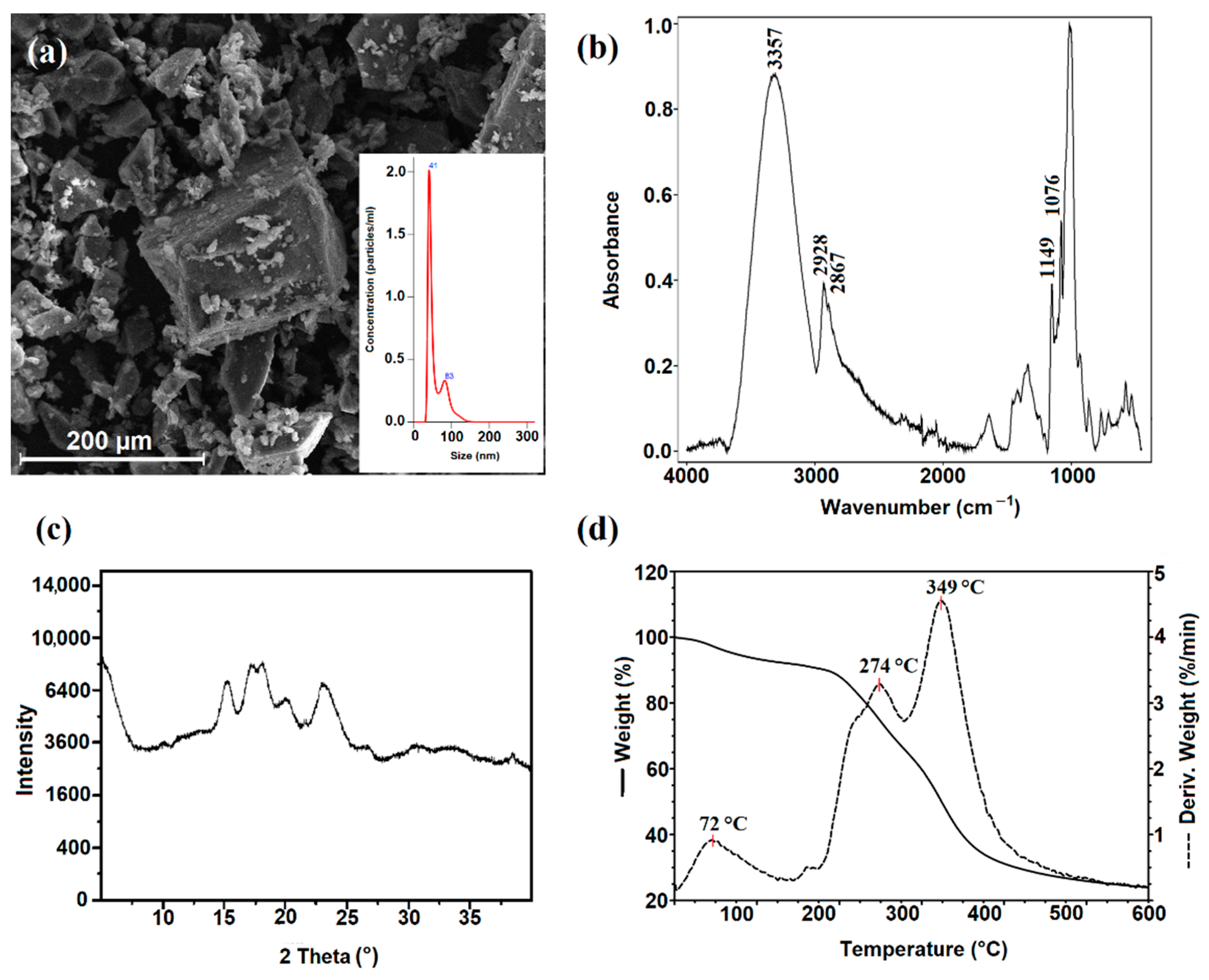
2.2. Characterization of Starch Nanocrystals
2.3. Synthesis of Dialdehyde Starch Nanocrystals
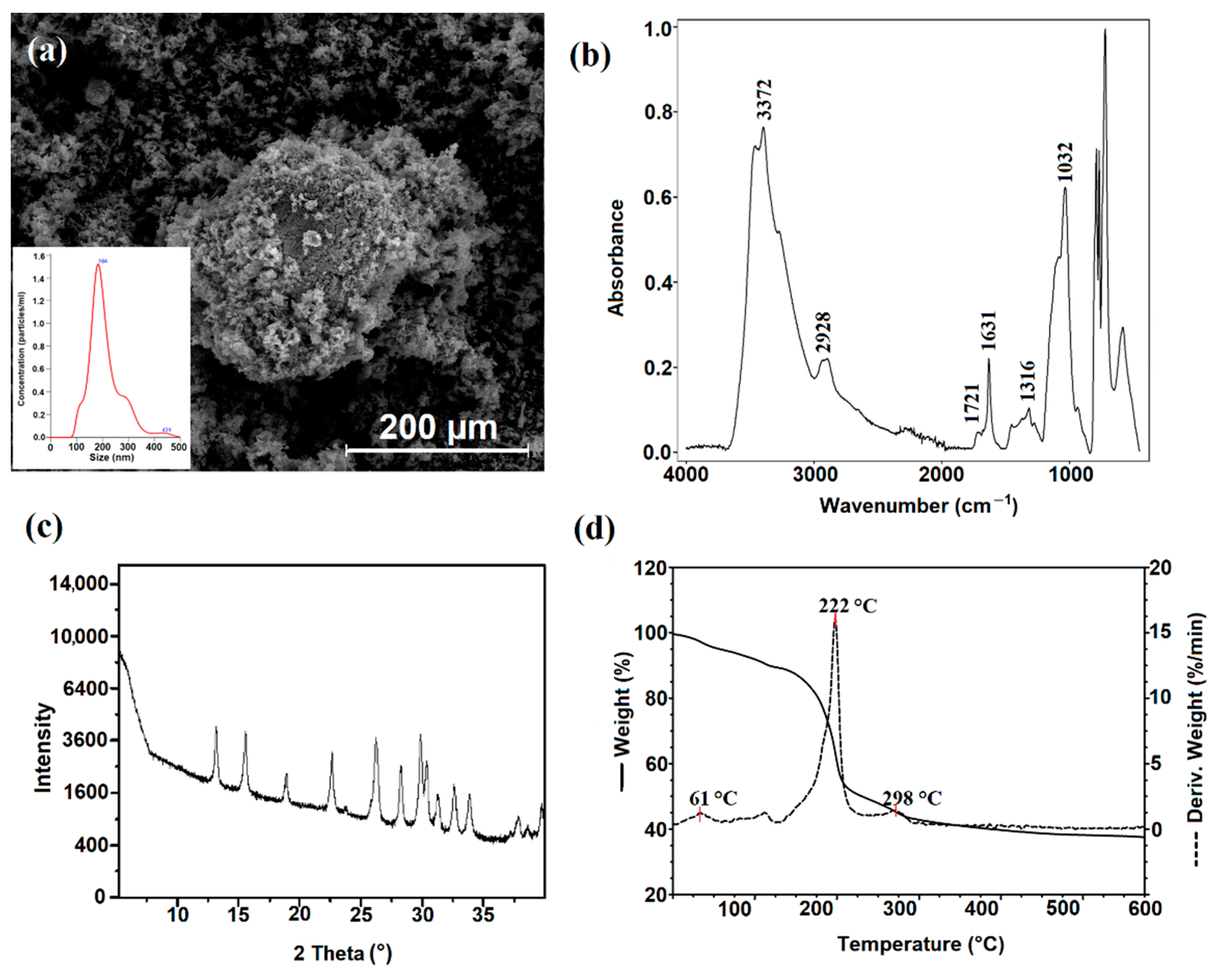
2.4. Characterization of Dialdehyde Starch Nanocrystals
2.5. Formation of Chitosan, Gelatin, and Chitosan–Gelatin Films by Cross-Linking with Dialdehyde Starch Nanocrystals
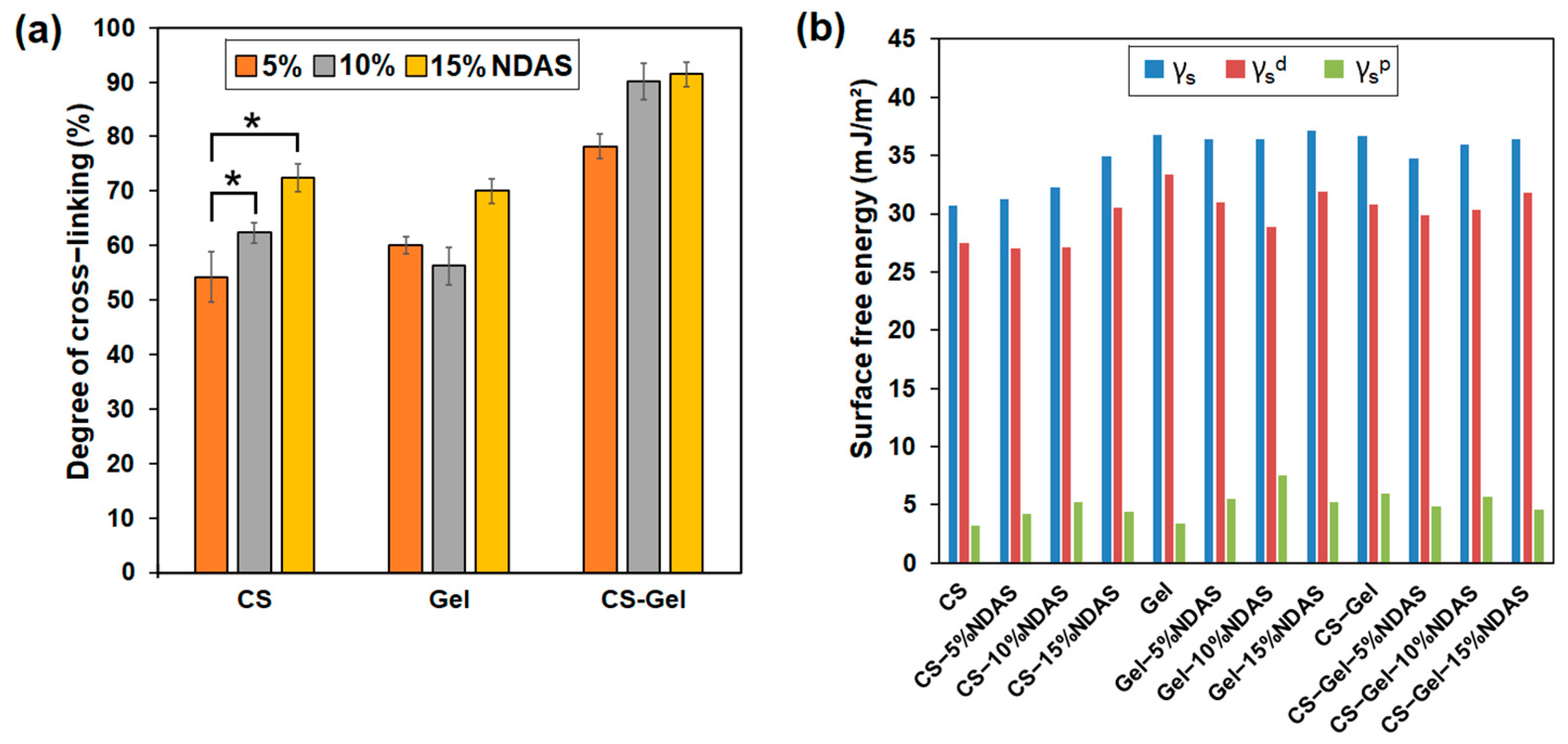
2.5.1. Determination of Cross-Linking Degree of the Films
2.5.2. Contact Angle Measurement
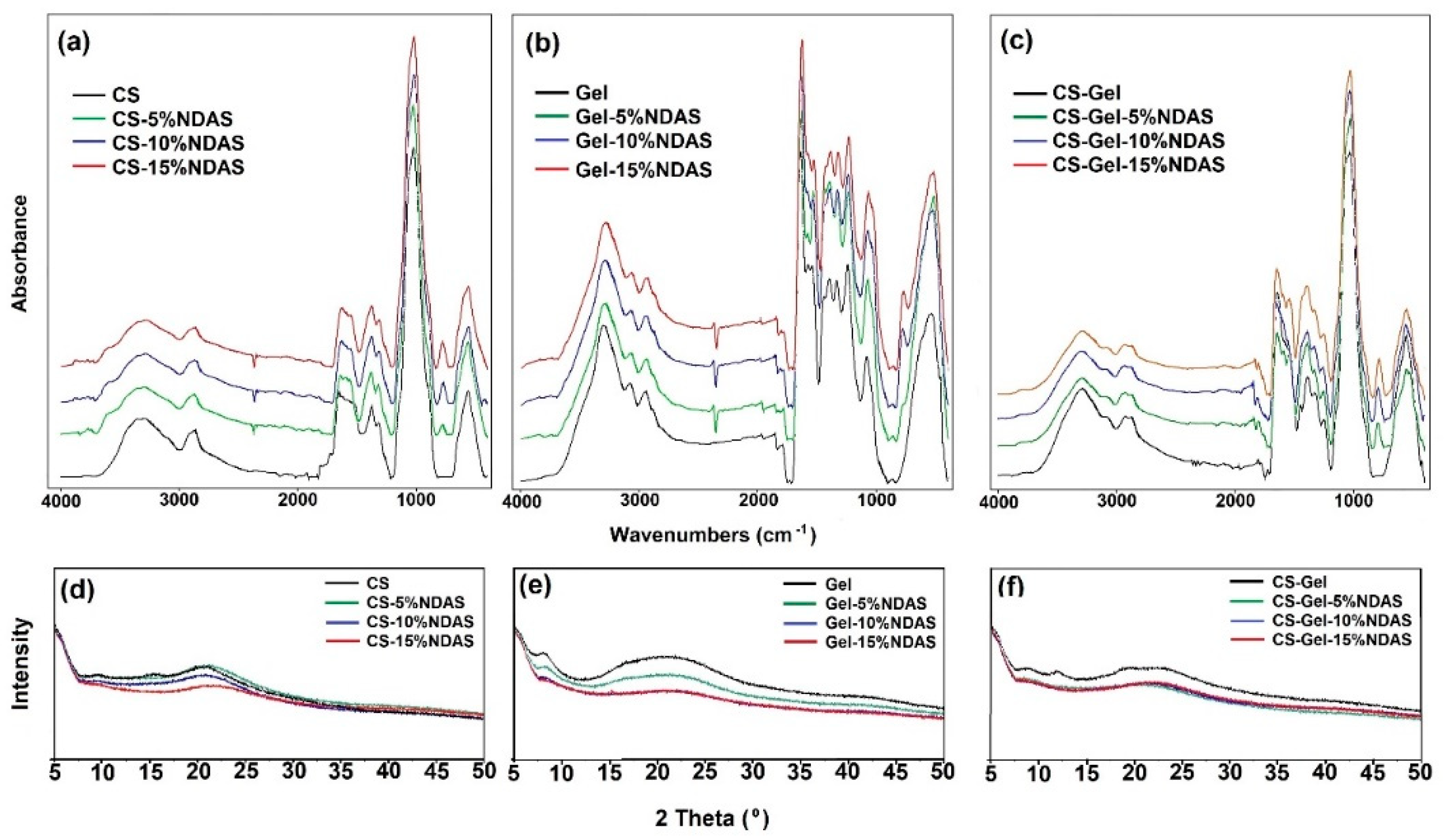
2.5.3. ATR-FTIR Spectroscopy and X-ray Diffraction (XRD)
2.5.4. Thermal Analysis
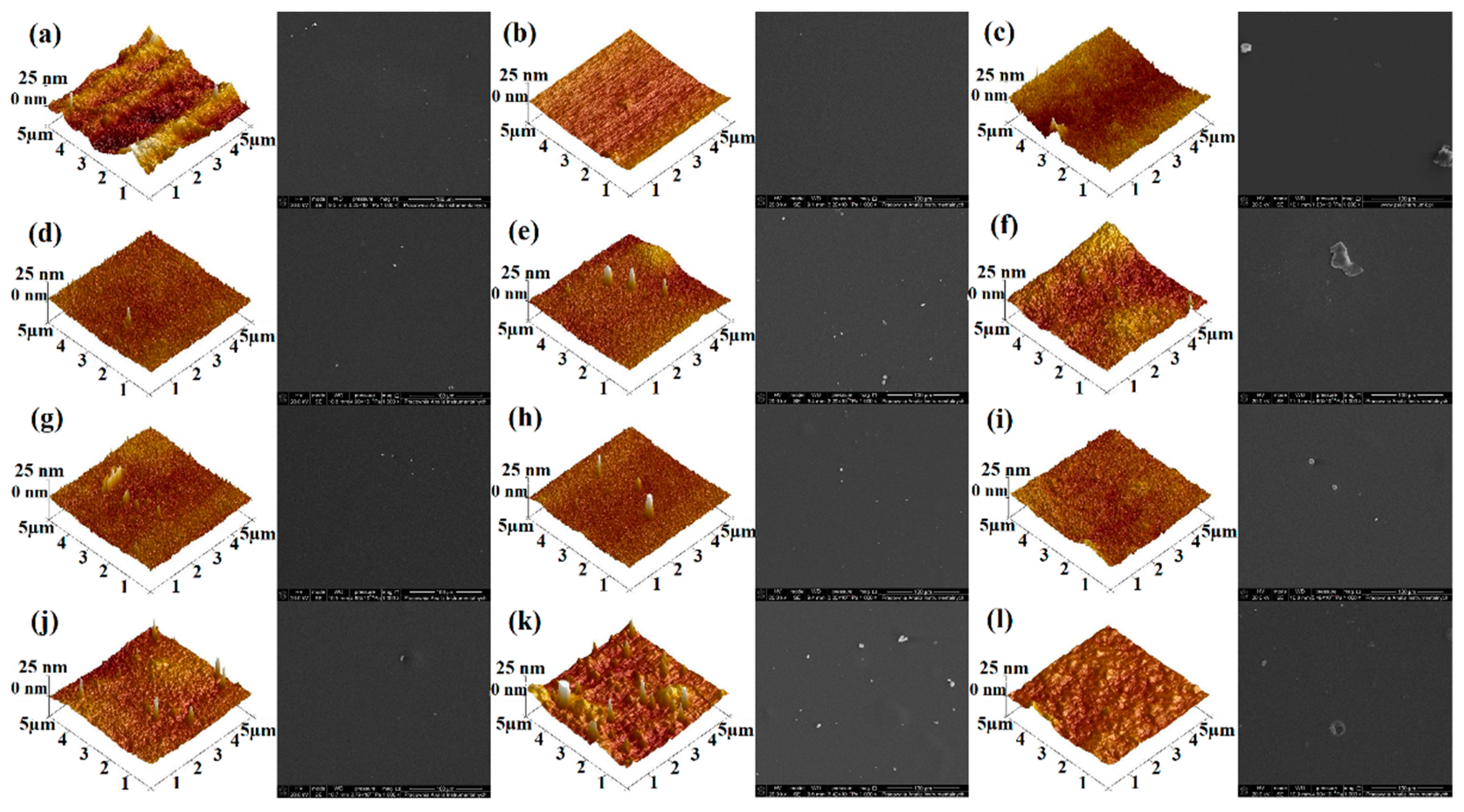
2.5.5. SEM and AFM
2.5.6. Mechanical Properties
2.5.7. Swelling Ability
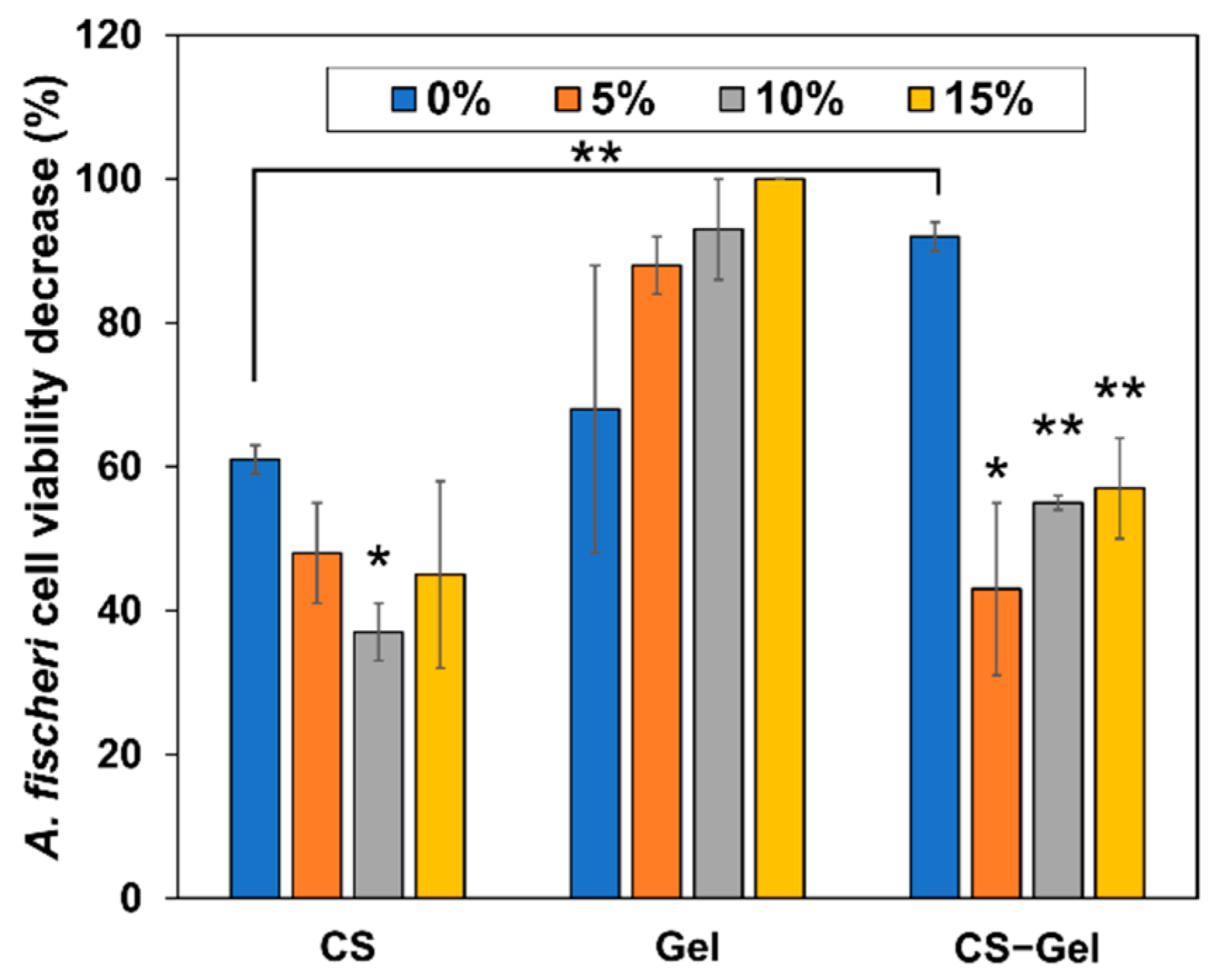
2.5.8. Toxicity Assessment
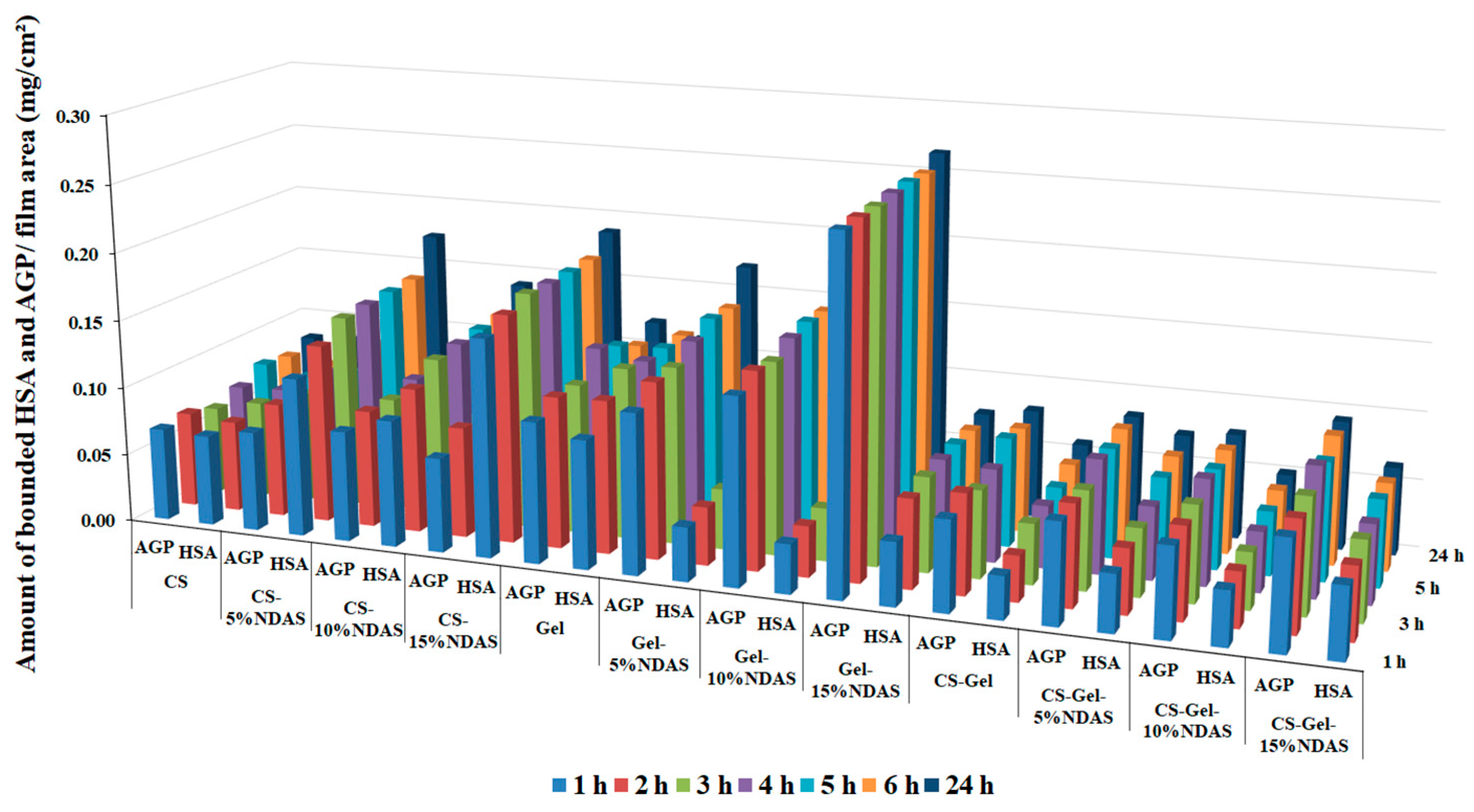
2.5.9. Protein Adsorption Study
3. Materials and Methods
3.1. Materials
3.2. Starch Nanocrystal Synthesis
3.3. Dialdehyde Starch Nanocrystal Synthesis
3.4. Biopolymer Preparation
3.5. Characterization of Starch Nanocrystals, Dialdehyde Starch Nanocrystals, and Cross-Linked Biopolymers
3.5.1. Determination of the Content of Aldehyde Groups
3.5.2. Particle Size Distribution Analysis
3.5.3. Determination of the Cross-Linking Degree of the Films
3.5.4. Contact Angle Measurement
3.5.5. Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) Spectroscopy and X-ray Diffraction (XRD)
3.5.6. Thermal Analysis
3.5.7. Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM)
3.5.8. Mechanical Properties
3.5.9. Swelling Ability
3.5.10. Toxicity Assessment
3.5.11. Protein Adsorption Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. Biomed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. Novel Asymmetric Wettable AgNPs/Chitosan Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2016, 8, 3958–3968. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Applications of Chitosan as Food Packaging Materials. In Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Crini, G., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 81–123. [Google Scholar]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Devel. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [Green Version]
- Dash, R.; Foston, M.; Ragauskas, A.J. Improving the mechanical and thermal properties of gelatin hydrogels cross-linked by cellulose nanowhiskers. Carbohydr. Polym. 2013, 91, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Chan, O.C.M.; So, K.F. Effects of photochemical crosslinking on the microstructure of collagen and a feasibility study on controlled protein release. Acta Biomater. 2008, 4, 1627–1636. [Google Scholar] [CrossRef]
- He, L.; Mu, C.; Shi, J.; Zhang, Q.; Shi, B.; Lin, W. Modification of collagen with a natural cross-linker, procyanidin. Int. J. Biol. Macromol. 2011, 48, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Boekema, B.K.H.L.; Vlig, M.; Olde Damink, L.; Middelkoop, E.; Eummelen, L.; Bühren, A.V.; Ulrich, M.M.W. Effect of pore size and cross-linking of a novel collagen-elastin dermal substitute on wound healing. J. Mater. Sci. Mater. Med. 2014, 25, 423–433. [Google Scholar] [CrossRef]
- Chen, R.-N.; Ho, H.-O.; Sheu, M.-T. Characterization of collagen matrices crosslinked using microbial transglutaminase. Biomaterials 2005, 26, 4229–4235. [Google Scholar] [CrossRef] [PubMed]
- Ermis, M.; Calamak, S.; Calibasi Kocal, G.; Guven, S.; Durmus, N.G.; Rizvi, I.; Hasan, T.; Hasirci, N.; Hasirci, V.; Demirci, U. Chapter 15—Hydrogels as a New Platform to Recapitulate the Tumor Microenvironment. In Handbook of Nanomaterials for Cancer Theranostics; Conde, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 463–494. [Google Scholar]
- Takigawa, T.; Endo, Y. Effects of Glutaraldehyde Exposure on Human Health. J. Occup. Health 2006, 48, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, E.; Hanna, K.; Bazin, C.; Keck, G.; Clément, B.; Perrodin, Y. Fate of glutaraldehyde in hospital wastewater and combined effects of glutaraldehyde and surfactants on aquatic organisms. Environ. Int. 2005, 31, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yoo, J.J.; Lim, G.J.; Atala, A.; Stitzel, J. In vitro evaluation of electrospun nanofiber scaffolds for vascular graft application. J. Biomed. Mater. Res. A 2007, 83A, 999–1008. [Google Scholar] [CrossRef]
- Rho, K.S.; Jeong, L.; Lee, G.; Seo, B.-M.; Park, Y.J.; Hong, S.-D.; Roh, S.; Cho, J.J.; Park, W.H.; Min, B.-M. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 2006, 27, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in polysaccharide and protein films and coatings for food contact—A review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Islam, M.S.; Chen, L.; Sisler, J.; Tam, K.C. Cellulose nanocrystal (CNC)–inorganic hybrid systems: Synthesis, properties and applications. J. Mater. Chem. B 2018, 6, 864–883. [Google Scholar] [CrossRef] [PubMed]
- Perez Herrera, M.; Vasanthan, T.; Chen, L. Rheology of starch nanoparticles as influenced by particle size, concentration and temperature. Food Hydrocoll. 2017, 66, 237–245. [Google Scholar] [CrossRef]
- Angellier, H.; Molina-Boisseau, S.; Lebrun, L.; Dufresne, A. Processing and Structural Properties of Waxy Maize Starch Nanocrystals Reinforced Natural Rubber. Macromolecules 2005, 38, 3783–3792. [Google Scholar] [CrossRef]
- Zheng, H.; Ai, F.; Chang, P.R.; Huang, J.; Dufresne, A. Structure and properties of starch nanocrystal-reinforced soy protein plastics. Polym. Compos. 2009, 30, 474–480. [Google Scholar] [CrossRef]
- Potthast, A.; Schiehser, S.; Rosenau, T.; Kostic, M. Oxidative modifications of cellulose in the periodate system—Reduction and beta-elimination reactions 2nd ICC 2007, Tokyo, Japan, October 25–29, 2007. Holzforschung 2009, 63, 12–17. [Google Scholar] [CrossRef]
- Fan, L.; Sun, Y.; Xie, W.; Zheng, H.; Liu, S. Oxidized pectin cross-linked carboxymethyl chitosan: A new class of hydrogels. J. Biomater. Sci. Polym. Ed. 2012, 23, 2119–2132. [Google Scholar] [CrossRef] [PubMed]
- Amirian, J.; Zeng, Y.; Shekh, M.I.; Sharma, G.; Stadler, F.J.; Song, J.; Du, B.; Zhu, Y. In-situ crosslinked hydrogel based on amidated pectin/oxidized chitosan as potential wound dressing for skin repairing. Carbohydr. Polym. 2021, 251, 117005. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.-Q.; Zhang, S.-C.; Chen, G.-G.; Hao, X.; Bian, J.; Peng, F. Xylan-based hydrogels for potential skin care application. Int. J. Biol. Macromol. 2020, 158, 244–250. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Bajpai, M.; Shah, F.F. Alginate dialdehyde (AD)-crosslinked casein films: Synthesis, characterization and water absorption behavior. Des. Monomers Polym. 2016, 19, 406–419. [Google Scholar] [CrossRef] [Green Version]
- Kuchaiyaphum, P.; Chotichayapong, C.; Butwong, N.; Bua-ngern, W. Silk Fibroin/Poly (vinyl alcohol) Hydrogel Cross-Linked with Dialdehyde Starch for Wound Dressing Applications. Macromol. Res. 2020, 28, 844–850. [Google Scholar] [CrossRef]
- Thomas, L.V.; Vg, R.; Nair, P.D. Effect of stiffness of chitosan-hyaluronic acid dialdehyde hydrogels on the viability and growth of encapsulated chondrocytes. Int. J. Biol. Macromol. 2017, 104, 1925–1935. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Liu, C.; Zheng, X.; Tang, K. Tuning structure and properties of gelatin edible films through pullulan dialdehyde crosslinking. LWT 2021, 138, 110607. [Google Scholar] [CrossRef]
- Lucas de Lima, E.; Fittipaldi Vasconcelos, N.; da Silva Maciel, J.; Karine Andrade, F.; Silveira Vieira, R.; Andrade Feitosa, J.P. Injectable hydrogel based on dialdehyde galactomannan and N-succinyl chitosan: A suitable platform for cell culture. J. Mater. Sci. Mater. Med. 2019, 31, 5. [Google Scholar] [CrossRef]
- Pietrucha, K.; Safandowska, M. Dialdehyde cellulose-crosslinked collagen and its physicochemical properties. Process Biochem. 2015, 50, 2105–2111. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, L.; Zhang, B.; He, M.; Shi, W.; Yang, S.; Cui, Y.; Yin, G. Preparation and Characterization of Edible Dialdehyde Carboxymethyl Cellulose Crosslinked Feather Keratin Films for Food Packaging. Polymers 2020, 12, 158. [Google Scholar] [CrossRef] [Green Version]
- Ngwabebhoh, F.A.; Zandraa, O.; Patwa, R.; Saha, N.; Capáková, Z.; Saha, P. Self-crosslinked chitosan/dialdehyde xanthan gum blended hypromellose hydrogel for the controlled delivery of ampicillin, minocycline and rifampicin. Int. J. Biol. Macromol. 2021, 167, 1468–1478. [Google Scholar] [CrossRef]
- Terracciano, R.; Zhang, A.; Butler, E.B.; Demarchi, D.; Hafner, J.H.; Grattoni, A.; Filgueira, C.S. Effects of Surface Protein Adsorption on the Distribution and Retention of Intratumorally Administered Gold Nanoparticles. Pharmaceutics 2021, 13, 216. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Riyazuddeen; Rabbani, G.; Khan, R.H. Spectroscopic and calorimetric studies of interaction of methimazole with human serum albumin. J. Lumin. 2014, 151, 219–223. [Google Scholar] [CrossRef]
- Matsumoto, K.; Sukimoto, K.; Nishi, K.; Maruyama, T.; Suenaga, A.; Otagiri, M. Characterization of ligand binding sites on the alpha1-acid glycoprotein in humans, bovines and dogs. Drug Metab. Pharmacokinet. 2002, 17, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Bteich, M. An overview of albumin and alpha-1-acid glycoprotein main characteristics: Highlighting the roles of amino acids in binding kinetics and molecular interactions. Heliyon 2019, 5, e02879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Q.; Wang, R.; Jiang, Y.; Liu, B. Study on the interaction between active components from traditional Chinese medicine and plasma proteins. Chem. Cent. J. 2018, 12, 48. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Ji, Y.; Sun, Q.; Fu, Y.; Xu, Y.; Jin, L. Fabrication of Cellulose Nanocrystal/Chitosan Hydrogel for Controlled Drug Release. Nanomaterials 2019, 9, 253. [Google Scholar] [CrossRef]
- Kwak, H.W.; Lee, H.; Park, S.; Lee, M.E.; Jin, H.-J. Chemical and physical reinforcement of hydrophilic gelatin film with di-aldehyde nanocellulose. Int. J. Biol. Macromol. 2020, 146, 332–342. [Google Scholar] [CrossRef]
- Chen, Y.; Hao, Y.; Ting, K.; Li, Q.; Gao, Q. Preparation and emulsification properties of dialdehyde starch nanoparticles. Food Chem. 2019, 286, 467–474. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, G.; Gao, Q. Starch Nanoparticles-Graphene Aerogels with High Supercapacitor Performance and Efficient Adsorption. ACS Sustain. Chem. Eng. 2019, 7, 14064–14073. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale 2012, 4, 3274–3294. [Google Scholar] [CrossRef] [PubMed]
- Condés, M.C.; Añón, M.C.; Mauri, A.N.; Dufresne, A. Amaranth protein films reinforced with maize starch nanocrystals. Food Hydrocoll. 2015, 47, 146–157. [Google Scholar] [CrossRef]
- Jayakody, L.; Hoover, R. The effect of lintnerization on cereal starch granules. Food Res. Int. 2002, 35, 665–680. [Google Scholar] [CrossRef]
- Sanchez de la Concha, B.B.; Agama-Acevedo, E.; Nuñez-Santiago, M.C.; Bello-Perez, L.A.; Garcia, H.S.; Alvarez-Ramirez, J. Acid hydrolysis of waxy starches with different granule size for nanocrystal production. J. Cereal Sci. 2018, 79, 193–200. [Google Scholar] [CrossRef]
- Silva, A.P.M.; Oliveira, A.V.; Pontes, S.M.A.; Pereira, A.L.S.; Souza Filho, M.d.s.M.; Rosa, M.F.; Azeredo, H.M.C. Mango kernel starch films as affected by starch nanocrystals and cellulose nanocrystals. Carbohydr. Polym. 2019, 211, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Ding, W.; Liu, J.; Li, Y.; Kennedy, J.F.; Gu, Q.; Shao, S. Preparation and characterization of organic-soluble acetylated starch nanocrystals. Carbohydr. Polym. 2010, 80, 1078–1084. [Google Scholar] [CrossRef]
- Duan, B.; Sun, P.; Wang, X.; Yang, C. Preparation and properties of starch nanocrystals/carboxymethyl chitosan nanocomposite films. Starch Stärke 2011, 63, 528–535. [Google Scholar] [CrossRef]
- Sessini, V.; Raquez, J.-M.; Kenny, J.M.; Dubois, P.; Peponi, L. Melt-processing of bionanocomposites based on ethylene-co-vinyl acetate and starch nanocrystals. Carbohydr. Polym. 2019, 208, 382–390. [Google Scholar] [CrossRef]
- Oyeyinka, S.A.; Singh, S.; Adebola, P.O.; Gerrano, A.S.; Amonsou, E.O. Physicochemical properties of starches with variable amylose contents extracted from bambara groundnut genotypes. Carbohydr. Polym. 2015, 133, 171–178. [Google Scholar] [CrossRef]
- Mukurumbira, A.; Mariano, M.; Dufresne, A.; Mellem, J.J.; Amonsou, E.O. Microstructure, thermal properties and crystallinity of amadumbe starch nanocrystals. Int. J. Biol. Macromol. 2017, 102, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Md Shahrodin, N.S.; Rahmat, A.R.; Arsad, A. Synthesis and Characterization of Cassava Starch Nanocrystals by Hydrolysis Method. Adv. Mat. Res. 2015, 1113, 446–452. [Google Scholar] [CrossRef]
- LeCorre, D.; Bras, J.; Dufresne, A. Influence of botanic origin and amylose content on the morphology of starch nanocrystals. J. Nanopart. Res. 2011, 13, 7193–7208. [Google Scholar] [CrossRef]
- Wegrzynowska-Drzymalska, K.; Grebicka, P.; Mlynarczyk, D.T.; Chelminiak-Dudkiewicz, D.; Kaczmarek, H.; Goslinski, T.; Ziegler-Borowska, M. Crosslinking of Chitosan with Dialdehyde Chitosan as a New Approach for Biomedical Applications. Materials 2020, 13, 3413. [Google Scholar] [CrossRef]
- Chauhan, K.; Kaur, J.; Kumari, A.; Kumari, A.; Chauhan, G.S. Efficient method of starch functionalization to bis-quaternary structure unit. Int. J. Biol. Macromol. 2015, 80, 498–505. [Google Scholar] [CrossRef]
- Zeng, L.; Qin, C.; Wang, L.; Li, W. Volatile compounds formed from the pyrolysis of chitosan. Carbohydr. Polym. 2011, 83, 1553–1557. [Google Scholar] [CrossRef]
- Gierszewska, M.; Jakubowska, E.; Olewnik-Kruszkowska, E. Effect of chemical crosslinking on properties of chitosan-montmorillonite composites. Polym. Test. 2019, 77, 105872. [Google Scholar] [CrossRef]
- Sionkowska, A.; Michalska-Sionkowska, M.; Walczak, M. Preparation and characterization of collagen/hyaluronic acid/chitosan film crosslinked with dialdehyde starch. Int. J. Biol. Macromol. 2020, 149, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Ezati, P.; Rhim, J.-W. pH-responsive chitosan-based film incorporated with alizarin for intelligent packaging applications. Food Hydrocoll. 2020, 102, 105629. [Google Scholar] [CrossRef]
- Hoffmann, B.; Seitz, D.; Mencke, A.; Kokott, A.; Ziegler, G. Glutaraldehyde and oxidised dextran as crosslinker reagents for chitosan-based scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2009, 20, 1495–1503. [Google Scholar] [CrossRef]
- Ilkar Erdagi, S.; Asabuwa Ngwabebhoh, F.; Yildiz, U. Genipin crosslinked gelatin-diosgenin-nanocellulose hydrogels for potential wound dressing and healing applications. Int. J. Biol. Macromol. 2020, 149, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, N.; Steinhagen, S.; Toth, G.; Pavia, H.; Edlund, U. Ulvan dialdehyde-gelatin hydrogels for removal of heavy metals and methylene blue from aqueous solution. Carbohydr. Polym. 2020, 249, 116841. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wu, Y.; Gu, Q.-s.; El-Hamshary, H.; El-Newehy, M.; Mo, X. Injectable photo crosslinked enhanced double-network hydrogels from modified sodium alginate and gelatin. Int. J. Biol. Macromol. 2017, 96, 569–577. [Google Scholar] [CrossRef]
- Bin Ahmad, M.; Lim, J.J.; Shameli, K.; Ibrahim, N.A.; Tay, M.Y. Synthesis of Silver Nanoparticles in Chitosan, Gelatin and Chitosan/Gelatin Bionanocomposites by a Chemical Reducing Agent and Their Characterization. Molecules 2011, 16, 7237–7248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osorio-Madrazo, A.; David, L.; Trombotto, S.; Lucas, J.-M.; Peniche-Covas, C.; Domard, A. Highly crystalline chitosan produced by multi-steps acid hydrolysis in the solid-state. Carbohydr. Polym. 2011, 83, 1730–1739. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, H.; Wang, R.; Chai, Y.; Ji, W. Fabrication and performance characterization of the membrane from self-dispersed gelatin-coupled cellulose microgels. Cellulose 2019, 26, 3255–3269. [Google Scholar] [CrossRef]
- Quero, F.; Padilla, C.; Campos, V.; Luengo, J.; Caballero, L.; Melo, F.; Li, Q.; Eichhorn, S.J.; Enrione, J. Stress transfer and matrix-cohesive fracture mechanism in microfibrillated cellulose-gelatin nanocomposite films. Carbohydr. Polym. 2018, 195, 89–98. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wang, X.; Xue, H. The performance of chitosan/gelatin composite microspheres in the wash-off procedure of reactive dyeing. Color. Technol. 2016, 132, 353–360. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chełminiak, D.; Kaczmarek, H.; Kaczmarek-Kędziera, A. Effect of side substituents on thermal stability of the modified chitosan and its nanocomposites with magnetite. J. Therm. Anal. Calorim. 2016, 124, 1267–1280. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Zhao, S.; Lu, W.; Chen, N.; Zhu, D.; Li, Y. Preparation and characterization of enzymatically cross-linked gelatin/cellulose nanocrystal composite hydrogels. RSC Adv. 2021, 11, 10794–10803. [Google Scholar] [CrossRef]
- Pulieri, E.; Chiono, V.; Ciardelli, G.; Vozzi, G.; Ahluwalia, A.; Domenici, C.; Vozzi, F.; Giusti, P. Chitosan/gelatin blends for biomedical applications. J. Biomed. Mater. Res. A 2008, 86A, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Sutirman, Z.A.; Sanagi, M.M.; Abd Karim, K.J.; Wan Ibrahim, W.A. Preparation of methacrylamide-functionalized crosslinked chitosan by free radical polymerization for the removal of lead ions. Carbohydr. Polym. 2016, 151, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek-Szczepańska, B.; Mazur, O.; Michalska-Sionkowska, M.; Łukowicz, K.; Osyczka, A.M. The Preparation and Characterization of Chitosan-Based Hydrogels Cross-Linked by Glyoxal. Materials 2021, 14, 2449. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, Z. Effects of gelatin-polyphenol and gelatin–genipin cross-linking on the structure of gelatin hydrogels. Int. J. Food Prop. 2017, 20 (Suppl. 3), S2822–S2832. [Google Scholar] [CrossRef]
- Nieto-Suárez, M.; López-Quintela, M.A.; Lazzari, M. Preparation and characterization of crosslinked chitosan/gelatin scaffolds by ice segregation induced self-assembly. Carbohydr. Polym. 2016, 141, 175–183. [Google Scholar] [CrossRef]
- Kim, S.; Nimni, M.E.; Yang, Z.; Han, B. Chitosan/gelatin–based films crosslinked by proanthocyanidin. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 75B, 442–450. [Google Scholar] [CrossRef]
- Yap, W.F.; Yunus, W.M.M.; Talib, Z.A.; Yusof, N.A. X-ray photoelectron spectroscopy and atomic force microscopy studies on crosslinked chitosan thin film. Int. J. Phys. Sci. 2011, 6, 2744–2749. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Grabska-Zielińska, S.; Skopińska-Wiśniewska, J.; Jakubowska, E. Examining the Impact of Squaric Acid as a Crosslinking Agent on the Properties of Chitosan-Based Films. Int. J. Mol. Sci. 2021, 22, 3329. [Google Scholar] [CrossRef]
- Abbasi, A.; Eslamian, M.; Heyd, D.; Rousseau, D. Controlled Release of DSBP from Genipin-Crosslinked Gelatin Thin Films. Pharm. Dev. Technol. 2008, 13, 549–557. [Google Scholar] [CrossRef]
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, structural and antimicrobial properties of poly vinyl alcohol–chitosan biodegradable films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Haider, S.; Raza, M.A.; Shah, S.A.; Razak, S.I.A.; Kadir, M.R.A.; Subhan, F.; Haider, A. Smart and pH-sensitive rGO/Arabinoxylan/chitosan composite for wound dressing: In-vitro drug delivery, antibacterial activity, and biological activities. Int. J. Biol. Macromol. 2021, 192, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Kudłacik-Kramarczyk, S.; Drabczyk, A.; Głąb, M.; Gajda, P.; Jaromin, A.; Czopek, A.; Zagórska, A.; Tyliszczak, B. Synthesis and Physicochemical Evaluation of Bees’ Chitosan-Based Hydrogels Modified with Yellow Tea Extract. Materials 2021, 14, 3379. [Google Scholar] [CrossRef] [PubMed]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative Study of Gelatin Hydrogels Modified by Various Cross-Linking Agents. Materials 2021, 14, 396. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Monteiro, F.J.; Carvalho, A.; Łukowicz, K.; Osyczka, A.M. Characterization of gelatin and chitosan scaffolds cross-linked by addition of dialdehyde starch. Biomed. Mater. 2017, 13, 015016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-H.; Liu, W.-S.; Sun, J.-F.; Hou, G.-G.; Chen, Q.; Cong, W.; Zhao, F. Non-toxic O-quaternized chitosan materials with better water solubility and antimicrobial function. Int. J. Biol. Macromol. 2016, 84, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Sajomsang, W. Synthetic methods and applications of chitosan containing pyridylmethyl moiety and its quaternized derivatives: A review. Carbohydr. Polym. 2010, 80, 631–647. [Google Scholar] [CrossRef]
- Karami Juyani, A.; Saberi, M.; Kaka, G.; Jafari, M.; Riyahi, S.; Taghizadeh, M. Toxicity Study of Gelatin-chitosan Films with Bone Marrow Stromalcells Cultured in Rat. Int. J. Pediatr. 2014, 2, 87. [Google Scholar] [CrossRef]
- Szurkowska, K.; Drobniewska, A.; Kolmas, J. Dual Doping of Silicon and Manganese in Hydroxyapatites: Physicochemical Properties and Preliminary Biological Studies. Materials 2019, 12, 2566. [Google Scholar] [CrossRef] [Green Version]
- Tammaro, M.; Salluzzo, A.; Rimauro, J.; Schiavo, S.; Manzo, S. Experimental investigation to evaluate the potential environmental hazards of photovoltaic panels. J. Hazard. Mater. 2016, 306, 395–405. [Google Scholar] [CrossRef]
- Isidori, M.; Ferrara, M.; Lavorgna, M.; Nardelli, A.; Parrella, A. In situ monitoring of urban air in Southern Italy with the tradescantia micronucleus bioassay and semipermeable membrane devices (SPMDs). Chemosphere 2003, 52, 121–126. [Google Scholar] [CrossRef]
- Fahmy, A.; Kamoun, E.; El-Eisawy, R.; El-Fakharany, E.; Taha, T.; El-Damhougy, B.; Ahmed, F. Poly(vinyl alcohol)-hyaluronic Acid Membranes for Wound Dressing Applications: Synthesis and in vitro Bio-Evaluations. J. Braz. Chem. Soc. 2015, 26. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, L.; Chen, Y.; Wang, J.; Chen, Y.; Mao, C.; Yang, M. Polydopamine modification of silk fibroin membranes significantly promotes their wound healing effect. Biomater. Sci. 2019, 7, 5232–5237. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Dhiman, A. Designing bio-mimetic moxifloxacin loaded hydrogel wound dressing to improve antioxidant and pharmacology properties. RSC Adv. 2015, 5, 44666–44678. [Google Scholar] [CrossRef]
- Yang, T.; Zheng, J.; Zheng, B.-S.; Liu, F.; Wang, S.; Tang, C.-H. High internal phase emulsions stabilized by starch nanocrystals. Food Hydrocoll. 2018, 82, 230–238. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Z.; Sheng, L.; Ma, M.; Xu, Q.; Jin, Y. Structure-property of crosslinked chitosan/silica composite films modified by genipin and glutaraldehyde under alkaline conditions. Carbohydr. Polym. 2019, 215, 348–357. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Kuderko, J.; Bajek, A.; Maj, M.; Sionkowska, A.; Ziegler-Borowska, M. Collagen/elastin hydrogels cross-linked by squaric acid. Mater. Sci. Eng. C 2016, 60, 100–108. [Google Scholar] [CrossRef]
- Johnson, B.T. Microtox® Acute Toxicity Test. In Small-Scale Freshwater Toxicity Investigations: Toxicity Test Methods; Blaise, C., Férard, J.-F., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2005; pp. 69–105. [Google Scholar]


| Sample | First Stage | Second Stage | Third Stage | Residue 600 °C (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Tmax (°C) | Δm (%) | To (°C) | Tmax (°C) | Δm (%) | To (°C) | Tmax (°C) | Δm (%) | ||
| CS | 30, 60 | 8 | - | - | - | 145 | 286 | 51 | 40 |
| CS-5%NDAS | 22, 53 | 13 | 134 | 145 | 3 | 162 | 274 | 49 | 34 |
| CS-10%NDAS | 31, 52 | 13 | 121 | 142 | 4 | 165 | 277 | 50 | 33 |
| CS-15%NDAS | 32, 55 | 12 | 125 | 141 | 4 | 161 | 258 | 47 | 37 |
| Gel | 34, 56 | 9 | - | - | - | 168 | 307 | 62 | 27 |
| Gel-5%NDAS | 38, 61 | 11 | 141 | 163 | 3 | 175 | 312 | 64 | 23 |
| Gel-10%NDAS | 35, 59 | 8 | 138 | 164 | 3 | 197 | 312 | 58 | 30 |
| Gel-15%NDAS | 37, 60 | 9 | 124 | 147 | 4 | 185 | 306 | 55 | 32 |
| CS-Gel | 26, 70 | 15 | - | - | - | 152 | 290 | 57 | 28 |
| CS-Gel-5%NDAS | 38, 57 | 13 | 135 | 150 | 2 | 168 | 284 | 54 | 31 |
| CS-Gel-10%NDAS | 32, 57 | 12 | 134 | 148 | 3 | 168 | 285 | 51 | 34 |
| CS-Gel-15%NDAS | 33, 57 | 11 | 131 | 144 | 4 | 165 | 270 | 51 | 34 |
| Roughness Parameters (nm) | Sample | |||
|---|---|---|---|---|
| CS | CS-5%NDAS | CS-10%NDAS | CS-15%NDAS | |
| Rq | 9.24 | 1.73 | 2.29 | 6.20 |
| Ra | 7.49 | 1.34 | 1.78 | 4.90 |
| Rmax | 89.9 | 28.1 | 31.3 | 33.9 |
| Gel | Gel-5%NDAS | Gel-10%NDAS | Gel-15%NDAS | |
| Rq | 1.83 | 2.48 | 3.26 | 4.70 |
| Ra | 1.48 | 1.61 | 2.36 | 2.64 |
| Rmax | 10.2 | 33.6 | 86.7 | 106.0 |
| CS-Gel | CS-Gel-5%NDAS | CS-Gel-10%NDAS | CS-Gel-15%NDAS | |
| Rq | 4.65 | 3.16 | 2.06 | 1.45 |
| Ra | 3.14 | 2.54 | 1.58 | 1.07 |
| Rmax | 65.6 | 27.1 | 18.3 | 14.8 |
| Amount of Adsorbed Serum Protein (mg/cm2) | Incubation Time (h) | Sample | |||
|---|---|---|---|---|---|
| CS | CS-5%NDAS | CS-10%NDAS | CS-15%NDAS | ||
| HSA | 1 | 0.067 | 0.116 | 0.093 | 0.159 |
| 4 | 0.067 | 0.131 | 0.106 | 0.167 | |
| 24 | 0.072 | 0.144 | 0.119 | 0.174 | |
| AGP | 1 | 0.068 | 0.073 | 0.081 | 0.069 |
| 4 | 0.070 | 0.084 | 0.086 | 0.081 | |
| 24 | 0.064 | 0.082 | 0.085 | 0.081 | |
| Gel | Gel-5%NDAS | Gel-10%NDAS | Gel-15%NDAS | ||
| HSA | 1 | 0.094 | 0.039 | 0.036 | 0.046 |
| 4 | 0.112 | 0.042 | 0.037 | 0.065 | |
| 24 | 0.126 | 0.044 | 0.039 | 0.069 | |
| AGP | 1 | 0.103 | 0.117 | 0.136 | 0.255 |
| 4 | 0.111 | 0.129 | 0.144 | 0.256 | |
| 24 | 0.111 | 0.130 | 0.141 | 0.256 | |
| CS-Gel | CS-Gel-5%NDAS | CS-Gel-10%NDAS | CS-Gel-15%NDAS | ||
| HSA | 1 | 0.031 | 0.042 | 0.039 | 0.052 |
| 4 | 0.033 | 0.047 | 0.040 | 0.053 | |
| 24 | 0.044 | 0.049 | 0.041 | 0.058 | |
| AGP | 1 | 0.066 | 0.073 | 0.065 | 0.079 |
| 4 | 0.073 | 0.074 | 0.067 | 0.080 | |
| 24 | 0.064 | 0.072 | 0.070 | 0.084 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wegrzynowska-Drzymalska, K.; Mylkie, K.; Nowak, P.; Mlynarczyk, D.T.; Chelminiak-Dudkiewicz, D.; Kaczmarek, H.; Goslinski, T.; Ziegler-Borowska, M. Dialdehyde Starch Nanocrystals as a Novel Cross-Linker for Biomaterials Able to Interact with Human Serum Proteins. Int. J. Mol. Sci. 2022, 23, 7652. https://doi.org/10.3390/ijms23147652
Wegrzynowska-Drzymalska K, Mylkie K, Nowak P, Mlynarczyk DT, Chelminiak-Dudkiewicz D, Kaczmarek H, Goslinski T, Ziegler-Borowska M. Dialdehyde Starch Nanocrystals as a Novel Cross-Linker for Biomaterials Able to Interact with Human Serum Proteins. International Journal of Molecular Sciences. 2022; 23(14):7652. https://doi.org/10.3390/ijms23147652
Chicago/Turabian StyleWegrzynowska-Drzymalska, Katarzyna, Kinga Mylkie, Pawel Nowak, Dariusz T. Mlynarczyk, Dorota Chelminiak-Dudkiewicz, Halina Kaczmarek, Tomasz Goslinski, and Marta Ziegler-Borowska. 2022. "Dialdehyde Starch Nanocrystals as a Novel Cross-Linker for Biomaterials Able to Interact with Human Serum Proteins" International Journal of Molecular Sciences 23, no. 14: 7652. https://doi.org/10.3390/ijms23147652






