Four Novel PAX9 Variants and the PAX9-Related Non-Syndromic Tooth Agenesis Patterns
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Variant Detection and Analysis
2.3. Sanger Sequencing and Clone Sequencing
2.4. Construction of Plasmids
2.5. Cell Culture and Transfection
2.6. Western Blot Analysis
2.7. Real-Time PCR and mRNA Stability Studies
2.8. Subcellular Localization Assay
2.9. Luciferase Reporter Assay
2.10. Statistical Analysis
3. Results
3.1. Clinical Findings and Variant Detection
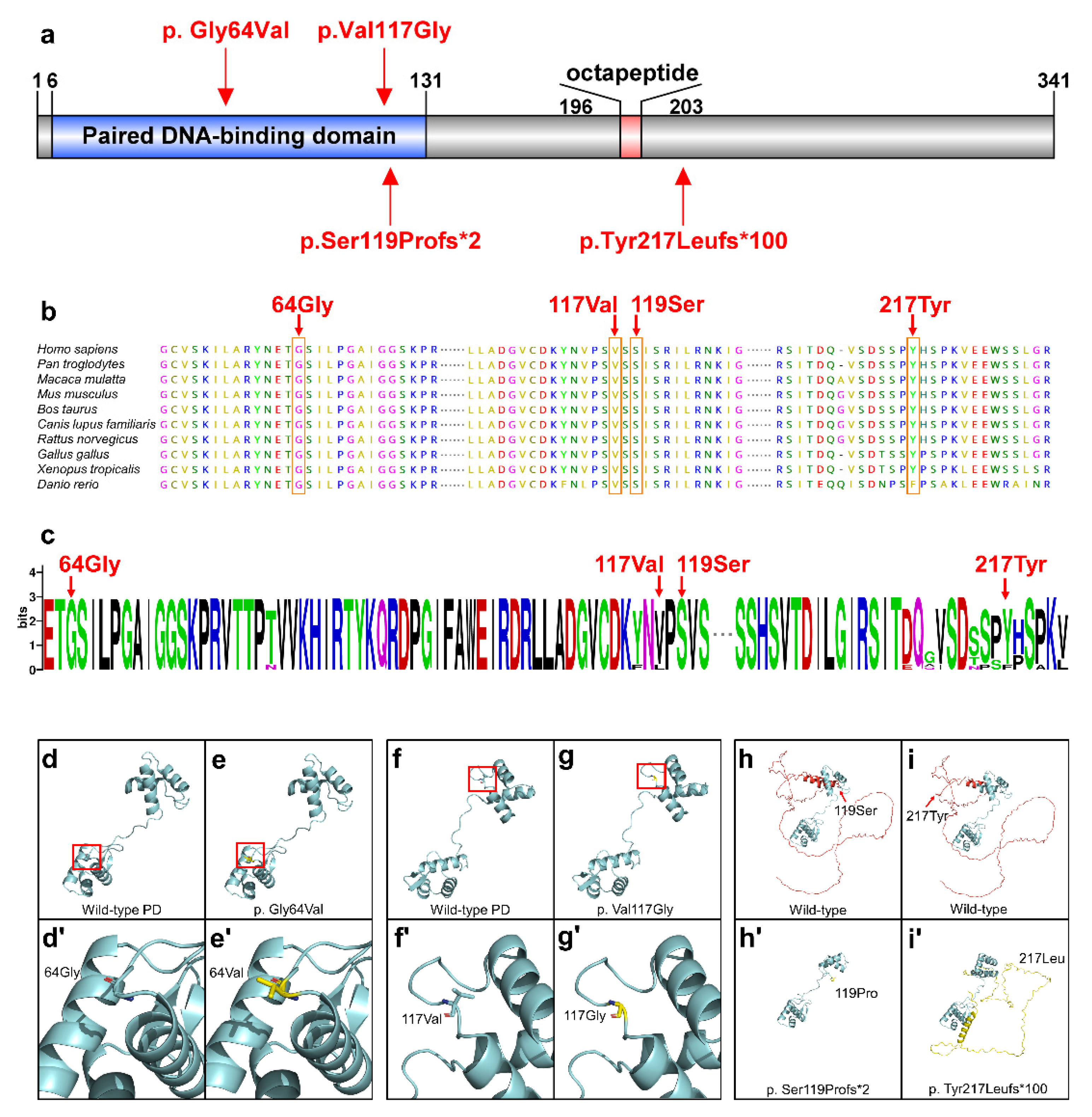
3.2. Conservation and Bioinformatics Analysis
3.3. Functional Analyses of PAX9 Variants
3.4. Statistical Analysis of the PAX9-Related Non-Syndromic Tooth Agenesis Pattern
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nieminen, P. Genetic Basis of Tooth Agenesis. J. Exp. Zool. 2009, 312B, 320–342. [Google Scholar] [CrossRef] [PubMed]
- Stockton, D.W.; Das, P.; Goldenberg, M.; D’Souza, R.N.; Patel, P.I. Mutation of PAX9 Is Associated with Oligodontia. Nat. Genet. 2000, 24, 18–19. [Google Scholar] [CrossRef]
- Ye, X.; Attaie, A. Genetic Basis of Nonsyndromic and Syndromic Tooth Agenesis. J. Pediatr. Genet. 2016, 5, 198–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polder, B.J.; Van’t Hof, M.A.; Van der Linden, F.P.G.M.; Kuijpers-Jagtman, A.M. A Meta-Analysis of the Prevalence of Dental Agenesis of Permanent Teeth. Community Dent. Oral Epidemiol. 2004, 32, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Rølling, S.; Poulsen, S. Oligodontia in Danish Schoolchildren. Acta Odontol. Scand. 2001, 59, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Maeda, T. Prevalence and Genetic Basis of Tooth Agenesis. Jpn. Dent. Sci. Rev. 2009, 45, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Ding, T.; Zhan, Y.; Feng, H. A Novel AXIN2 Missense Mutation Is Associated with Non-Syndromic Oligodontia. PLoS ONE 2015, 10, e0138221. [Google Scholar] [CrossRef]
- Yu, P.; Yang, W.; Han, D.; Wang, X.; Guo, S.; Li, J.; Li, F.; Zhang, X.; Wong, S.-W.; Bai, B.; et al. Mutations in WNT10B Are Identified in Individuals with Oligodontia. Am. J. Hum. Genet. 2016, 99, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.-W.; Liu, H.-C.; Han, D.; Chang, H.-G.; Zhao, H.-S.; Wang, Y.-X.; Feng, H.-L. A Novel Non-Stop Mutation in MSX1 Causing Autosomal Dominant Non-Syndromic Oligodontia. Mutagenesis 2014, 29, 319–323. [Google Scholar] [CrossRef] [Green Version]
- Suda, N.; Ogawa, T.; Kojima, T.; Saito, C.; Moriyama, K. Non-Syndromic Oligodontia with a Novel Mutation of PAX9. J. Dent. Res. 2011, 90, 382–386. [Google Scholar] [CrossRef]
- Song, S.; Han, D.; Qu, H.; Gong, Y.; Wu, H.; Zhang, X.; Zhong, N.; Feng, H. EDA Gene Mutations Underlie Non-Syndromic Oligodontia. J. Dent. Res. 2009, 88, 126–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inagaki, Y.; Ogawa, T.; Tabata, M.J.; Nagata, Y.; Watanabe, R.; Kawamoto, T.; Moriyama, K.; Tanaka, T. Identification of OPN3 as Associated with Non-Syndromic Oligodontia in a Japanese Population. J. Hum. Genet. 2021, 66, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhao, R.; He, H.; Zhang, J.; Feng, H.; Lin, L. WNT10A Variants Are Associated with Non-Syndromic Tooth Agenesis in the General Population. Hum. Genet. 2014, 133, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, S.N.; Yasue, A.; Masuda, K.; Watanabe, K.; Horiuchi, S.; Imoto, I.; Tanaka, E. Novel PAX9 Mutations Cause Non-Syndromic Tooth Agenesis. J. Dent. Res. 2014, 93, 245–249. [Google Scholar] [CrossRef]
- Wong, S.-W.; Han, D.; Zhang, H.; Liu, Y.; Zhang, X.; Miao, M.Z.; Wang, Y.; Zhao, N.; Zeng, L.; Bai, B.; et al. Nine Novel PAX9 Mutations and a Distinct Tooth Agenesis Genotype-Phenotype. J. Dent. Res. 2018, 97, 155–162. [Google Scholar] [CrossRef]
- Sun, K.; Yu, M.; Yeh, I.; Zhang, L.; Liu, H.; Cai, T.; Feng, H.; Liu, Y.; Han, D. Functional Study of Novel PAX9 Variants: The Paired Domain and Non-syndromic Oligodontia. Colorectal Dis. 2021, 27, 1468–1477. [Google Scholar] [CrossRef]
- Fauzi, N.H.; Ardini, Y.D.; Zainuddin, Z.; Lestari, W. A Review on Non-Syndromic Tooth Agenesis Associated with PAX9 Mutations. Jpn. Dent. Sci. Rev. 2018, 54, 30–36. [Google Scholar] [CrossRef]
- Mostowska, A.; Biedziak, B.; Trzeciak, W.H. A Novel Mutation in PAX9 Causes Familial Form of Molar Oligodontia. Eur. J. Hum. Genet. 2006, 14, 173–179. [Google Scholar] [CrossRef]
- Peters, H.; Neubüser, A.; Kratochwil, K.; Balling, R. Pax9-Deficient Mice Lack Pharyngeal Pouch Derivatives and Teeth and Exhibit Craniofacial and Limb Abnormalities. Genes Dev. 1998, 12, 2735–2747. [Google Scholar] [CrossRef] [Green Version]
- Prasad, M.K.; Geoffroy, V.; Vicaire, S.; Jost, B.; Dumas, M.; Le Gras, S.; Switala, M.; Gasse, B.; Laugel-Haushalter, V.; Paschaki, M.; et al. A Targeted Next-Generation Sequencing Assay for the Molecular Diagnosis of Genetic Disorders with Orodental Involvement. J. Med. Genet. 2016, 53, 98–110. [Google Scholar] [CrossRef] [Green Version]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, D.R.; Bond, J.P.; Tarone, R.E.; Harris, C.C.; Makalowski, W.; Boguski, M.S.; Greenblatt, M.S. Evolutionary Conservation and Somatic Mutation Hotspot Maps of P53: Correlation with P53 Protein Structural and Functional Features. Oncogene 1999, 18, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, S.; Zhou, J.; Gao, Y.; Baek, J.-A.; Martin, J.F.; Lan, Y.; Jiang, R. Roles of Bmp4 during Tooth Morphogenesis and Sequential Tooth Formation. Development 2013, 140, 423–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Gao, Y.; Zhang, Z.; Zhang, Y.; Maltby, K.M.; Liu, Z.; Lan, Y.; Jiang, R. Osr2 Acts Downstream of Pax9 and Interacts with Both Msx1 and Pax9 to Pattern the Tooth Developmental Field. Dev. Biol. 2011, 353, 344–353. [Google Scholar] [CrossRef] [Green Version]
- Mazzoni, C.; Falcone, C. MRNA Stability and Control of Cell Proliferation. Biochem. Soc. Trans. 2011, 39, 1461–1465. [Google Scholar] [CrossRef]
- Rawa, K.; Szczesny, R.J.; Owczarek, E.P.; Adamowicz-Salach, A.; Klukowska, A.; Demkow, U.; Plochocka, D.; Szczesny, P.; Gora, M.; Dziembowski, A.; et al. Two Novel C-Terminal Frameshift Mutations in the β-Globin Gene Lead to Rapid MRNA Decay. BMC Med. Genet. 2017, 18, 65. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Liu, Q.; Zhang, J.; Yang, Y.; Tian, Y.; Zeng, J.; Yin, P.; Mei, L.; Xiong, W.-C.; Li, X.-J.; et al. PRRT2 Frameshift Mutation Reduces Its MRNA Stability Resulting Loss of Function in Paroxysmal Kinesigenic Dyskinesia. Biochem. Biophys. Res. Commun. 2020, 522, 553–559. [Google Scholar] [CrossRef]
- Zarraga, I.G.; Zhang, L.; Stump, M.R.; Gong, Q.; Vincent, G.M.; Zhou, Z. Nonsense-Mediated MRNA Decay Caused by a Frameshift Mutation in a Large Kindred of Type 2 Long QT Syndrome. Heart Rhythm. 2011, 8, 1200–1206. [Google Scholar] [CrossRef] [Green Version]
- Kong, H.; Wang, Y.; Patel, M.; Mues, G.; D’Souza, R.N. Regulation of Bmp4 Expression in Odontogenic Mesenchyme: From Simple to Complex. Cells Tissues Organs 2011, 194, 156–160. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, T.; Kapadia, H.; Feng, J.Q.; Raghow, R.; Peters, H.; D’Souza, R.N. Functional Consequences of Interactions between Pax9 and Msx1 Genes in Normal and Abnormal Tooth Development. J. Biol. Chem. 2006, 281, 18363–18369. [Google Scholar] [CrossRef]
- Wang, Y.; Groppe, J.C.; Wu, J.; Ogawa, T.; Mues, G.; D’Souza, R.N.; Kapadia, H. Pathogenic Mechanisms of Tooth Agenesis Linked to Paired Domain Mutations in Human PAX9. Hum. Mol. Genet. 2009, 18, 2863–2874. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Number | Exon | Nucleotide Change | Protein Change | Variation Type | SIFT a | PROVEAN b | PolyPhen-2 c | Fathmm d | Mutation Taster e | ACMG Classification (Evidence of Pathogenicity) |
|---|---|---|---|---|---|---|---|---|---|---|
| #836 II 2 | 2 | c.350T>G | p. Val117Gly | Missense | 0.000 Damaging | −6.789 Deleterious | 0.981 (probably damaging) | −5.97 Damaging | Disease-causing | Pathogenic PS2 + PS3 + PM1 + PM2 + PP1 + PP2 + PP3 + PP4 |
| #821 III 1 | 2 | c.352delC | p. Ser119Pro fs*2 | Frameshift | Disease-causing | Pathogenic PVS1 + PS3 + PM1 + PM2 + PM4 | ||||
| #622 II 2 | 2 | c.191G>T | p. Gly64Val | Missense | 0.000 Damaging | −8.976 Deleterious | 1.000 (probably damaging) | −6.84 Damaging | Disease-causing | Pathogenic PS3 + PM1 + PM2 + PP1 + PP2 + PP3 |
| #350 III 1 | 3 | c.648_649insC | p. Tyr217Leu fs*100 | Frameshift | Disease-causing | Pathogenic PVS1 + PS3 + PM2 + PM4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Liu, H.; Su, L.; Zheng, J.; Feng, H.; Liu, Y.; Yu, M.; Han, D. Four Novel PAX9 Variants and the PAX9-Related Non-Syndromic Tooth Agenesis Patterns. Int. J. Mol. Sci. 2022, 23, 8142. https://doi.org/10.3390/ijms23158142
Liu H, Liu H, Su L, Zheng J, Feng H, Liu Y, Yu M, Han D. Four Novel PAX9 Variants and the PAX9-Related Non-Syndromic Tooth Agenesis Patterns. International Journal of Molecular Sciences. 2022; 23(15):8142. https://doi.org/10.3390/ijms23158142
Chicago/Turabian StyleLiu, Haochen, Hangbo Liu, Lanxin Su, Jinglei Zheng, Hailan Feng, Yang Liu, Miao Yu, and Dong Han. 2022. "Four Novel PAX9 Variants and the PAX9-Related Non-Syndromic Tooth Agenesis Patterns" International Journal of Molecular Sciences 23, no. 15: 8142. https://doi.org/10.3390/ijms23158142






