Abstract
The endocrine-related cancers and hormones are undoubtedly highly interconnected. How hormones support or repress tumor induction and progression has been extensively profiled. Furthermore, advances in understanding the role of glutamine metabolism in mediating tumorigenesis and development, coupled with these in-depth studies on hormone (e.g., estrogen, progesterone, androgen, prostaglandin, thyroid hormone, and insulin) regulation of glutamine metabolism, have led us to think about the relationship between these three factors, which remains to be elucidated. Accordingly, in this review, we present an updated overview of glutamine metabolism traits and its influence on endocrine oncology, as well as its upstream hormonal regulation. More importantly, this hormone/glutamine metabolism axis may help in the discovery of novel therapeutic strategies for endocrine-related cancer.
Keywords:
glutamine; hormone; estrogen; progesterone; androgen; prostaglandin; thyroid hormone; insulin 1. Introduction
Endocrine-related cancers generally indicate sex steroid-responsive cancers, including breast cancers, endometrial cancers, prostate cancers, and testis cancers, but there are other cancers, like thyroid and ovary cancers, which are also responsive to sex hormones [,,]. Indeed, they are unique as most are hormone-dependent or produce hormones. Hormones, such as estrogen and androgen, play a pivotal role in these tumors, promoting carcinogenesis, angiogenesis, and invasiveness, as well as apoptosis [,,]. For example, estrogen functions as the promoter of breast cancer and ovarian cancer through the ligand-activated transcription factor ER on the cell nucleus, and it can also stimulate endometrial glands and stromal cells to grow and proliferate during the menstrual cycle. DNA damage and mutations accumulate and increase the possibility of breast and ovarian cancer occurrence due to the imbalance between estrogen and progesterone during menstrual cycles. Meanwhile, prolonged exposure to estrogen is the leading cause of type I endometrial cancer [,,], which usually expresses a high level of estrogen receptors (ER). Moreover, there is an interesting phenomenon whereby ERα activation may induce thyroid cancer, while wild-type ERβ (ERβ1) has protective effects against thyroid cancer []. Androgen and its receptors are reported to be associated with the carcinogenesis of the testes and prostate [,,]. The imbalance between sex hormones and hormone receptors is the leading cause of testis cancer due to their considerable roles in normal organogenesis and spermatogenesis in testes and development in prostate [,]. Meanwhile, thyroid hormones triiodothyronine (T3) and thyroxine (T4) show effects on cancer proliferation, apoptosis, invasiveness, and angiogenesis through activation of the plasma membrane receptor integrin αvβ3 [,].
Besides, the metabolism of cancer cells has recently obtained increasing attention due to its emerging role in the occurrence and development of cancers, and endocrine-related tumors are no exception. It refers to glycolysis, lipid metabolism, glutamine metabolism, and so on. The activated aerobic glycolysis (Warburg effect) in the cancer cells is related to oncogenic effectors and loss of tumor suppressors. Inhibition of cancer cell glycolytic capacity has become a promising strategy for anti-tumor research []. Cancer cells also utilize lipid metabolism to provide their energy source, membrane components, and signaling molecules for proliferation. Cytoplasmic acetyl-CoA for lipid synthesis is produced from the ATP citrate lyase (ACLY)-catalyzed citrate and acetyl-CoA synthetase (ACSS)-catalyzed acetate, which is supplied by glucose and glutamine in the tricarboxylic acid (TCA) cycle and reductive carboxylation []. Interestingly, it has been found that cancer cells can also use glutamine as a carbon source (not emphasized in the Warburg effect) and glutamine metabolism provides intermediates in the TCA cycle to support other biosynthetic pathways [].
Glutamine (Gln) is one of the dispensable amino acids as it can be synthesized by the human body stably, but some cells are dependent on Gln, and they will die under glutamine depletion, including both normal and cancer cells. It contributes as an energy source for biosynthesis and homeostasis, especially under catabolic stress conditions and is consumed by the kidney, gastrointestinal tract, and immune compartment [,,,]. As shown in Figure 1, glutamine metabolism can fuel ATP production with participation in the TCA cycle. It enters mammalian cells with the help of a vital amino acid transporter, SLC1A5 (also called ASCT2), and then is transformed into glutamate (Glu) in the mitochondria through a deamination reaction with the catalysis of two different kinds of glutaminase, GLS (kidney type glutaminase) and GLS2 (liver type glutaminase). Glu is then converted to α-ketoglutarate (α-KG), catalyzed by one of the two enzymes: glutamate dehydrogenase (GLUD1 or GLUD2) or aminotransferase. α-KG enters into the TCA cycle and participates in the generation of ATP with production of NADH and FADH2. In addition, malate, one of the TCA cycle intermediates, leaves the cycle to produce pyruvate and NADPH. Another product, oxaloacetate (OAA), can be converted to aspartate for nucleotide synthesis. Citrate will further participate in the synthesis of acetyl-CoA and lipids through the cataplerosis of citrate [,,,,,,,,,]. Glutamine also serves as a biosynthetic precursor and reducing agent in regulating the amino acid pools. In addition to glutamate, it also acts as a nitrogen donor for the synthesis of other dispensable amino acids, including alanine, aspartate, and serine, under the catalysis of aminotransferases like alanine aminotransferase (ALT, also known as GPT, glutamate–pyruvate transaminase), aspartate aminotransferase (AAT, also known as GOT, glutamate–oxaloacetate transaminase), and phosphoserine aminotransferase 1 (PSAT1) [,,,,]. Moreover, Gln-derived glutamate can interact with cystine through the homodimer of SLC7A11 and SCL3A2, and glycine is further added to the complex with glutathione synthetase, which finally forms the tripeptide glutathione, showing inhibition of the reactive oxygen species (ROS) by neutralization of peroxide free radicals. Although ROS-mediated cell signaling pathways have shown pro-oncogenic effects physiologically, excess levels of ROS can damage the macromolecules. As mentioned above, glutamine metabolism also produces NADPH, which is necessary for the conversion of oxidized glutathione to reduced glutathione that neutralizes ROS [,,,].
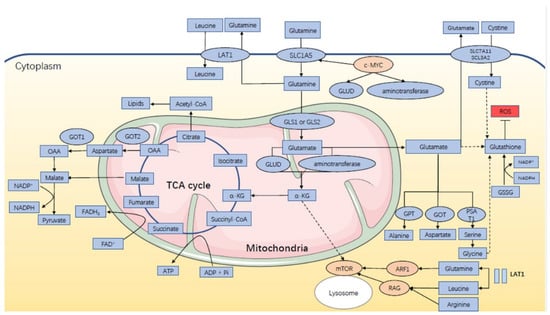
Figure 1.
Glutamine metabolism in endocrine-related cancers.
Arguably, glutamine metabolism is indispensable for tumor proliferation, and with the emerging understanding of the role of hormones in Gln metabolism and endocrine oncology, we, therefore, decided to dive deeper into these issues to untangle the connection among the three. Here, we review the specific mechanisms and functions of glutamine metabolism in endocrine-related tumors. Additionally, we also discuss how hormones may contribute to the process and corresponding treatment for endocrine-related cancers.
In mammalian cells, SLC1A5 transports glutamine into the cells while LAT1 mediates the efflux of glutamine from the cells for leucine uptake. This “glutamine recycling” will activate mTOR via RAG and ARF1. Intracellular glutamine can be converted to glutamate by mitochondrial enzyme GLS1 or GLS2, and then glutamate is further catabolized to α-KG, either via GLUD or aminotransferase. α-KG enters into the TCA cycle, during which ATP is produced, and its intermediate, malate, leaves the cycle to generate pyruvate and NADPH. OAA can also be converted to aspartate to join in the generation of pyruvate and NADPH. Citrate further participates in the synthesis of lipids. Meanwhile, glutamate helps for the synthesis of alanine, aspartate, and serine under the catalysis of aminotransferase GPT, GOT, and PSAT1. It is also directly or indirectly involved in the biosynthesis of glutathione, which can be converted from GSSG with NADPH consumed and helps for ROS scavenging. Besides, c-MYC can regulate the activity of SLA1C5, GDH, and aminotransferase to influence glutamine metabolism. Abbreviations: LAT1: L-type amino acid transporter 1; SLC1A5: solute carrier family 1 member 5; SLC7A11: solute carrier family 7 member 11; SCL3A2: solute carrier family 3 member 2; GLUD: glutamate dehydrogenase; GLS: glutaminase; GOT: glutamate–oxaloacetate transaminase; OAA: oxaloacetate; TCA: tricarboxylic acid; NADPH: reduced nicotinamide adenine nucleotide phosphate; FADH2: reduced flavin adenine dinucleotide; ATP: adenosine triphosphate; α-KG: α-ketoglutarate; GPT: glutamate–pyruvate transaminase; PSAT1: phosphoserine aminotransferase 1; ARF1: ADP ribosylation factor 1; RAG: Ras-related GTPase; GSSG: glutathione disulfide; ROS: reactive oxygen species.
2. Glutamine Metabolism Characteristics in Endocrine-Related Cancers
Gln is a non-essential amino acid (NEAA) abundant in the human body, and high glutamine consumption has been discovered in various cancers, including pancreatic cancer, acute myeloid leukemia, and endocrine-related cancers (e.g., ovarian cancer, breast cancer, thyroid cancer) [,,,,,,]. Herein, we try to discuss the glutamine metabolism characteristics in the endocrine-related cancers.
2.1. Incorporation into the TCA Cycle
In endocrine-related cancers, cancer cells uptake glutamine via ASCT2 [,,,]; intracellular glutamine can be utilized first by converting to glutamate catalyzed by mitochondrial enzyme GLS, and then glutamate is further catabolized to TCA cycle substrate α-KG, either via GLUD or transaminase [,,,,,]. As Gln feeds into the TCA cycle, ATP is generated and energy is supplied to cancerous cells. It is unrestrained proliferation, unbridled implantation, and invasive cancer cells’ capabilities that are bound to be accompanied by an increase in ATP and high energy requirements. As expected, Gln has been reported to become the primary carbon source for the maintenance of the TCA cycle in aggressive, therapy-resistant, and advanced prostate cancer []. Additionally, evidence has been provided that Gln significantly increases TCA cycle metabolite abundances in highly invasive ovarian cancer cells (SKOV3) compared to lowly invasive ovarian cancer cells (OVCAR3), and Gln addition doubles the ATP content in highly invasive subtypes []. Alternatively, the corresponding transporters and enzymes have been found to be highly expressed in these cancer patient samples, such as SLC1A5 [], GLS [,,], and GLUD1 []. Therefore, as with other cancer types, glutaminolysis is also a considerable metabolic pathway of endocrine-related cancer cells that provides TCA cycle intermediates, increases the TCA cycle process, and gives more energy.
2.2. Fatty Acid Synthesis
It has been reported that the LNCaP cell, an androgen-sensitive line of prostate cancer cells, can directly utilize glutamine for fatty acid production, and so glutamine was identified as a precursor of fatty acids and cholesterol in PC cells [], which has been confirmed by subsequent studies [,]. This pathway may be due to a process referred to as reductive carboxylation. During the course, a portion of the TCA cycle reverses itself, transforming α-KG into citrate, which can then be metabolized into cytosolic acetyl-CoA, participating in the de novo lipogenesis in the oncogenic contexts with a deficit of glucose-derived acetyl-CoA [,,].
2.3. NADPH Synthesis
Yang et al. claimed that Gln acts as an alternative fuel for NADPH, which decreases ROS, in highly invasive OVCA cells (SKOV3 and SKOV3ip), but not in lowly invasive OVCA cells (OVCAR3) []. This procedure has also been obtained in mouse breast cancer cells (4T1) [] and human prostate cancer cells (PC-3) []. It has been reported that NADPH is a designated donor of reducing equivalents, generated from NADP+, used in cellular biosynthetic reactions. As it is indispensable for sustaining reductive biosynthesis and maintaining redox homeostasis, a proliferating cell must allocate part of its carbon substrates to be applied to NADPH generation [,]. Accumulating data suggest that oxaloacetate from the oxidation of glutamine-derived a-ketoglutarate can be catabolized to malate. Following this, malate can be further oxidized to pyruvate in a reaction that concurrently produces NADPH under glucose-deprived conditions. The synthesis process has been found in tumor cells, including ovarian, breast, and prostate cancers [,,].
2.4. Glutathione Synthesis
Recent studies have found that Gln-derived Glu and α-KG are essential for the biosynthesis of glutathione (GSH), ROS scavenging, and protection against oxidative stress in prostate cancer cells, and glutaminolysis thus serves as a critical regulator of prostate cancer radiosensitivity and radioresistance [,]. GSH, a prime cellular antioxidant tripeptide, consists of glutamate, cysteine, and glycine. Therefore, in addition to fueling the TCA cycle as described above, Gln-derived glutamate also partially participates in the synthesis of GSH [,,]. In particular, it as been suggested that NADPH can be consumed by tumor cells as the substrate with the conversion of glutathione disulfide (GSSG) to GSH by glutathione reductase (GR) [,,]. Collectively, by Gln breaking down, GSH is generated, accounting for the maintenance of redox status and protecting cells from oxidative stress.
2.5. Facilitating Leucine Transport
SLC1A5, mentioned above, functions as an antiporter that transports Gln into the intracellular area, whereas SLC7A5 (LAT1) then mediates the efflux of Gln from the cells to facilitate uptake of its substrate leucine, an essential amino acid [,]. Given that intracellular leucine and SLC7A5 have been proven to be responsible for the growth of ER+ breast cancer cells [], intracellular glutamine is aberrantly exported from the cell to support the leucine-dependent cell proliferation []. Besides, this “glutamine recycling” has been found in PC-3 cells as well. The researchers’ explanation for this is the activation of the mammalian target of rapamycin complex 1 (mTORC1), which is stimulated under the activation of the Ras-related GTPase (RAG) complex and recruited on lysosomes facilitated by ADP ribosylation factor 1 (ARF1) and may indirectly affect mitochondrial respiration and favor cell growth [].
3. Hormone Regulation of Glutamine Metabolism
Hormones have shown different roles in participating in the glutamine metabolism and, therefore, in regulating further biological responses. Here, we will discuss the effects of hormone regulation on glutamine metabolism, including estrogen, progesterone, androgen, prostaglandin, and thyroid hormone, as shown in Table 1.

Table 1.
Effects of regulation of hormones on glutamine metabolism.
3.1. Estrogen and Progesterone
Glutamate and glutamine remain balanced in the human body under the synthesis of glutamine catalyzed by glutamine synthetase and hydrolysis of glutamine with GLS catalysis. The conversion of glutamate to glutamine mainly happens when glutamate is uptaken by astroglial cells, which are located in glial cells in neural system under the catalysis of glutamine synthetase. The glutamine formed in astroglia is transported with facilitated diffusion via Na+ and H+-coupled electroneutral systems—N transporters. Research by Haghighat et al. has proven that estrogen enhances glutamine synthetase activity and glutamine formation in C-6 glial cells, which furthermore influences glutamine metabolism. Vallejo et al. suggest that progesterone displays neuroprotective effects and the glutamine synthetase is significantly increased with the treatment of progesterone. Grasso et al. also showed that progesterone interacts with glucocorticoid receptors and induces glutamine synthetase [,,,,]. Studies from Zlotnik et al. proved that the blood glutamate level is inversely correlated to the increasing level of plasma estrogen and progesterone during pregnancy and menstrual cycles []. Interestingly, there has also been research that found that mitochondrial glutamine and glutamate metabolism may contribute to progesterone biosynthesis by providing NADPH [].
Estrogen can also directly facilitate glutamine metabolism by increasing aminotransferase activity. Research by Zhou et al. proved that estrogen can increase Gln metabolism of estrogen-sensitive uterine endometrial carcinoma cells by upregulating the c-MYC pathway and, therefore, increasing the GLS level, which transfers cellular glutamine to glutamate []. The oncogene, c-MYC, as a master transcriptional factor, modulates approximately 10–15% of genes in the genome and regulates the expression of SLC7A11, SLC1A5, SLC6A14, SLC7A5, GLS, and so on [,,]. A study carried out by Chen et al. also found that for ER-positive and aromatase inhibitor-sensitive breast cancers, estrogen can bind to ER and further upregulate the c-MYC pathway to increase glutamine metabolism. Interestingly, although glutamine metabolism is independent of estrogen in aromatase inhibitor-resistant breast cancer, the cross-talk between ER and HER2 upregulates the c-MYC pathway and further increases the expression level of GLS, SLC1A5, and glutamine consumption. In line with Chen’s study, estrogen has been proven to promote ovarian cancer development through either receptor-dependent or receptor-independent pathways by binding to the estrogen receptor α and activating estrogen transcriptive genes like c-MYC, HER2, and growth factors, which lead to cell differentiation and division [,,,]. Estrogen-related receptors (ERR) can also control glutathione production and downstream regulate glutamine metabolism participation in the TCA cycle and also influence ROS, which is essential in cancer development [,,,]. On the other hand, a weak positive correlation between GLS with PI3KCa within the low proliferative luminal breast cancer was put forward by Masisi’s group. PIK3Ca mutations, shown to be linked to hormone receptor-positive breast cancer [], are involved in metabolic reprogramming, specifically enhancing glutamine uptake and glutamate generation via regulation of pyruvate dehydrogenase activity []. Therefore, glutamine metabolism can be regulated in estrogen-dependent manners. Additionally, the synergistic and/or antagonistic effects and detailed mechanisms of progesterone and estrogen in glutamine metabolism need to be further researched.
3.2. Androgen and Prostaglandin
It has been suggested that androgen and prostaglandin are implicated in carcinogenesis and the progression of a lot of cancers, especially prostate cancer, and almost all prostate cancers are androgen dependent [,]. Barfeld et al. have reported that androgen regulates biosynthesis and metabolism in PC, including the c-MYC signaling pathway []. As androgen is stimulated, the expression of c-MYC in PC cells will be suppressed []. The steroid-activated androgen receptor (AR) can also decrease the c-MYC signaling pathway, which accounts for driving reinforced glutaminolysis in cancer cells through regulating glutamine transporters and GLS1 directly or indirectly (via miRNA) [,,]. However, White’s team claimed that AR signaling could upregulate the expression of glutamine transporters SLC1A4 and SLC1A5, which are overexpressed in prostate cancer. MYC only acts as a context-dependent regulator of SLC1A4 and SLC1A5 levels while mTORC1 is involved in the maximum AR-mediated glutamine uptake []. Of note, Xu and his colleague found that AR upregulated GLS1 and increased the glutamine utilization, and therefore, androgen deprivation is considered as an important method to prevent glutamine uptake by cancer cells []. More interestingly, the gut microbe has been reported to be involved in androgen-regulated glucose homeostasis and circulating glutamine/glutamate ratio [].
In addition, prostaglandin E2 (PGE2) can mediate the Ca2+-dependent release of glutamate from neuron cells under the coactivation of the AMPA/kainate and metabotropic glutamate receptors (mGluRs) [,]. GABA-transaminase (GABA-T) can convert glutamate (Glu)–γ-aminobutyric acid (GABA) to succinate as a TCA cycle intermediate and further convert α-KG to resynthesize intramitochondrial glutamate for the TCA cycle, and AAT converts glutamate to α-KG and aspartate. Notably, prostaglandins E1 (PGE1) can induce morphological changes in astrocytes with dibutyryl cyclic AMP (dBc AMP) and further increase two enzymes, GABA-T and AAT, in the GABA cycle and glutamate metabolism [,,].
3.3. Thyroid Hormones
Thyroid hormone signaling is necessary for skeletal muscle development and research has proven that alanine and glutamine are synthesized and released in skeletal muscle, indicating that the thyroid hormone may contribute to the regulation of glutamine metabolism [,]. Glutamine synthetase is prominent in converting glutamate to glutamine, as discussed before, and it is developmentally regulated in the oligodendrocyte lineage. T3 has been found to control glutamine synthetase levels and, therefore, regulate oligodendrocyte maturation and progenitor cell proliferation []. Cicatiello et al. also suggested that the thyroid hormone regulated glutamine metabolism by upregulating the GPT2 gene. GPT2 is an aminotransferase that transfers nitrogen from glutamate to pyruvate to make alanine and α-ketoglutarate. In turn, the mutation of glutamine also interferes with the function of thyroid hormone nuclear receptors [,]. Besides, research by Ou et al. described a positive association between GABA and T3, as well as Gln and T4 concentrations in the serum of manganese-exposed rats []. Parry-Billings et al. held the hypothesis that the thyroid hormone can influence glutamine release by skeletal muscle. More specifically, T3 induces the rate of glutamine release from skeletal muscle, through which, T3 may affect the function of the immune system [,].
Other thyroid-related hormones also influence glutamine metabolism. Thyroid-stimulating hormones are produced by pars tuberalis (PT). Aizawa et al. found that the ionotropic glutamic acid receptor (iGluR) KA2 was highly expressed in PT, as well as glutamine transporter (ATA2) and GLS2. These findings suggest that glutamine is highly ingested by PT and converted to glutamate by GLS []. Garber et al. showed that a high level of parathyroid hormones has direct effects on skeletal muscle formation. The parathyroid hormone regulates glutamine metabolism by increasing glutamine uptake and using GLS1 to convert glutamine to glutamate. The parathyroid hormone can also augment glutamine utilization through both phosphate-dependent glutaminase (PDG) and GDH pathways to increase α-KG formation in glutamine metabolism and increase extracellular glutamate transport [,].
3.4. Insulin
Insulin has been found to boost the expression of glutamine synthetase (GS) mediated by sterol regulatory element-binding protein 1 (SREBP1), and increased GS expression further enhances glutamine-dependent anabolic pathways to promote nucleotide and protein synthesis, lipid droplet (LD) formation, and lipogenesis in breast cancer cells []. Therefore, insulin can impact on breast cancer cell growth, proliferation, and migration, which has also been proved by other studies [,].
4. Functional Roles of Glutamine Metabolism on Endocrine-Related Cancers
With the field of glutamine metabolism becoming a hotspot for research, it has become evident that Gln metabolism plays a prominent role in energy production, biosynthesis, and redox homeostasis, and so is vital for establishing and maintaining a tumorigenic status by becoming involving in cancer-related signaling pathways, such as c-MYC, mTOR, Akt, Ras, and AMPK pathways [,,,,,,,]. Then, what about endocrine tumors? We will further clarify the specific roles of glutamine metabolism in endocrine-related cancers as follows and as shown in Figure 2 and Table 2.
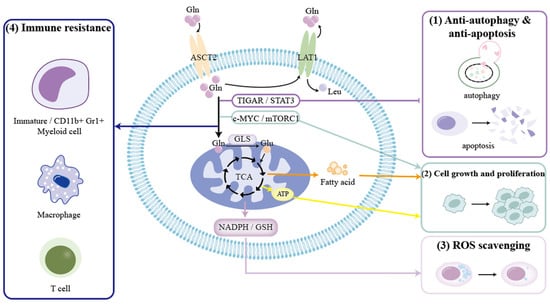
Figure 2.
Effect of glutamine metabolism on endocrine-related cancers.

Table 2.
Influence of glutamine metabolism on endocrine-related cancers.
- (1)
- Gln metabolism is able to protect endocrine-related cancer cells against apoptosis and autophagy through the TIGAR and STAT3 signaling pathways [,].
- (2)
- Gln metabolism helps tumor cell growth and proliferation via the c-MYC and mTORC1 signaling pathways, and by producing ATP and fatty acid [,,,].
- (3)
- Gln metabolism can generate NADPH and GSH to decrease the generation of ROS and maintain the redox state [,,,].
- (4)
- Gln metabolism can act on immature myeloid cells, CD11b+Gr1+ myeloid cells, macrophages, and T lymphocytes to realize antitumor immune response [,,,,].
Abbreviations: Gln: glutamine; Leu: leucine; ASCT2: amino acid transporter-2; LAT1: L-type amino acid transporter 1; TIGAR: TP53-induced glycolysis and apoptosis regulator; STAT3: signal transducer and activator of transcription 3; mTORC1: mammalian target of rapamycin complex 1; GLS: glutaminase; TCA: tricarboxylic acid; ATP: adenosine triphosphate; NADPH: reduced nicotinamide adenine nucleotide phosphate; GSH: glutathione; ROS: reactive oxygen species; NAA: N-acetylaspartate.
4.1. Breast Cancer
Glutamine metabolism has been shown to favor proliferation and be associated with aggressive breast cancer [,,,], which is partly due to the hyperactivation of mTORC1, a critical regulator of cell signaling and metabolic pathways, promoting protein translation and fatty acids biosynthesis, improving cell growth, and inhibiting catabolism or autophagy [,]. Another important reason may be that glutaminolysis supplies the cancer cells with intermediates for the synthesis of macromolecules, redox balance, and mitochondrial energy metabolism required for growth [,,,,]. In addition, it has been demonstrated that glutamine can augment mitochondrial mass and decrease the expression of autophagy markers in the human breast adenocarcinoma cell line (MCF7 cells). Moreover, Gln is able to protect MCF7 cells against apoptosis via the upregulation of TIGAR, a multi-functional protein that suppresses glycolysis, apoptosis, and autophagy []. Of note, Wu et al. have suggested that the metabolism of glutamine is the master metabolic pathway controlling the proliferation and activation of immature myeloid cells using a patient-derived and 4T1 breast cancer preclinical model []. It has also been found that restraint of glutaminolysis in 4T1 breast cancer preclinical model contributes to immunogenic tumor cell death []. In triple-negative breast cancer (TNBC), glutamine metabolism has been discovered to refrain tumor-infiltrating T lymphocyte numbers and function, and so the team concluded that increased glutamine metabolism combined with the declined cytotoxic potential of T cells within tumors may result in the nonresponsiveness of a significant proportion of TNBC patients to immunotherapies [,]. Besides, Dias et al. also showed that in TNBC, the GLS2 level increased significantly and sequentially promoted tumor proliferation and metastasis. These reports suggest that an active glutamine metabolism promotes the development of TNBC under the regulation of non-hormone factors. Gln metabolism is also enhanced to facilitate the growth of advanced hormone receptor-positive breast cancer, the development of which is independent of estrogen [,,]. By considering the above-mentioned facts, it is clear that Gln metabolism is conducive to the propagation, anti-apoptotic and anti-autophagic ability, and immune resistance of breast cancer, thus hastening tumor progression.
4.2. Uterine Endometrial Carcinoma
Nowadays, the emerging understanding of estrogen and glutamine in regulating the development of uterine endometrial carcinoma (UEC) has been established [,,]. The intracellular glutamine has been described as mediating the ability of LAT1 to enhance the growth of UEC cells. LAT1, a reciprocal transporter, plays a growth-promoting role in UEC [] via uptake of leucine in exchange for efflux of glutamine, which further activates the mTORC1 pathway to facilitate the proliferation []. Zhou et al. have proposed that estrogen enables the activation of glutaminolysis, which in turn suppresses autophagy in endometrial cancer cells of estrogen-sensitive UEC cell line Ishikawa cells []. This process relies on the upregulation of GLS, activated by c-MYC under the regulation of estrogen, which is consistent with previous reports []. Additionally, they found Gln can promote the growth of both Ishikawa and KLE cells regardless of estrogen sensitivity. In Ishikawa cells, estrogen’s ability to improve cellular viability is an accounting factor for this effect []. Overall, glutamine metabolism accelerates tumor growth and inhibits autophagy in uterine endometrial cancer.
4.3. Ovarian Cancer
Ovarian cancer (OVCA) is regarded as the highest mortality of gynecological malignancies, for the reason that most metastases have already occurred with a poor prognosis by the time they are detected [,,], for which glutamine metabolism occupies a nonnegligible position. First of all, Gln metabolism has been extensively elucidated to not only help in the proliferation and metastasis of OVCA cells, but also has been implicated in ovarian cancer aggressiveness and increased invasion [,,,,,,]. In addition to promoting biosynthesis, energy supply, relieving oxidative stress, and maintaining redox, the mTOR/S6 and MAPK pathways also play an important role, as inhibiting mTOR activity by rapamycin or blockade of S6 expression via siRNA will restrict the activity of GDH and GLS, resulting in a reduction in Gln-induced cell proliferation [,]. Of note, Gln is capable of pushing OVCA cells from stage G1 to stage S to affect cell cycle progression and ultimately induce cell growth []. Besides, the activation of STAT3 by glutamine in OVCA has been already unraveled, and it has been reported that Gln deprivation regulates STAT3 phosphorylation, thereby causing the cancer metabolic switch []. STAT3 has been shown to function in cell differentiation, antiapoptotic response, cancer metastasis, and regulating cancer hallmarks, such that it potentiates drug resistance and correlates with highly invasive OVCA cells [,,,,,,]. Recently, Menga’s team uncovered the unprecedented role of glutaminolysis in regulating macrophage polarization in highly invasive OVCA and they clarified that this effect links to N-acetylaspartate (NAA) synthesis as its anti-inflammatory and protumoral properties []. Furthermore, glutamine metabolism has also been proven to modulate the metabolism and function of immunosuppressive CD11b+Gr1+ myeloid cells in ovarian cancer, finally influencing the immune environment of tumors and the antitumor immune response []. According to the above analysis, we suppose that the proliferation, migratory and invasive abilities, immune resistance, and anti-apoptosis of OVCA cells are dependent on glutaminolysis to some extent.
4.4. Prostate Cancer
Prostate cancer (PC) is a hormone-dependent disease, known for its metabolism deregulation, metabolic rewiring, and reliance on glutamine [,,,,]. Glutamine has been previously found to decrease the generation of ROS and produce GSH in PC to maintain the redox state, reduce the risk of oxidative stress, and, more importantly, mediate PC radioresistance [,,,]. In addition, the biosynthesis and energy production of glutamine metabolism can be observed in PC, and consequently, glutaminolysis has been found to be involved in the cell growth, division and invasion, and the progression of the tumor [,,,,]. To conclude, glutamine metabolism benefits prostate cancer mainly in promoting the development of the tumor and radiation resistance.
4.5. Thyroid Cancer
In thyroid cancer (TC), the alteration of glutamine level and Gln metabolism-related protein expression have been demonstrated to be implicated in tumorigenesis [,]. Like in other endocrine related cancers, glutaminolysis plays a prominent role in the development and progression of papillary thyroid cancer (PTC), owing a great deal to the key enzyme, GLS []. It has been well described that blocking GLS can inhibit glutamine metabolism; limit mitochondrial respiration; induce apoptosis and autophagy via the mTORC1 signaling pathway; and end with the growth, viability, and invasion of PTC cells getting impaired. Accordingly, glutaminolysis is associated with the formation and progression of TC. However, given the limited number of studies to date, further research is needed and the relevant mechanism remains to be elucidated.
5. Hormone/Glutamine Metabolism Axis in Endocrine-Related Cancer Intervention
Currently, the use of hormone/glutamine metabolic axis for anti-tumor therapy has become increasingly common, as listed in Table 3. CB-839, a GLS inhibitor, is regarded as a putative treatment for tamoxifen-resistant LCC9 breast cancer cells [] and UECC []. It has been found that CB-839 effectively increases glutamine in LCC9 cells, which have been proved to be dependent on upregulated pro-survival autophagy [], and so it is estimated that the efficacy of CB-839 links to the disruption of amino acid metabolism following autophagy []. As for the uterine endometrial carcinoma cell (UECC), CB-839 significantly suppresses glutaminolysis and abrogates estrogen-induced cell viability and the inhibitory effect of autophagy in estrogen-sensitive UECC in vitro and in vivo []. Alternatively, chemical inhibition or shRNA knockdown of ASCT2 has been shown to be an effective therapeutic target in PC. Given that ASCT2 is androgen-regulated, this intervention will target PC at multiple sites along the androgen/glutamine metabolism axis []. Meanwhile, the glutaminase inhibitor BPTES can decrease the viability and migration of PC cells while increasing caspase-3 activity. There is an additive effect on suppressing androgen-sensitive LNCaP cell viability when BPTES and anti-androgen bicalutamide coexist []. Besides, chemical inhibition of glutamine transport by benzylserine (BenSer) or GPNA has also been proved to work by limiting UECC growth []. BenSer has also been described as inhibiting breast cancer cell growth and viability by blocking ASCT2 and LAT1 activity []. To sum up, Gln metabolism as a target of intervention in the treatment of endocrine-related cancers has potential value and deserves further exploration.

Table 3.
Drug intervention in endocrine-related cancers.
6. Conclusions
In conclusion, as shown above, hormone-regulated glutaminolysis is involved in various aspects of endocrine-related cancers, such as tumor growth, invasion, oxidative stress, autophagy, and so on. The hormone/glutamine metabolism axis plays a momentous role in tumor genesis and development, and the targeted intervention measures have already achieved certain progress in tumor treatment. Through the summarized literature review of these findings, we believe that future research directions in this field can focus on the aspects as follows. Firstly, although there have been some studies on the regulatory effect of glutamine metabolism on endocrine oncology, the local regulatory mechanism is still inadequate and needs further study. Secondly, the regulation of glutamine metabolism on the immune microenvironment of endocrine related cancers and related mechanisms are also worth exploring. Thirdly, there are many other factors (e.g., hypoxia, epigenetics, cytokines) involved in the regulation of glutamine metabolism to affect non-endocrine-related cancers and endocrine-related cancers. Hormones can be regarded as a therapeutic target due to their regulatory effects on glutamine metabolism in endocrine-related cancers, which is different to non-endocrine-related cancers. Therefore, more drug development efforts ought to be made to exploit the novel therapeutic strategies targeting glutamine metabolism, especially in combination with hormone-targeted interventions, for patients with endocrine-related cancer.
Author Contributions
F.X. and J.S. performed the literature research, wrote the manuscript, and prepared the tables and figures. X.Q. edited the figures, Z.Z., M.C., and Z.L. revised the manuscript. J.Y. edited the manuscript. M.L. designed and wrote the review, supervised, and critically reviewed the complete manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (NSFC) (No. 92057119, 31970798), the Program for Zhuoxue of Fudan University (JIF157602) and the Support Project for Original Personalized Research of Fudan University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Belfiore, A.; Perks, C.M. Grand Challenges in Cancer Endocrinology: Endocrine Related Cancers, an Expanding Concept. Front. Endocrinol. 2013, 4, 141. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.E.; Ross, R.K.; Pike, M.C.; Casagrande, J.T. Endogenous hormones as a major factor in human cancer. Cancer Res. 1982, 42, 3232–3239. [Google Scholar] [PubMed]
- Henderson, B.E.; Feigelson, H.S. Hormonal carcinogenesis. Carcinogenesis 2000, 21, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Lin, C.-Y. Oestrogen receptors in breast cancer: Basic mechanisms and clinical implications. Ecancermedicalscience 2013, 7, 370. [Google Scholar] [CrossRef]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen Signaling in Endometrial Cancer: A Key Oncogenic Pathway with Several Open Questions. Horm. Cancer 2019, 10, 51–63. [Google Scholar] [CrossRef]
- Michaud, J.E.; Billups, K.L.; Partin, A.W. Testosterone and prostate cancer: An evidence-based review of pathogenesis and oncologic risk. Ther. Adv. Urol. 2015, 7, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Burke, W.M.; Orr, J.; Leitao, M.; Salom, E.; Gehrig, P.; Olawaiye, A.B.; Brewer, M.; Boruta, D.; Villella, J.; Herzog, T.; et al. Endometrial cancer: A review and current management strategies: Part I. Gynecol. Oncol. 2014, 134, 385–392. [Google Scholar] [CrossRef]
- Pike, M.C.; Peters, R.K.; Cozen, W.; Probst-Hensch, N.M.; Wan, P.C.; Mack, T.M.; Felix, J.C. Estrogen-Progestin Replacement Therapy and Endometrial Cancer. JNCI J. Natl. Cancer Inst. 1997, 89, 1110–1116. [Google Scholar] [CrossRef]
- Fisher, B.; Costantino, J.P.; Redmond, C.K.; Fisher, E.R.; Wickerham, D.L.; Cronin, W.M.; Contributors, O.N. Endometrial Cancer in Tamoxifen-Treated Breast Cancer Patients: Findings From the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. JNCI J. Natl. Cancer Inst. 1994, 86, 527–537. [Google Scholar] [CrossRef]
- Liu, Y.; Su, L.; Xiao, H. Review of Factors Related to the Thyroid Cancer Epidemic. Int. J. Endocrinol. 2017, 2017, 5308635. [Google Scholar] [CrossRef]
- Grassetti, D.; Giannandrea, F.; Paoli, D.; Masciandaro, P.; Figura, V.; Carlini, T.; Rizzo, F.; Lombardo, F.; Lenzi, A.; Gandini, L. Androgen receptor polymorphisms and testicular cancer risk. Andrology 2014, 3, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Heinlein, C.A.; Chang, C. Androgen Receptor in Prostate Cancer. Endocr. Rev. 2004, 25, 276–308. [Google Scholar] [CrossRef] [PubMed]
- Stuchbery, R.; McCoy, P.J.; Hovens, C.M.; Corcoran, N.M. Androgen synthesis in prostate cancer: Do all roads lead to Rome? Nat. Rev. Urol. 2016, 14, 49–58. [Google Scholar] [CrossRef]
- Nakagawa, H.; Ueda, T.; Ito, S.; Shiraishi, T.; Taniguchi, H.; Kayukawa, N.; Nakanishi, H.; Ushijima, S.; Kanazawa, M.; Nakamura, T.; et al. Androgen suppresses testicular cancer cell growth in vitro and in vivo. Oncotarget 2016, 7, 35224–35232. [Google Scholar] [CrossRef] [PubMed]
- Krashin, E.; Piekiełko-Witkowska, A.; Ellis, M.; Ashur-Fabian, O. Thyroid Hormones and Cancer: A Comprehensive Review of Preclinical and Clinical Studies. Front. Endocrinol. 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Lee, E.J.; Huang, M.G.; Park, Y.I.; Khullar, A.; A Plodkowski, R. Diagnosis and treatment of patients with thyroid cancer. Am. Health Drug Benefits 2015, 8, 30–40. [Google Scholar] [PubMed]
- Jang, M.; Kim, S.S.; Lee, J. Cancer cell metabolism: Implications for therapeutic targets. Exp. Mol. Med. 2013, 45, e45. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Liu, R.; Meng, Y.; Xing, D.; Xu, D.; Lu, Z. Lipid metabolism and cancer. J. Exp. Med. 2020, 218, e20201606. [Google Scholar] [CrossRef]
- Wang, J.-B.; Erickson, J.W.; Fuji, R.; Ramachandran, S.; Gao, P.; Dinavahi, R.; Wilson, K.F.; Ambrosio, A.L.; Dias, S.M.; Dang, C.V.; et al. Targeting Mitochondrial Glutaminase Activity Inhibits Oncogenic Transformation. Cancer Cell 2010, 18, 207–219. [Google Scholar] [CrossRef]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef]
- Jiang, J.; Srivastava, S.; Zhang, J. Starve Cancer Cells of Glutamine: Break the Spell or Make a Hungry Monster? Cancers 2019, 11, 804. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.; Macedo Rogero, M.; Keane, K.N.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Cluntun, A.A.; Lukey, M.J.; Cerione, R.A.; Locasale, J.W. Glutamine Metabolism in Cancer: Understanding the Heterogeneity. Trends Cancer 2017, 3, 169–180. [Google Scholar] [CrossRef]
- Xiao, D.; Zeng, L.; Yao, K.; Kong, X.; Wu, G.; Yin, Y. The glutamine-alpha-ketoglutarate (AKG) metabolism and its nutritional implications. Amino Acids 2016, 48, 2067–2080. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, N.P.; Watford, M. Regulation of Glutaminase Activity and Glutamine Metabolism. Annu. Rev. Nutr. 1995, 15, 133–159. [Google Scholar] [CrossRef] [PubMed]
- Moreadith, R.W.; Lehninger, A.L. The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P)+-dependent malic enzyme. J. Biol. Chem. 1984, 259, 6215–6221. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef]
- Son, J.; Lyssiotis, C.A.; Ying, H.; Wang, X.; Hua, S.; Ligorio, M.; Perera, R.M.; Ferrone, C.R.; Mullarky, E.; Shyh-Chang, N.; et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013, 496, 101–105. [Google Scholar] [CrossRef]
- Ward, P.S.; Patel, J.; Wise, D.R.; Abdel-Wahab, O.; Bennett, B.D.; Coller, H.A.; Cross, J.R.; Fantin, V.R.; Hedvat, C.V.; Perl, A.E.; et al. The Common Feature of Leukemia-Associated IDH1 and IDH2 Mutations Is a Neomorphic Enzyme Activity Converting α-Ketoglutarate to 2-Hydroxyglutarate. Cancer Cell 2010, 17, 225–234. [Google Scholar] [CrossRef]
- Awapara, J.; Seale, B. DISTRIBUTION OF TRANSAMINASES IN RAT ORGANS. J. Biol. Chem. 1952, 194, 497–502. [Google Scholar] [CrossRef]
- Welbourne, T.C. Ammonia production and glutamine incorporation into glutathione in the functioning rat kidney. Can. J. Biochem. 1979, 57, 233–237. [Google Scholar] [CrossRef]
- Fan, J.; Kamphorst, J.J.; Mathew, R.; Chung, M.K.; White, E.; Shlomi, T.; Rabinowitz, J.D. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol. Syst. Biol. 2013, 9, 712. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Moss, T.; Mangala, L.S.; Marini, J.; Zhao, H.; Wahlig, S.; Armaiz-Pena, G.; Jiang, D.; Achreja, A.; Win, J.; et al. Metabolic shifts toward glutamine regulate tumor growth, invasion and bioenergetics in ovarian cancer. Mol. Syst. Biol. 2014, 10, 728. [Google Scholar] [CrossRef]
- Van Geldermalsen, M.; Wang, Q.; Nagarajah, R.; Marshall, A.D.; Thoeng, A.; Gao, D.; Ritchie, W.; Feng, Y.; Bailey, C.G.; Deng, N.; et al. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene 2015, 35, 3201–3208. [Google Scholar] [CrossRef]
- Demas, D.M.; Demo, S.; Fallah, Y.; Clarke, R.; Nephew, K.P.; Althouse, S.; Sandusky, G.; He, W.; Shajahan-Haq, A.N. Glutamine Metabolism Drives Growth in Advanced Hormone Receptor Positive Breast Cancer. Front. Oncol. 2019, 9, 686. [Google Scholar] [CrossRef]
- Wise, D.R.; Thompson, C.B. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef]
- Gregory, M.A.; Nemkov, T.; Park, H.J.; Zaberezhnyy, V.; Gehrke, S.; Adane, B.; Jordan, C.T.; Hansen, K.C.; D’Alessandro, A.; DeGregori, J. Targeting Glutamine Metabolism and Redox State for Leukemia Therapy. Clin. Cancer Res. 2019, 25, 4079–4090. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, X.; Fan, C.; Wang, H.; Wang, R.; Feng, C.; Guan, H. Targeting glutaminase-mediated glutamine dependence in papillary thyroid cancer. Klin. Wochenschr. 2018, 96, 777–790. [Google Scholar] [CrossRef]
- Timmerman, L.A.; Holton, T.; Yuneva, M.; Louie, R.J.; Padró, M.; Daemen, A.; Hu, M.; Chan, D.A.; Ethier, S.P.; Veer, L.J.V.; et al. Glutamine Sensitivity Analysis Identifies the xCT Antiporter as a Common Triple-Negative Breast Tumor Therapeutic Target. Cancer Cell 2013, 24, 450–465. [Google Scholar] [CrossRef]
- Saito, Y.; Soga, T. Amino acid transporters as emerging therapeutic targets in cancer. Cancer Sci. 2021, 112, 2958–2965. [Google Scholar] [CrossRef]
- Abooshahab, R.; Hooshmand, K.; Razavi, F.; Dass, C.R.; Hedayati, M. A glance at the actual role of glutamine metabolism in thyroid tumorigenesis. Excli. J. 2021, 20, 1170–1183. [Google Scholar] [CrossRef]
- Mukha, A.; Kahya, U.; Dubrovska, A. Targeting glutamine metabolism and autophagy: The combination for prostate cancer radiosensitization. Autophagy 2021, 17, 3879–3881. [Google Scholar] [CrossRef]
- Katt, W.P.; Cerione, R.A. Glutaminase regulation in cancer cells: A druggable chain of events. Drug Discov. Today 2013, 19, 450–457. [Google Scholar] [CrossRef]
- Ferreira, A.P.S.; Cassago, A.; Gonçalves, K.; Dias, M.M.; Adamoski, D.; Ascenção, C.F.R.; Honorato, R.V.; Oliveira, J.; Ferreira, I.M.; Fornezari, C.; et al. Active Glutaminase C Self-assembles into a Supratetrameric Oligomer That Can Be Disrupted by an Allosteric Inhibitor. J. Biol. Chem. 2013, 288, 28009–28020. [Google Scholar] [CrossRef]
- Scalise, M.; Pochini, L.; Galluccio, M.; Indiveri, C. Glutamine transport. From energy supply to sensing and beyond. Biochim. Biophys. Acta 2016, 1857, 1147–1157. [Google Scholar] [CrossRef]
- Zhou, W.-J.; Zhang, J.; Yang, H.-L.; Wu, K.; Xie, F.; Wu, J.-N.; Wang, Y.; Yao, L.; Zhuang, Y.; Xiang, J.-D.; et al. Estrogen inhibits autophagy and promotes growth of endometrial cancer by promoting glutamine metabolism. Cell Commun. Signal. 2019, 17, 99. [Google Scholar] [CrossRef]
- Xu, L.; Yin, Y.; Li, Y.; Chen, X.; Chang, Y.; Zhang, H.; Liu, J.; Beasley, J.; McCaw, P.; Zhang, H.; et al. A glutaminase isoform switch drives therapeutic resistance and disease progression of prostate cancer. Proc. Natl. Acad. Sci. USA 2021, 118, e2012748118. [Google Scholar] [CrossRef]
- Wang, Q.; Hardie, R.A.; Hoy, A.J.; Van Geldermalsen, M.; Gao, D.; Fazli, L.; Sadowski, M.C.; Balaban, S.; Schreuder, M.; Nagarajah, R.; et al. Targeting ASCT2 -mediated glutamine uptake blocks prostate cancer growth and tumour development. J. Pathol. 2015, 236, 278–289. [Google Scholar] [CrossRef]
- Craze, M.L.; El-Ansari, R.; Aleskandarany, M.A.; Cheng, K.W.; Alfarsi, L.; Masisi, B.; Diez-Rodriguez, M.; Nolan, C.C.; Ellis, I.O.; Rakha, E.A.; et al. Glutamate dehydrogenase (GLUD1) expression in breast cancer. Breast Cancer Res. Treat. 2018, 174, 79–91. [Google Scholar] [CrossRef]
- Yang, T.; Wu, X.; Hu, J.; Hu, M.; Xu, H.; Jiang, H.; Ding, Q. Maternal High-Fat Diet Promotes the Development and Progression of Prostate Cancer in Transgenic Adenocarcinoma Mouse Prostate Offspring. Cell. Physiol. Biochem. 2018, 47, 1862–1870. [Google Scholar] [CrossRef]
- Marín-Aguilera, M.; Pereira, M.V.; Jiménez, N.; Reig, Ò.; Cuartero, A.; Victoria, I.; Aversa, C.; Ferrer-Mileo, L.; Prat, A.; Mellado, B. Glutamine and Cholesterol Plasma Levels and Clinical Outcomes of Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Taxanes. Cancers 2021, 13, 4960. [Google Scholar] [CrossRef]
- Wise, D.R.; Ward, P.S.; Shay, J.E.S.; Cross, J.R.; Gruber, J.J.; Sachdeva, U.M.; Platt, J.M.; DeMatteo, R.G.; Simon, M.C.; Thompson, C.B. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Proc. Natl. Acad. Sci. USA 2011, 108, 19611–19616. [Google Scholar] [CrossRef] [PubMed]
- Metallo, C.M.; Gameiro, P.A.; Bell, E.L.; Mattaini, K.R.; Yang, J.; Hiller, K.; Jewell, C.M.; Johnson, Z.R.; Irvine, D.J.; Guarente, L.; et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 2012, 481, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Mullen, A.R.; Wheaton, W.W.; Jin, E.S.; Chen, P.-H.; Sullivan, L.B.; Cheng, T.; Yang, Y.; Linehan, W.M.; Chandel, N.S.; DeBerardinis, R.J. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 2011, 481, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; You, D.; Zhu, X.; Cai, L.; Zeng, S.; Hu, X. Lactate and glutamine support NADPH generation in cancer cells under glucose deprived conditions. Redox Biol. 2021, 46, 102065. [Google Scholar] [CrossRef]
- Igelmann, S.; Lessard, F.; Uchenunu, O.; Bouchard, J.; Fernandez-Ruiz, A.; Rowell, M.-C.; Lopes-Paciencia, S.; Papadopoli, D.; Fouillen, A.; Ponce, K.J.; et al. A hydride transfer complex reprograms NAD metabolism and bypasses senescence. Mol. Cell 2021, 81, 3848–3865.e19. [Google Scholar] [CrossRef]
- Tao, R.; Zhao, Y.; Chu, H.; Wang, A.; Zhu, J.; Chen, X.; Zou, Y.; Shi, M.; Liu, R.; Su, N.; et al. Genetically encoded fluorescent sensors reveal dynamic regulation of NADPH metabolism. Nat. Methods 2017, 14, 720–728. [Google Scholar] [CrossRef]
- Fan, J.; Ye, J.; Kamphorst, J.J.; Shlomi, T.; Thompson, C.B.; Rabinowitz, J.D. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 2014, 510, 298–302. [Google Scholar] [CrossRef]
- Michalak, K.P.; Maćkowska-Kędziora, A.; Sobolewski, B.; Woźniak, P. Key Roles of Glutamine Pathways in Reprogramming the Cancer Metabolism. Oxidative Med. Cell. Longev. 2015, 2015, 964321. [Google Scholar] [CrossRef]
- Mukha, A.; Kahya, U.; Linge, A.; Chen, O.; Löck, S.; Lukiyanchuk, V.; Richter, S.; Alves, T.C.; Peitzsch, M.; Telychko, V.; et al. GLS-driven glutamine catabolism contributes to prostate cancer radiosensitivity by regulating the redox state, stemness and ATG5-mediated autophagy. Theranostics 2021, 11, 7844–7868. [Google Scholar] [CrossRef]
- Ogor, P.; Yoshida, T.; Koike, M.; Kakizuka, A. VCP relocalization limits mitochondrial activity, GSH depletion and ferroptosis during starvation in PC3 prostate cancer cells. Genes Cells 2021, 26, 570–582. [Google Scholar] [CrossRef]
- Schömel, N.; Hancock, S.E.; Gruber, L.; Olzomer, E.M.; Byrne, F.L.; Shah, D.; Hoehn, K.L.; Turner, N.; Grösch, S.; Geisslinger, G.; et al. UGCG influences glutamine metabolism of breast cancer cells. Sci. Rep. 2019, 9, 15665. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, Y.D.; Ganapathy, V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim. Biophys. Acta 2015, 1863, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Zhang, J.; Pavlova, N.N.; Thompson, C.B. Cancer cell metabolism: The essential role of the nonessential amino acid, glutamine. EMBO J. 2017, 36, 1302–1315. [Google Scholar] [CrossRef]
- Xu, D.; Hemler, M.E. Metabolic Activation-related CD147-CD98 Complex. Mol. Cell. Proteom. 2005, 4, 1061–1071. [Google Scholar] [CrossRef]
- Saito, Y.; Li, L.; Coyaud, E.; Luna, A.; Sander, C.; Raught, B.; Asara, J.M.; Brown, M.; Muthuswamy, S.K. LLGL2 rescues nutrient stress by promoting leucine uptake in ER+ breast cancer. Nature 2019, 569, 275–279. [Google Scholar] [CrossRef]
- Gruvberger, S.; Ringnér, M.; Chen, Y.; Panavally, S.; Saal, L.; A, B.; Fernö, M.; Peterson, C.; Meltzer, P.S. Estrogen receptor status in breast cancer is associated with remarkably distinct gene expression patterns. Cancer Res. 2001, 61, 5979–5984. [Google Scholar]
- Haghighat, N. Estrogen (17β-Estradiol) Enhances Glutamine Synthetase Activity in C6-Glioma Cells. Neurochem. Res. 2005, 30, 661–667. [Google Scholar] [CrossRef]
- Hertz, L. Functional interactions between neurons and astrocytes I. Turnover and metabolism of putative amino acid transmitters. Prog. Neurobiol. 1979, 13, 277–323. [Google Scholar] [CrossRef]
- Zelewski, B.B.J.P. Mitochondrial glutamine and glutamate metabolism in human placenta and its possible link with progesterone biosynthesis. Placenta 1993, 14, 77–86. [Google Scholar]
- Zhang, P.; Cao, L.; Fan, P.; Mei, Y.; Wu, M. Lnc RNA - MIF, a c-Myc-activated long non-coding RNA, suppresses glycolysis by promoting Fbxw7-mediated c-Myc degradation. EMBO Rep. 2016, 17, 1204–1220. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Tchernyshyov, I.; Chang, T.-C.; Lee, Y.-S.; Kita, K.; Ochi, T.; Zeller, K.I.; De Marzo, A.M.; Van Eyk, J.E.; Mendell, J.T.; et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 2009, 458, 762–765. [Google Scholar] [CrossRef]
- Ganguly, K.; Bhatia, R.; Rauth, S.; Kisling, A.; Atri, P.; Thompson, C.; Vengoji, R.; Krishn, S.R.; Shinde, D.; Thomas, V.; et al. Mucin 5AC Serves as the Nexus for β-Catenin/c-Myc Interplay to Promote Glutamine Dependency During Pancreatic Cancer Chemoresistance. Gastroenterology 2022, 162, 253–268.e13. [Google Scholar] [CrossRef]
- Mungenast, F.; Thalhammer, T. Estrogen Biosynthesis and Action in Ovarian Cancer. Front. Endocrinol. 2014, 5, 192. [Google Scholar] [CrossRef]
- Bossard, C.; Busson, M.; Vindrieux, D.; Gaudin, F.; Machelon, V.; Brigitte, M.; Jacquard, C.; Pillon, A.; Balaguer, P.; Balabanian, K.; et al. Potential Role of Estrogen Receptor Beta as a Tumor Suppressor of Epithelial Ovarian Cancer. PLoS ONE 2012, 7, e44787. [Google Scholar] [CrossRef]
- Cavalieri, E.L.; Rogan, E.G. Unbalanced metabolism of endogenous estrogens in the etiology and prevention of human cancer. J. Steroid Biochem. Mol. Biol. 2011, 125, 169–180. [Google Scholar] [CrossRef]
- Yager, J.D. Mechanisms of estrogen carcinogenesis: The role of E2/E1–quinone metabolites suggests new approaches to preventive intervention—A review. Steroids 2014, 99, 56–60. [Google Scholar] [CrossRef]
- Budczies, J.; Brockmöller, S.F.; Müller, B.M.; Barupal, D.K.; Richter-Ehrenstein, C.; Kleine-Tebbe, A.; Griffin, J.L.; Orešič, M.; Dietel, M.; Denkert, C.; et al. Comparative metabolomics of estrogen receptor positive and estrogen receptor negative breast cancer: Alterations in glutamine and beta-alanine metabolism. J. Proteom. 2013, 94, 279–288. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Warden, C.; Chen, S. Cross-talk between ER and HER2 regulates c-MYC-mediated glutamine metabolism in aromatase inhibitor resistant breast cancer cells. J. Steroid Biochem. Mol. Biol. 2015, 149, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Vernier, M.; Dufour, C.R.; McGuirk, S.; Scholtes, C.; Li, X.; Bourmeau, G.; Kuasne, H.; Park, M.; St-Pierre, J.; Audet-Walsh, E.; et al. Estrogen-related receptors are targetable ROS sensors. Genes Dev. 2020, 34, 544–559. [Google Scholar] [CrossRef] [PubMed]
- Yudkoff, M.; Nissim, I.; Hertz, L.; Pleasure, D.; Erecinska, M. Chapter 18: Nitrogen metabolism: Neuronal-astroglial relationships. Prog. Brain Res. 1992, 94, 213–224. [Google Scholar] [CrossRef]
- Sánchez-Vallejo, V.; Benlloch-Navarro, S.; López-Pedrajas, R.; Romero, F.; Miranda, M. Neuroprotective actions of progesterone in an in vivo model of retinitis pigmentosa. Pharmacol. Res. 2015, 99, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, D.; Simonides, W.S.; Dentice, M.; Zavacki, A.M.; Larsen, P.R. Thyroid hormones and skeletal muscle—new insights and potential implications. Nat. Rev. Endocrinol. 2013, 10, 206–214. [Google Scholar] [CrossRef]
- Baas, D.; Fressinaud, C.; Vitkovic, L.; Sarlieve, L.L. Glutamine synthetase expression and activity are regulated by 3,5,3′-TRIODO-l-THYRONINE and hydrocortisone in rat oligodendrocyte cultures. Int. J. Dev. Neurosci. 1998, 16, 333–340. [Google Scholar] [CrossRef]
- Cicatiello, A.G.; Sagliocchi, S.; Nappi, A.; Di Cicco, E.; Miro, C.; Murolo, M.; Stornaiuolo, M.; Dentice, M. Thyroid hormone regulates glutamine metabolism and anaplerotic fluxes by inducing mitochondrial glutamate aminotransferase GPT. Cell Rep. 2022, 38, 110562. [Google Scholar] [CrossRef]
- Aizawa, S.; Sakai, T.; Sakata, I. Glutamine and glutamic acid enhance thyroid-stimulating hormone β subunit mRNA expression in the rat pars tuberalis. J. Endocrinol. 2012, 212, 383–394. [Google Scholar] [CrossRef]
- Garber, A.J. Effects of Parathyroid Hormone on Skeletal Muscle Protein and Amino Acid Metabolism in the Rat. J. Clin. Investig. 1983, 71, 1806–1821. [Google Scholar] [CrossRef]
- Jain, S.; Chakraborty, G.; Raja, R.; Kale, S.; Kundu, G.C. Prostaglandin E2 Regulates Tumor Angiogenesis in Prostate Cancer. Cancer Res. 2008, 68, 7750–7759. [Google Scholar] [CrossRef]
- Kokontis, J.; Takakura, K.; Hay, N.; Liao, S. Increased androgen receptor activity and altered c-myc expression in prostate cancer cells after long-term androgen deprivation. Cancer Res. 1994, 54, 1566–1573. [Google Scholar] [PubMed]
- Wise, D.R.; DeBerardinis, R.J.; Mancuso, A.; Sayed, N.; Zhang, X.-Y.; Pfeiffer, H.K.; Nissim, I.; Daikhin, E.; Yudkoff, M.; McMahon, S.B.; et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA 2008, 105, 18782–18787. [Google Scholar] [CrossRef] [PubMed]
- Barfeld, S.J.; Itkonen, H.; Urbanucci, A.; Mills, I.G. Androgen-regulated metabolism and biosynthesis in prostate cancer. Endocr. Relat. Cancer 2014, 21, T57–T66. [Google Scholar] [CrossRef]
- Gao, A.; Su, J.; Liu, R.; Zhao, S.; Li, W.; Xu, X.; Li, D.; Shi, J.; Bin Gu, B.; Zhang, J.; et al. Sexual dimorphism in glucose metabolism is shaped by androgen-driven gut microbiome. Nat. Commun. 2021, 12, 7080. [Google Scholar] [CrossRef]
- Bezzi, P.; Carmignoto, P.; Pasti, L.; Vesce, S.; Rossi, D.M.; Rizzini, B.L.; Pozzan, T.; Volterra, A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 1998, 391, 281–285. [Google Scholar] [CrossRef]
- Finetti, F.; Travelli, C.; Ercoli, J.; Colombo, G.; Buoso, E.; Trabalzini, L. Prostaglandin E2 and Cancer: Insight into Tumor Progression and Immunity. Biology 2020, 9, 434. [Google Scholar] [CrossRef]
- Tardy, M.; Fages, C.; Rolland, B.; Bardakdjian, J.; Gonnard, P. Effect of prostaglandins and dibutyryl cyclic AMP on the morphology of cells in primary astroglial cultures and on metabolic enzymes of GABA and glutamate metabolism. Experientia 1981, 37, 19–21. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.C.; Hopkins, I.B.; Lindauer, S.L.; Bamford, P. Aspartate aminotransferase in synaptic and nonsynaptic mitochondria: Differential effect of compounds that influence transient hetero-enzyme complex (metabolon) formation. Neurochem. Int. 2006, 48, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Stegen, S.; Devignes, C.; Torrekens, S.; Van Looveren, R.; Carmeliet, P.; Carmeliet, G. Glutamine Metabolism in Osteoprogenitors Is Required for Bone Mass Accrual and PTH -Induced Bone Anabolism in Male Mice. J. Bone Miner. Res. 2020, 36, 604–616. [Google Scholar] [CrossRef]
- Hackney, J.F.; Holbrook, N.J.; Grasso, R.J. Progesterone as a partial glucocorticoid agonist in L929 mouse fibroblasts: Effects on cell growth, glutamine synthetase induction and glucocorticoid receptors. J. Steroid Biochem. 1981, 14, 971–977. [Google Scholar] [CrossRef]
- Zlotnik, A.; Gruenbaum, B.F.; Mohar, B.; Kuts, R.; Gruenbaum, S.E.; Ohayon, S.; Boyko, M.; Klin, Y.; Sheiner, E.; Shaked, G.; et al. The Effects of Estrogen and Progesterone on Blood Glutamate Levels: Evidence from Changes of Blood Glutamate Levels During the Menstrual Cycle in Women. Biol. Reprod. 2011, 84, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.V.; Matthews, S.B.; Fettig, L.M.; Riley, D.; Finlay-Schultz, J.; Paul, K.V.; Jackman, M.; Kabos, P.; MacLean, P.S.; Sartorius, C.A. Estrogens and Progestins Cooperatively Shift Breast Cancer Cell Metabolism. Cancers 2022, 14, 1776. [Google Scholar] [CrossRef] [PubMed]
- Masisi, B.K.; El Ansari, R.; Alfarsi, L.; Craze, M.L.; Jewa, N.; Oldfield, A.; Cheung, H.; Toss, M.; Rakha, E.A.; Green, A.R. The Biological and Clinical Significance of Glutaminase in Luminal Breast Cancer. Cancers 2021, 13, 3963. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.-H.E.; Tredwell, G.; Ellis, J.K.; Lam, E.; Keun, H.C. Metabolomic characterisation of the effects of oncogenic PIK3CA transformation in a breast epithelial cell line. Sci. Rep. 2017, 7, srep46079. [Google Scholar] [CrossRef] [PubMed]
- Saraon, P.; Drabovich, A.P.; Jarvi, K.A.; Diamandis, E.P. Mechanisms of Androgen-Independent Prostate Cancer. EJIFCC 2014, 25, 42–54. [Google Scholar]
- White, M.A.; Lin, C.; Rajapakshe, K.; Dong, J.; Shi, Y.; Tsouko, E.; Mukhopadhyay, R.; Jasso, D.; Dawood, W.; Coarfa, C.; et al. Glutamine Transporters Are Targets of Multiple Oncogenic Signaling Pathways in Prostate Cancer. Mol. Cancer Res. 2017, 15, 1017–1028. [Google Scholar] [CrossRef]
- Waagepetersen, H.; Schousboe, A. Glial GABA and Glutamate Metabolism. Adv. Neurobiol. 2015, 11, 13–30. [Google Scholar] [CrossRef]
- Garber, A.J.; E Karl, I.; Kipnis, D.M. Alanine and glutamine synthesis and release from skeletal muscle. I. Glycolysis and amino acid release. J. Biol. Chem. 1976, 251, 826–835. [Google Scholar] [CrossRef]
- Bhat, M.K.; McPhie, P.; Cheng, S.-Y. Critical Role of Glutamine 252 in the Hormone-Dependent Transcriptional Activity of the Thyroid Hormone β1 Nuclear Receptor. Biochemistry 1997, 36, 4233–4239. [Google Scholar] [CrossRef]
- Ou, C.-Y.; He, Y.-H.; Sun, Y.; Yang, L.; Shi, W.-X.; Li, S.-J. Effects of Sub-Acute Manganese Exposure on Thyroid Hormone and Glutamine (Gln)/Glutamate (Glu)-γ- Aminobutyric Acid (GABA) Cycle in Serum of Rats. Int. J. Environ. Res. Public Health 2019, 16, 2157. [Google Scholar] [CrossRef]
- Domingues, J.T.; Cattani, D.; Cesconetto, P.A.; de Almeida, B.A.N.; Pierozan, P.; dos Santos, K.; Razzera, G.; Silva, F.R.M.B.; Pessoa-Pureur, R.; Zamoner, A. Reverse T3 interacts with αvβ3 integrin receptor and restores enzyme activities in the hippocampus of hypothyroid developing rats: Insight on signaling mechanisms. Mol. Cell. Endocrinol. 2018, 470, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Parry-Billings, M.; Dimitriadis, G.D.; Leighton, B.; Bond, J.; Bevan, S.J.; Opara, E.; A Newsholme, E. Effects of hyperthyroidism and hypothyroidism on glutamine metabolism by skeletal muscle of the rat. Biochem. J. 1990, 272, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Jhu, J.-W.; Yan, J.-B.; Lin, Z.-H.; Lin, S.-C.; Peng, I.-C. SREBP1-Induced Glutamine Synthetase Triggers a Feedforward Loop to Upregulate SREBP1 through Sp1 O-GlcNAcylation and Augments Lipid Droplet Formation in Cancer Cells. Int. J. Mol. Sci. 2021, 22, 9814. [Google Scholar] [CrossRef]
- Biello, F.; Platini, F.; D’Avanzo, F.; Cattrini, C.; Mennitto, A.; Genestroni, S.; Martini, V.; Marzullo, P.; Aimaretti, G.; Gennari, A. Insulin/IGF Axis in Breast Cancer: Clinical Evidence and Translational Insights. Biomolecules 2021, 11, 125. [Google Scholar] [CrossRef]
- Silva, C.; Andrade, N.; Rodrigues, I.; Ferreira, A.C.; Soares, M.L.; Martel, F. The pro-proliferative effect of interferon-γ in breast cancer cell lines is dependent on stimulation of ASCT2-mediated glutamine cellular uptake. Life Sci. 2021, 286, 120054. [Google Scholar] [CrossRef]
- Fendt, S.-M.; Bell, E.L.; Keibler, M.A.; Davidson, S.M.; Wirth, G.J.; Fiske, B.; Mayers, J.R.; Schwab, M.; Bellinger, G.; Csibi, A.; et al. Metformin Decreases Glucose Oxidation and Increases the Dependency of Prostate Cancer Cells on Reductive Glutamine Metabolism. Cancer Res. 2013, 73, 4429–4438. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cui, H. Targeting Glutamine Induces Apoptosis: A Cancer Therapy Approach. Int. J. Mol. Sci. 2015, 16, 22830–22855. [Google Scholar] [CrossRef] [PubMed]
- Hensley, C.T.; Wasti, A.T.; DeBerardinis, R.J. Glutamine and cancer: Cell biology, physiology, and clinical opportunities. J. Clin. Investig. 2013, 123, 3678. [Google Scholar] [CrossRef]
- Jin, L.; Alesi, G.N.; Kang, S. Glutaminolysis as a target for cancer therapy. Oncogene 2015, 35, 3619–3625. [Google Scholar] [CrossRef]
- Lukey, M.J.; Wilson, K.F.; A Cerione, R. Therapeutic strategies impacting cancer cell glutamine metabolism. Futur. Med. Chem. 2013, 5, 1685–1700. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-H.; Lin, Z.; Flomenberg, N.; Pestell, R.G.; Howell, A.; Sotgia, F.; Lisanti, M.P.; Martinez-Outschoorn, U.E. Glutamine fuels a vicious cycle of autophagy in the tumor stroma and oxidative mitochondrial metabolism in epithelial cancer cells. Cancer Biol. Ther. 2011, 12, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Holst, J. L-type amino acid transport and cancer: Targeting the mTORC1 pathway to inhibit neoplasia. Am. J. Cancer Res. 2015, 5, 1281–1294. [Google Scholar] [PubMed]
- Yuan, L.; Sheng, X.; Willson, A.K.; Roque, D.R.; E Stine, J.; Guo, H.; Jones, H.M.; Zhou, C.; Bae-Jump, V.L. Glutamine promotes ovarian cancer cell proliferation through the mTOR/S6 pathway. Endocr. Relat. Cancer 2015, 22, 577–591. [Google Scholar] [CrossRef]
- Stine, J.E.; Guo, H.; Sheng, X.; Han, X.; Schointuch, M.N.; Gilliam, T.P.; Gehrig, P.A.; Zhou, C.; Bae-Jump, V.L. The HMG-CoA reductase inhibitor, simvastatin, exhibits anti-metastatic and anti-tumorigenic effects in ovarian cancer. Oncotarget 2015, 7, 946–960. [Google Scholar] [CrossRef]
- Fu, Y.-M.; Zhang, H.; Ding, M.; Li, Y.-Q.; Fu, X.; Yu, Z.-X.; Meadows, G.G. Selective amino acid restriction targets mitochondria to induce apoptosis of androgen-independent prostate cancer cells. J. Cell. Physiol. 2006, 209, 522–534. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Y.-M.; Meadows, G.G. Differential effects of specific amino acid restriction on glucose metabolism, reduction/oxidation status and mitochondrial damage in DU145 and PC3 prostate cancer cells. Oncol. Lett. 2011, 2, 349–355. [Google Scholar] [CrossRef]
- Wu, W.-C.; Sun, H.-W.; Chen, J.; OuYang, H.-Y.; Yu, X.-J.; Chen, H.-T.; Shuang, Z.-Y.; Shi, M.; Wang, Z.; Zheng, L. Immunosuppressive Immature Myeloid Cell Generation Is Controlled by Glutamine Metabolism in Human Cancer. Cancer Immunol. Res. 2019, 7, 1605–1618. [Google Scholar] [CrossRef]
- Edwards, D.N.; Ngwa, V.M.; Raybuck, A.L.; Wang, S.; Hwang, Y.; Kim, L.C.; Cho, S.H.; Paik, Y.; Wang, Q.; Zhang, S.; et al. Selective glutamine metabolism inhibition in tumor cells improves antitumor T lymphocyte activity in triple-negative breast cancer. J. Clin. Investig. 2021, 131, 140100. [Google Scholar] [CrossRef]
- Marra, A.; Viale, G.; Curigliano, G. Recent advances in triple negative breast cancer: The immunotherapy era. BMC Med. 2019, 17, 90. [Google Scholar] [CrossRef]
- Menga, A.; Favia, M.; Spera, I.; Vegliante, M.C.; Gissi, R.; De Grassi, A.; Laera, L.; Campanella, A.; Gerbino, A.; Carrà, G.; et al. N-acetylaspartate release by glutaminolytic ovarian cancer cells sustains protumoral macrophages. EMBO Rep. 2021, 22, e51981. [Google Scholar] [CrossRef] [PubMed]
- Udumula, M.P.; Sakr, S.; Dar, S.; Alvero, A.B.; Ali-Fehmi, R.; Abdulfatah, E.; Li, J.; Jiang, J.; Tang, A.; Buekers, T.; et al. Ovarian cancer modulates the immunosuppressive function of CD11b+Gr1+ myeloid cells via glutamine metabolism. Mol. Metab. 2021, 53, 101272. [Google Scholar] [CrossRef] [PubMed]
- Arena, F. Clinical implications of recent studies using mTOR inhibitors to treat advanced hormone receptor-positive breast cancer. Cancer Manag. Res. 2014, 6, 389–395. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, S.; Kim, D.H.; Jung, W.-H.; Koo, J.S. Expression of glutamine metabolism-related proteins according to molecular subtype of breast cancer. Endocr. Relat. Cancer 2013, 20, 339–348. [Google Scholar] [CrossRef]
- Kim, J.Y.; Heo, S.-H.; Choi, S.K.; Song, I.H.; Park, I.A.; Kim, Y.-A.; Park, H.S.; Park, S.Y.; Bang, W.S.; Gong, G.; et al. Glutaminase expression is a poor prognostic factor in node-positive triple-negative breast cancer patients with a high level of tumor-infiltrating lymphocytes. Virchows Arch. 2017, 470, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Nicklin, P.; Bergman, P.; Zhang, B.; Triantafellow, E.; Wang, H.; Nyfeler, B.; Yang, H.; Hild, M.; Kung, C.; Wilson, C.; et al. Bidirectional Transport of Amino Acids Regulates mTOR and Autophagy. Cell 2009, 136, 521–534. [Google Scholar] [CrossRef]
- Kung, H.-N.; Marks, J.R.; Chi, J.-T. Glutamine Synthetase Is a Genetic Determinant of Cell Type–Specific Glutamine Independence in Breast Epithelia. PLoS Genet. 2011, 7, e1002229. [Google Scholar] [CrossRef]
- Mauro-Lizcano, M.; López-Rivas, A. Glutamine metabolism regulates FLIP expression and sensitivity to TRAIL in triple-negative breast cancer cells. Cell Death Dis. 2018, 9, 205. [Google Scholar] [CrossRef]
- Oh, M.-H.; Sun, I.-H.; Zhao, L.; Leone, R.D.; Sun, I.M.; Xu, W.; Collins, S.L.; Tam, A.J.; Blosser, R.L.; Patel, C.H.; et al. Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J. Clin. Investig. 2020, 130, 3865–3884. [Google Scholar] [CrossRef]
- Dias, M.M.; Adamoski, D.; dos Reis, L.M.; Ascenção, C.F.R.; De Oliveira, K.R.S.; Mafra, A.C.; Bastos, A.C.D.S.; Quintero, M.; Cassago, C.D.G.; Ferreira, I.M.; et al. GLS2 is protumorigenic in breast cancers. Oncogene 2019, 39, 690–702. [Google Scholar] [CrossRef]
- Dragan, M.; Nguyen, M.-U.; Guzman, S.; Goertzen, C.; Brackstone, M.; Dhillo, W.S.; Bech, P.R.; Clarke, S.; Abbara, A.; Tuck, A.B.; et al. G protein-coupled kisspeptin receptor induces metabolic reprograming and tumorigenesis in estrogen receptor-negative breast cancer. Cell Death Dis. 2020, 11, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.D.; Van Geldermalsen, M.; Otte, N.J.; Lum, T.; Vellozzi, M.; Thoeng, A.; Pang, A.; Nagarajah, R.; Zhang, B.K.; Wang, Q. (.; et al. ASCT2 regulates glutamine uptake and cell growth in endometrial carcinoma. Oncogenesis 2017, 6, e367. [Google Scholar] [CrossRef] [PubMed]
- Veliça, P.; Davies, N.J.; Rocha, P.P.; Schrewe, H.; Ride, J.P.; Bunce, C.M. Lack of functional and expression homology between human and mouse aldo-keto reductase 1C enzymes: Implications for modelling human cancers. Mol. Cancer 2009, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Kossaï, M.; Leary, A.; Scoazec, J.-Y.; Genestie, C. Ovarian Cancer: A Heterogeneous Disease. Pathobiology 2017, 85, 41–49. [Google Scholar] [CrossRef]
- Al-Alem, L.F.; Pandya, U.M.; Baker, A.T.; Bellio, C.; Zarrella, B.D.; Clark, J.; DiGloria, C.M.; Rueda, B.R. Ovarian cancer stem cells: What progress have we made? Int. J. Biochem. Cell Biol. 2018, 107, 92–103. [Google Scholar] [CrossRef]
- Yang, L.; Venneti, S.; Nagrath, D. Glutaminolysis: A Hallmark of Cancer Metabolism. Annu. Rev. Biomed. Eng. 2017, 19, 163. [Google Scholar] [CrossRef]
- Burke, W.M.; Jin, X.; Lin, H.-J.; Huang, M.; Liu, R.; Reynolds, R.K.; Lin, J. Inhibition of constitutively active Stat3 suppresses growth of human ovarian and breast cancer cells. Oncogene 2001, 20, 7925–7934. [Google Scholar] [CrossRef]
- Prasad, P.; Roy, S.S. Glutamine regulates ovarian cancer cell migration and invasion through ETS. Heliyon 2021, 7, e07064. [Google Scholar] [CrossRef]
- Duan, Z.; Foster, R.; Bell, D.A.; Mahoney, J.; Wolak, K.; Vaidya, A.; Hampel, C.; Lee, H.; Seiden, M.V. Signal Transducers and Activators of Transcription 3 Pathway Activation in Drug-Resistant Ovarian Cancer. Clin. Cancer Res. 2006, 12, 5055–5063. [Google Scholar] [CrossRef]
- Gest, C.; Mirshahi, P.; Li, H.; Pritchard, L.-L.; Joimel, U.; Blot, E.; Chidiac, J.; Poletto, B.; Vannier, J.-P.; Varin, R.; et al. Ovarian cancer: Stat3, RhoA and IGF-IR as therapeutic targets. Cancer Lett. 2012, 317, 207–217. [Google Scholar] [CrossRef]
- Turkson, J.; Jove, R. STAT proteins: Novel molecular targets for cancer drug discovery. Oncogene 2000, 19, 6613–6626. [Google Scholar] [CrossRef] [PubMed]
- Schindler, C.; Levy, D.E.; Decker, T. JAK-STAT Signaling: From Interferons to Cytokines. J. Biol. Chem. 2007, 282, 20059–20063. [Google Scholar] [CrossRef]
- Coloff, J.L.; Murphy, J.P.; Braun, C.R.; Harris, I.S.; Shelton, L.M.; Kami, K.; Gygi, S.P.; Selfors, L.M.; Brugge, J.S. Differential Glutamate Metabolism in Proliferating and Quiescent Mammary Epithelial Cells. Cell Metab. 2016, 23, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.D.; van Geldermalsen, M.; Otte, N.J.; Anderson, L.A.; Lum, T.; Vellozzi, M.A.; Zhang, B.K.; Thoeng, A.; Wang, Q.; Rasko, J.E.; et al. LAT1 is a putative therapeutic target in endometrioid endometrial carcinoma. Int. J. Cancer 2016, 139, 2529–2539. [Google Scholar] [CrossRef] [PubMed]
- Holinka, C.F.; Anzai, Y.; Hata, H.; Kimmel, N.; Kuramoto, H.; Gurpide, E. Proliferation and responsiveness to estrogen of human endometrial cancer cells under serum-free culture conditions. Cancer Res. 1989, 49, 3297–3301. [Google Scholar] [PubMed]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT. Nat. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- Kataoka, K.; Kim, D.J.; Carbajal, S.; Clifford, J.L.; DiGiovanni, J. Stage-specific disruption of Stat3 demonstrates a direct requirement during both the initiation and promotion stages of mouse skin tumorigenesis. Carcinogenesis 2008, 29, 1108–1114. [Google Scholar] [CrossRef]
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Gomes, A.J.P.D.S.; Given, R.; Soto, A.J.; Merseburger, A.S.; Özgüroglu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Burke, H.B.; Djakiew, D.; Euling, S.; Ho, S.-M.; Landolph, J.; Morrison, H.; Sonawane, B.; Shifflett, T.; Waters, D.J.; et al. Human prostate cancer risk factors. Cancer 2004, 101, 2371–2490. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.; Figtree, G.; Costa, D.; Eade, T.; Hruby, G.; Lim, S.; Elfiky, A.; Martine, N.; Rosenthal, D.; Clarke, S.; et al. Oxidative stress in prostate cancer patients: A systematic review of case control studies. Prostate Int. 2016, 4, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Schöpf, B.; Weissensteiner, H.; Schäfer, G.; Fazzini, F.; Charoentong, P.; Naschberger, A.; Rupp, B.; Fendt, L.; Bukur, V.; Giese, I.; et al. OXPHOS remodeling in high-grade prostate cancer involves mtDNA mutations and increased succinate oxidation. Nat. Commun. 2020, 11, 1487. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Lee, Y.K.; Koo, J.S. Expression of glutamine metabolism-related proteins in thyroid cancer. Oncotarget 2016, 7, 53628–53641. [Google Scholar] [CrossRef]
- Cook, K.L.; Shajahan, A.N.; Clarke, R. Autophagy and endocrine resistance in breast cancer. Expert Rev. Anticancer Ther. 2011, 11, 1283–1294. [Google Scholar] [CrossRef]
- Cardoso, H.J.; Figueira, M.I.; Vaz, C.V.; Carvalho, T.M.A.; Brás, L.A.; Madureira, P.A.; Oliveira, P.J.; Sardão, V.A.; Socorro, S. Glutaminolysis is a metabolic route essential for survival and growth of prostate cancer cells and a target of 5α-dihydrotestosterone regulation. Cell. Oncol. 2021, 44, 385–403. [Google Scholar] [CrossRef]
- Van Geldermalsen, M.; Quek, L.-E.; Turner, N.; Freidman, N.; Pang, A.; Guan, Y.F.; Krycer, J.R.; Ryan, R.; Wang, Q.; Holst, J. Benzylserine inhibits breast cancer cell growth by disrupting intracellular amino acid homeostasis and triggering amino acid response pathways. BMC Cancer 2018, 18, 689. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).