The Neuroprotective Effects of Exosomes Derived from TSG101-Overexpressing Human Neural Stem Cells in a Stroke Model
Abstract
:1. Introduction
2. Results
2.1. Establishment of F3.TSG Cells
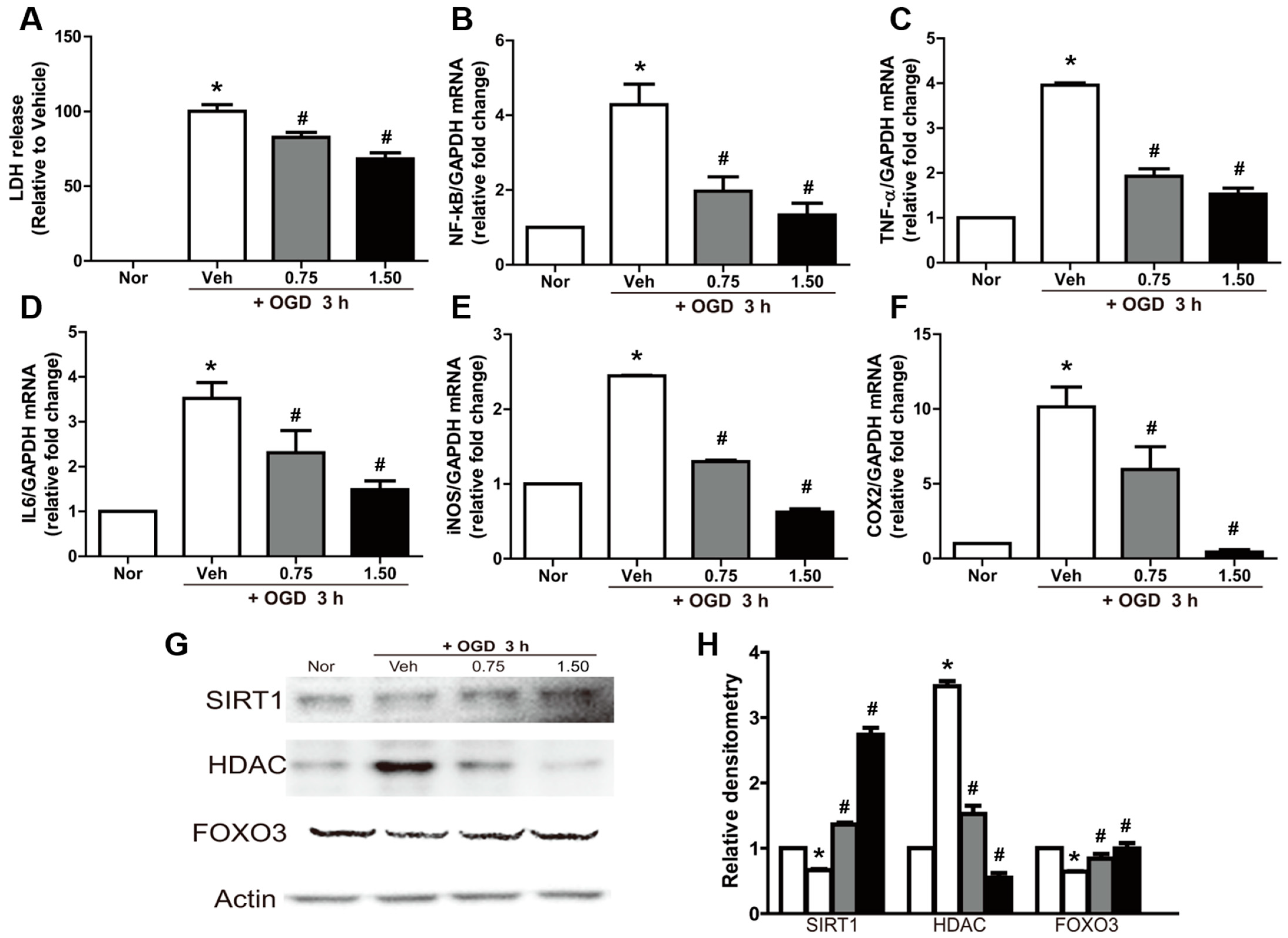
2.2. Neuroprotective and Anti-Inflammatory Effects of Exosomes in OGD-Exposed N2A Cells
2.3. Reversal of OGD-Induced Effects by Exosomes in N2A Cells
2.4. Neuroprotective Effects of Exosomes in MCAO Animals
3. Discussion
4. Materials and Methods
4.1. TSG101 Plasmid Construction
4.2. Establishment of the F3.TSG Cell Line
4.3. Cell Culture
4.4. Exosome Isolation
4.5. Nanoparticle Tracking Analysis
4.6. Transcriptome Sequencing Analysis
4.7. Oxygen–Glucose Deprivation (OGD) Culture of N2A Cells and Treatment with Exosomes
4.8. Lactate Dehydrogenase (LDH) Assay in N2A Cells
4.9. Animals
4.10. Treatment of the Middle Cerebral Artery Occlusion Model (MCAO) with Exosomes and Cells
4.11. 2,3,5-Triphenyltetrazolium Chloride (TTC) Staining
4.12. Quantitative Real-Time PCR Analysis of N2A Cells and Brain Tissues
4.13. Western Blot Analysis of N2A Cells and Brain Tissues
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bilic, I.; Dzamonja, G.; Lusic, I.; Matijaca, M.; Caljkusic, K. Risk factors and outcome differences between ischemic and hemorrhagic stroke. Acta Clin. Croat. 2009, 48, 399–403. [Google Scholar] [PubMed]
- Durukan, A.; Tatlisumak, T. Acute ischemic stroke: Overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol. Biochem. Behav. 2007, 87, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Teoh, H.L.; Wong, L.Y.; Su, J.; Ong, B.K.; Chan, B.P. Recanalization therapies in acute ischemic stroke: Pharmacological agents, devices, and combinations. Stroke Res. Treat. 2010, 2010, 672064. [Google Scholar] [CrossRef] [Green Version]
- Chapman, S.N.; Mehndiratta, P.; Johansen, M.C.; McMurry, T.L.; Johnston, K.C.; Southerland, A.M. Current perspectives on the use of intravenous recombinant tissue plasminogen activator (tPA) for treatment of acute ischemic stroke. Vasc. Health Risk Manag. 2014, 10, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.; Albers, G.W. Advanced imaging to extend the therapeutic time window of acute ischemic stroke. Ann. Neurol. 2013, 73, 4–9. [Google Scholar] [CrossRef]
- Hacke, W.; Schwab, S.; Horn, M.; Spranger, M.; De Georgia, M.; von Kummer, R. ‘Malignant’ middle cerebral artery territory infarction: Clinical course and prognostic signs. Arch. Neurol. 1996, 53, 309–315. [Google Scholar] [CrossRef]
- Kim, J.; Shin, K.; Cha, Y.; Ban, Y.H.; Park, S.K.; Jeong, H.S.; Park, D.; Choi, E.K.; Kim, Y.B. Neuroprotective effects of human neural stem cells over-expressing choline acetyltransferase in a middle cerebral artery occlusion model. J. Chem. Neuroanat. 2020, 103, 101730. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Joo, S.S.; Lee, H.J.; Choi, K.C.; Kim, S.U.; Kim, Y.B. Microtubule-associated protein 2, an early blood marker of ischemic brain injury. J. Neurosci. Res. 2012, 90, 461–467. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhang, Y.L.; Wu, Y.C. The Role of Sirt1 in Ischemic Stroke: Pathogenesis and Therapeutic Strategies. Front. Neurosci. 2018, 12, 833. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Patil, M.; Henderson, J.; Luong, H.; Annamalai, D.; Sreejit, G.; Krishnamurthy, P. The Art of Intercellular Wireless Communications: Exosomes in Heart Disease and Therapy. Front. Cell Dev. Biol. 2019, 7, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, J.S.; Lim, J.Y.; Yoon, D.W.; Pyo, S.; Kim, J. Exosome and Melatonin Additively Attenuates Inflammation by Transferring miR-34a, miR-124, and miR-135b. BioMed Res. Int. 2020, 2020, 1621394. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.J.; Song, M.; Huang, Q.H.; Guan, H.; Liu, X.D.; Xie, D.F.; Huang, B.; Huang, R.X.; Zhou, P.K. Exosome-packaged miR-1246 contributes to bystander DNA damage by targeting LIG4. Br. J. Cancer 2018, 119, 492–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, G.H.; Wu, J.; Mou, F.F.; Xie, W.H.; Wang, F.B.; Wang, Q.L.; Fang, J.; Xu, Y.W.; Dong, Y.R.; Liu, J.R.; et al. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018, 32, 654–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Yun, H.W.; Park, D.Y.; Choi, B.H.; Min, B.H. Three-Dimensional Spheroid Culture Increases Exosome Secretion from Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2018, 15, 427–436. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, X.; Yao, Q.; Liu, Y.; Zhang, H.; Dong, Z. HIF-1-mediated production of exosomes during hypoxia is protective in renal tubular cells. Am. J. Physiol.-Ren. Physiol. 2017, 313, F906–F913. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef]
- Colombo, M.; Moita, C.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Thery, C.; Raposo, G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, D.; Kirkbride, K.C.; Costello, K.; Clark, E.S.; Sinha, S.; Grega-Larson, N.; Tyska, M.J.; Weaver, A.M. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013, 5, 1159–1168. [Google Scholar] [CrossRef] [Green Version]
- Giordano, C.; Gelsomino, L.; Barone, I.; Panza, S.; Augimeri, G.; Bonofiglio, D.; Rovito, D.; Naimo, G.D.; Leggio, A.; Catalano, S.; et al. Leptin Modulates Exosome Biogenesis in Breast Cancer Cells: An Additional Mechanism in Cell-to-Cell Communication. J. Clin. Med. 2019, 8, 1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.; Choi, E.K.; Cho, T.H.; Joo, S.S.; Kim, Y.B. Human Neural Stem Cells Encoding ChAT Gene Restore Cognitive Function via Acetylcholine Synthesis, Abeta Elimination, and Neuroregeneration in APPswe/PS1dE9 Mice. Int. J. Mol. Sci. 2020, 21, 3958. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Park, D.; Ban, Y.H.; Cha, Y.; An, E.S.; Choi, J.; Choi, E.K.; Kim, Y.B. Improvement by Human Oligodendrocyte Progenitor Cells of Neurobehavioral Disorders in an Experimental Model of Neonatal Periventricular Leukomalacia. Cell Transplant. 2018, 27, 1168–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Yoon, E.J.; Choi, Y.; Park, D. Improvement of Cognitive Function in Ovariectomized Rats by Human Neural Stem Cells Overexpressing Choline Acetyltransferase via Secretion of NGF and BDNF. Int. J. Mol. Sci. 2022, 23, 5560. [Google Scholar] [CrossRef]
- Saksena, S.; Sun, J.; Chu, T.; Emr, S.D. ESCRTing proteins in the endocytic pathway. Trends Biochem. Sci. 2007, 32, 561–573. [Google Scholar] [CrossRef]
- Strickland, M.; Nyenhuis, D.; Watanabe, S.M.; Tjandra, N.; Carter, C.A. Novel Tsg101 Binding Partners Regulate Viral L Domain Trafficking. Viruses 2021, 13, 1147. [Google Scholar] [CrossRef]
- McDonald, B.; Martin-Serrano, J. Regulation of Tsg101 expression by the steadiness box: A role of Tsg101-associated ligase. Mol. Biol. Cell 2008, 19, 754–763. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, O.; Teis, D. The ESCRT machinery. Curr. Biol. 2012, 22, R116–R120. [Google Scholar] [CrossRef] [Green Version]
- Khodayari, N.; Oshins, R.; Alli, A.A.; Tuna, K.M.; Holliday, L.S.; Krotova, K.; Brantly, M. Modulation of calreticulin expression reveals a novel exosome-mediated mechanism of Z variant alpha1-antitrypsin disposal. J. Biol. Chem. 2019, 294, 6240–6252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangoda, L.; Liem, M.; Ang, C.S.; Keerthikumar, S.; Adda, C.G.; Parker, B.S.; Mathivanan, S. Proteomic Profiling of Exosomes Secreted by Breast Cancer Cells with Varying Metastatic Potential. Proteomics 2017, 17, 1600370. [Google Scholar] [CrossRef] [PubMed]
- Mrowczynski, O.D.; Madhankumar, A.B.; Sundstrom, J.M.; Zhao, Y.; Kawasawa, Y.I.; Slagle-Webb, B.; Mau, C.; Payne, R.A.; Rizk, E.B.; Zacharia, B.E.; et al. Exosomes impact survival to radiation exposure in cell line models of nervous system cancer. Oncotarget 2018, 9, 36083–36101. [Google Scholar] [CrossRef]
- Zheng, K.; Ma, J.; Wang, Y.; He, Z.; Deng, K. Sulforaphane Inhibits Autophagy and Induces Exosome-Mediated Paracrine Senescence via Regulating mTOR/TFE3. Mol. Nutr. Food Res. 2020, 64, e1901231. [Google Scholar] [CrossRef]
- Bilog, A.D.; Smulders, L.; Oliverio, R.; Labanieh, C.; Zapanta, J.; Stahelin, R.V.; Nikolaidis, N. Membrane Localization of HspA1A, a Stress Inducible 70-kDa Heat-Shock Protein, Depends on Its Interaction with Intracellular Phosphatidylserine. Biomolecules 2019, 9, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, T.G.; Martins, V.R.; Hajj, G.N.M. Unconventional Secretion of Heat Shock Proteins in Cancer. Int. J. Mol. Sci. 2017, 18, 946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.Y.; Xu, B.; Wu, Q.X.; Chen, W.L.; Cai, S.; Zhang, H.; Tang, Q.F. Cisplatin-Resistant Gastric Cancer Cells Promote the Chemoresistance of Cisplatin-Sensitive Cells via the Exosomal RPS3-Mediated PI3K-Akt-Cofilin-1 Signaling Axis. Front. Cell Dev. Biol. 2021, 9, 618899. [Google Scholar] [CrossRef]
- Ferrer, E.; Dunmore, B.J.; Hassan, D.; Ormiston, M.L.; Moore, S.; Deighton, J.; Long, L.; Yang, X.D.; Stewart, D.J.; Morrell, N.W. A Potential Role for Exosomal Translationally Controlled Tumor Protein Export in Vascular Remodeling in Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol. 2018, 59, 467–478. [Google Scholar] [CrossRef]
- Taylor, D.D.; Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008, 110, 13–21. [Google Scholar] [CrossRef]
- Chaudhuri, A.D.; Dastgheyb, R.M.; Yoo, S.W.; Trout, A.; Talbot, C.C., Jr.; Hao, H.; Witwer, K.W.; Haughey, N.J. TNFalpha and IL-1beta modify the miRNA cargo of astrocyte shed extracellular vesicles to regulate neurotrophic signaling in neurons. Cell Death Dis. 2018, 9, 363. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Cao, L.L.; Wang, X.P.; Guo, W.; Guo, R.B.; Sun, Y.Q.; Xue, T.F.; Cai, Z.Y.; Ji, J.; Cheng, H.; et al. Neuronal extracellular vesicle derived miR-98 prevents salvageable neurons from microglial phagocytosis in acute ischemic stroke. Cell Death Dis. 2021, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zou, X.; Zhang, R.; Xie, Y.; Feng, Z.; Li, F.; Han, J.; Sun, H.; Ouyang, Q.; Hua, S.; et al. Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke. Aging 2021, 13, 3060–3079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, S.; McElroy, C.L.; Wang, B.; Jin, D.; Uteshev, V.V.; Jin, K. Circulating Pro-Inflammatory Exosomes Worsen Stroke Outcomes in Aging. Circ. Res. 2021, 129, e121–e140. [Google Scholar] [CrossRef]
- Yang, J.; Wu, S.; Hou, L.; Zhu, D.; Yin, S.; Yang, G.; Wang, Y. Therapeutic Effects of Simultaneous Delivery of Nerve Growth Factor mRNA and Protein via Exosomes on Cerebral Ischemia. Mol. Ther. Nucleic Acids 2020, 21, 512–522. [Google Scholar] [CrossRef]
- Han, H.S.; Yenari, M.A. Cellular targets of brain inflammation in stroke. Curr. Opin. Investig. Drugs 2003, 4, 522–529. [Google Scholar] [PubMed]
- Wang, Q.; Tang, X.N.; Yenari, M.A. The inflammatory response in stroke. J. Neuroimmunol. 2007, 184, 53–68. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Park, D.; Lee, S.H.; Bae, D.K.; Yang, Y.H.; Kyung, J.; Kim, D.; Choi, E.K.; Hong, J.T.; Jeong, H.S.; et al. Neuroprotective Effects of a Butanol Fraction of Rosa hybrida Petals in a Middle Cerebral Artery Occlusion Model. Biomol. Ther. 2013, 21, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.; Tekwani, B.L. Histone Deacetylases Inhibitors in Neurodegenerative Diseases, Neuroprotection and Neuronal Differentiation. Front. Pharmacol. 2020, 11, 537. [Google Scholar] [CrossRef]
- Miller, K.M.; Tjeertes, J.V.; Coates, J.; Legube, G.; Polo, S.E.; Britton, S.; Jackson, S.P. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat. Struct. Mol. Biol. 2010, 17, 1144–1151. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Wu, J.; Li, J.; Zhang, L.; Xiong, T.; Tang, J.; Qu, Y.; Mu, D. Involvement of the JNK/FOXO3a/Bim Pathway in Neuronal Apoptosis after Hypoxic-Ischemic Brain Damage in Neonatal Rats. PLoS ONE 2015, 10, e0132998. [Google Scholar] [CrossRef] [Green Version]
- Abe, K. Therapeutic potential of neurotrophic factors and neural stem cells against ischemic brain injury. J. Cereb. Blood Flow Metab. 2000, 20, 1393–1408. [Google Scholar] [CrossRef] [PubMed]
- Alderson, R.F.; Alterman, A.L.; Barde, Y.A.; Lindsay, R.M. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron 1990, 5, 297–306. [Google Scholar] [CrossRef]
- Schabitz, W.R.; Sommer, C.; Zoder, W.; Kiessling, M.; Schwaninger, M.; Schwab, S. Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke 2000, 31, 2212–2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Ahlenius, H.; Thored, P.; Kobayashi, R.; Kokaia, Z.; Lindvall, O. Intracerebral infusion of glial cell line-derived neurotrophic factor promotes striatal neurogenesis after stroke in adult rats. Stroke 2006, 37, 2361–2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clatterbuck, R.E.; Price, D.L.; Koliatsos, V.E. Ciliary neurotrophic factor prevents retrograde neuronal death in the adult central nervous system. Proc. Natl. Acad. Sci. USA 1993, 90, 2222–2226. [Google Scholar] [CrossRef] [Green Version]
- Wen, T.C.; Matsuda, S.; Yoshimura, H.; Kawabe, T.; Sakanaka, M. Ciliary neurotrophic factor prevents ischemia-induced learning disability and neuronal loss in gerbils. Neurosci. Lett. 1995, 191, 55–58. [Google Scholar] [CrossRef]
- Park, D.; Joo, S.S.; Kim, T.K.; Lee, S.H.; Kang, H.; Lee, H.J.; Lim, I.; Matsuo, A.; Tooyama, I.; Kim, Y.B.; et al. Human neural stem cells overexpressing choline acetyltransferase restore cognitive function of kainic acid-induced learning and memory deficit animals. Cell Transplant. 2012, 21, 365–371. [Google Scholar] [CrossRef]
- Guo, L.; Santschi, P.H. Ultrafiltration and its applications to sampling and characterisation of aquatic colloids. In Environmental Colloids and Particles: Behaviour, Separation and Characterisation; IUPAC Series on Analytical and Physical Chemistry of Environmental Systems; John Wiley and Sons: Chichester, UK, 2007; Volume 10, pp. 159–221. [Google Scholar]
- Sokolova, V.; Ludwig, A.K.; Hornung, S.; Rotan, O.; Horn, P.A.; Epple, M.; Giebel, B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf. B Biointerfaces 2011, 87, 146–150. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Schmid-Elsaesser, R.; Zausinger, S.; Hungerhuber, E.; Baethmann, A.; Reulen, H.J. Monotherapy with dextromethorphan or tirilazad--but not a combination of both--improves outcome after transient focal cerebral ischemia in rats. Exp. Brain Res. 1998, 122, 121–127. [Google Scholar] [CrossRef]
- Choi, B.I.; Park, D.; Lee, S.H.; Bae, D.K.; Yang, G.; Yang, Y.H.; Kim, T.K.; Choi, E.K.; Lee, H.J.; Choi, K.C.; et al. Neurobehavioural deficits correlate with the cerebral infarction volume of stroke animals: A comparative study on ischaemia-reperfusion and photothrombosis models. Environ. Toxicol. Pharmacol. 2012, 33, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yon, J.M.; Kim, Y.B.; Park, D. The Ethanol Fraction of White Rose Petal Extract Abrogates Excitotoxicity-Induced Neuronal Damage In Vivo and In Vitro through Inhibition of Oxidative Stress and Proinflammation. Nutrients 2018, 10, 1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, E.-J.; Choi, Y.; Kim, T.M.; Choi, E.-K.; Kim, Y.-B.; Park, D. The Neuroprotective Effects of Exosomes Derived from TSG101-Overexpressing Human Neural Stem Cells in a Stroke Model. Int. J. Mol. Sci. 2022, 23, 9532. https://doi.org/10.3390/ijms23179532
Yoon E-J, Choi Y, Kim TM, Choi E-K, Kim Y-B, Park D. The Neuroprotective Effects of Exosomes Derived from TSG101-Overexpressing Human Neural Stem Cells in a Stroke Model. International Journal of Molecular Sciences. 2022; 23(17):9532. https://doi.org/10.3390/ijms23179532
Chicago/Turabian StyleYoon, Eun-Jung, Yunseo Choi, Tae Myoung Kim, Ehn-Kyoung Choi, Yun-Bae Kim, and Dongsun Park. 2022. "The Neuroprotective Effects of Exosomes Derived from TSG101-Overexpressing Human Neural Stem Cells in a Stroke Model" International Journal of Molecular Sciences 23, no. 17: 9532. https://doi.org/10.3390/ijms23179532





