Pomegranate Extract Augments Energy Expenditure Counteracting the Metabolic Stress Associated with High-Fat-Diet-Induced Obesity
Abstract
:1. Introduction
2. Results
2.1. Study Workflow
2.2. PomE Extract Improves the Systemic Glucose Homeostasis and Insulin Sensitivity after HFD Induced Obesity
2.3. PomE Augments the Systemic Energy Expenditure after HFD-Induced Obesity
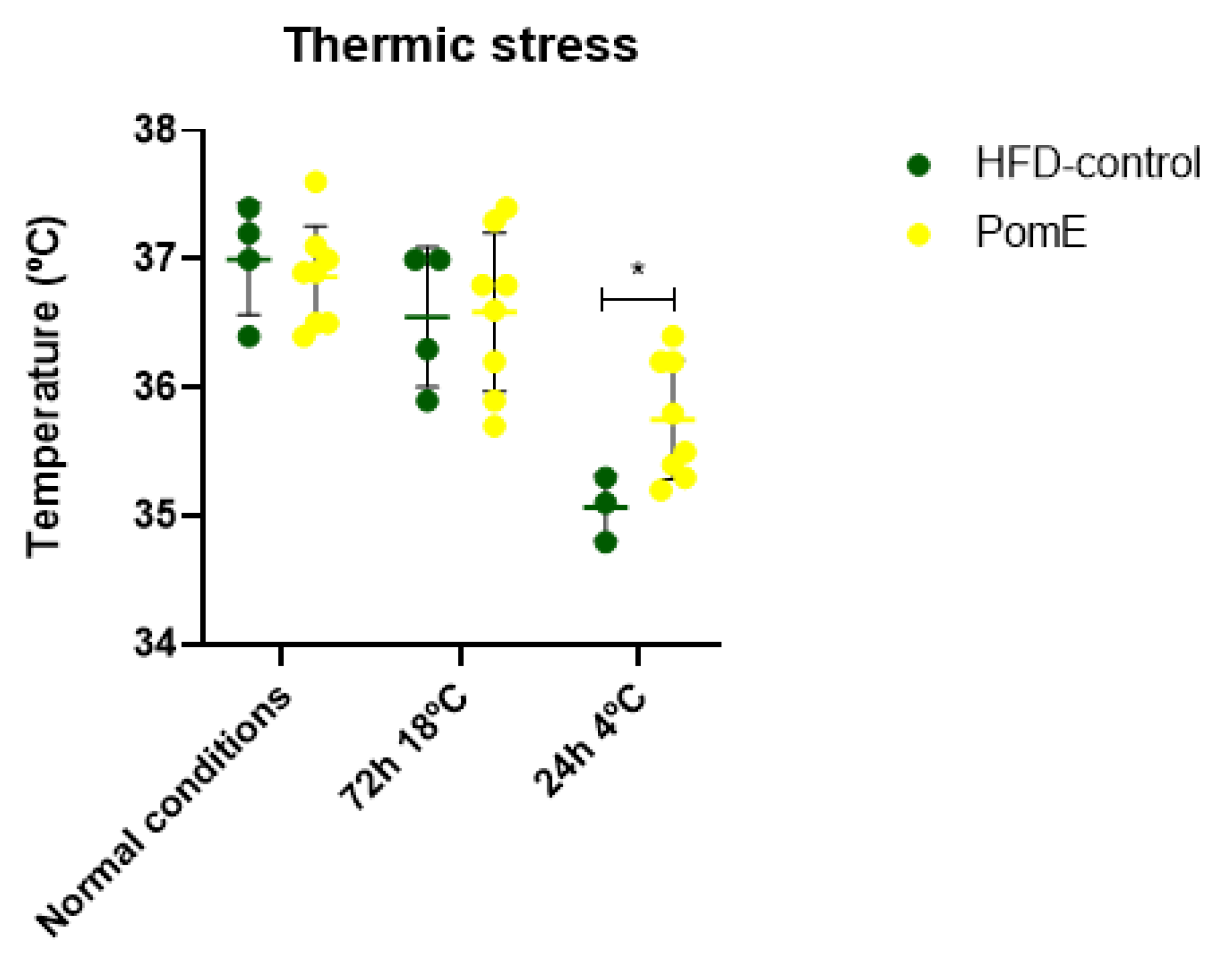
2.4. PomE Preserves the Body Temperature after Cold Exposition
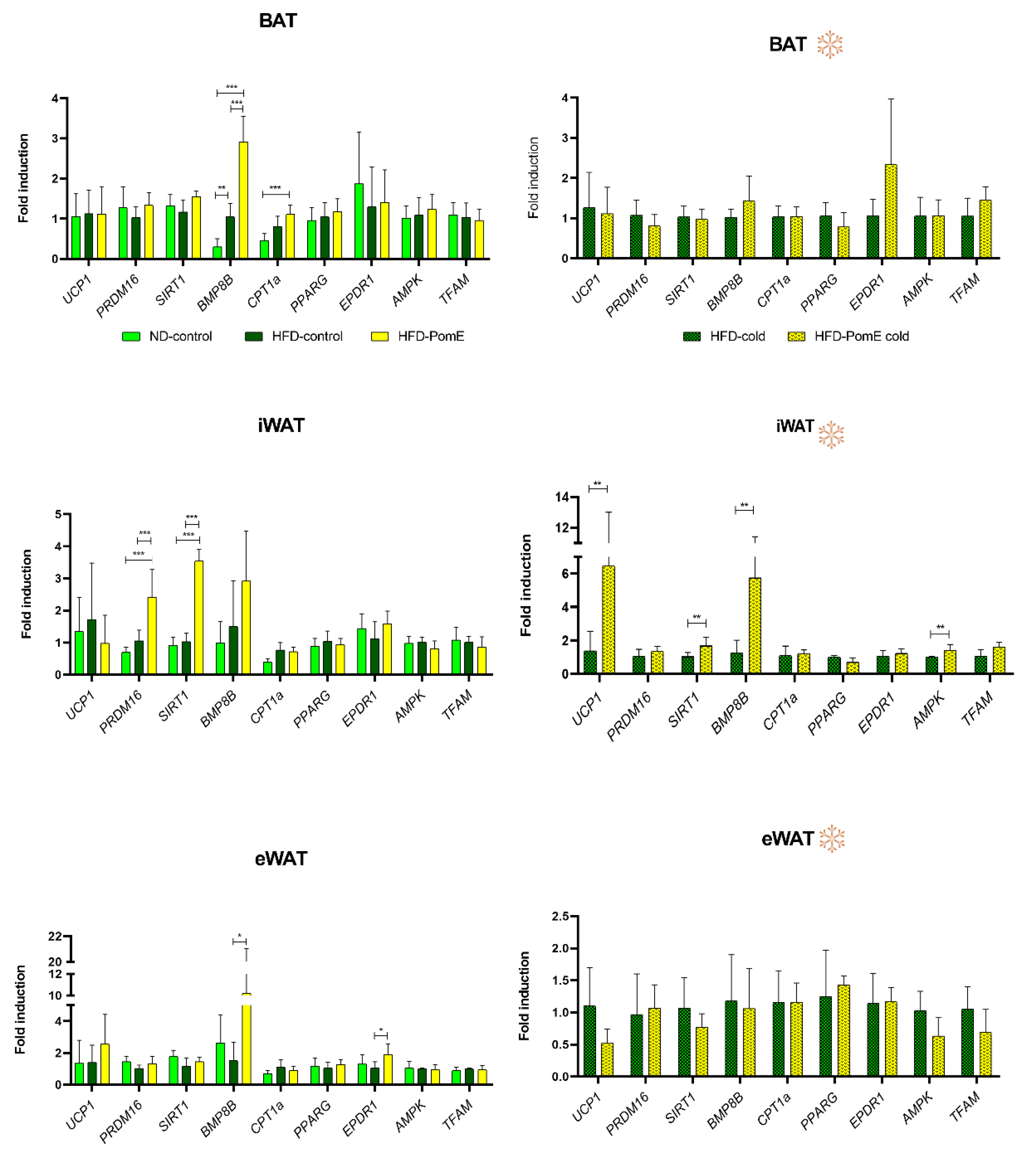
2.5. PomE Augments the Expression of Genes Implicated in Browning of WAT In Vivo
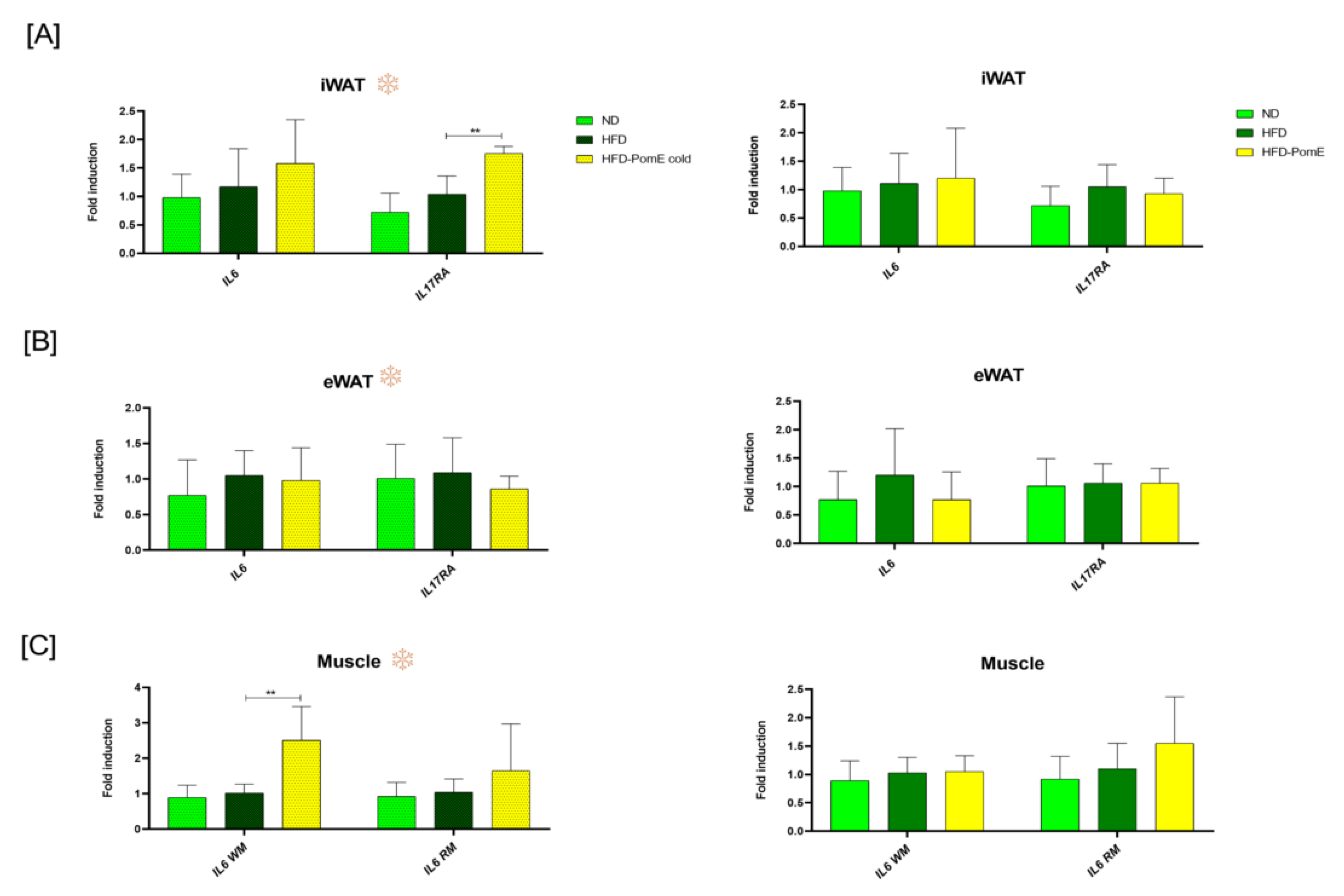
2.6. PomE Modulates the Expression of Inflammatory Myokines in Muscle Tissues
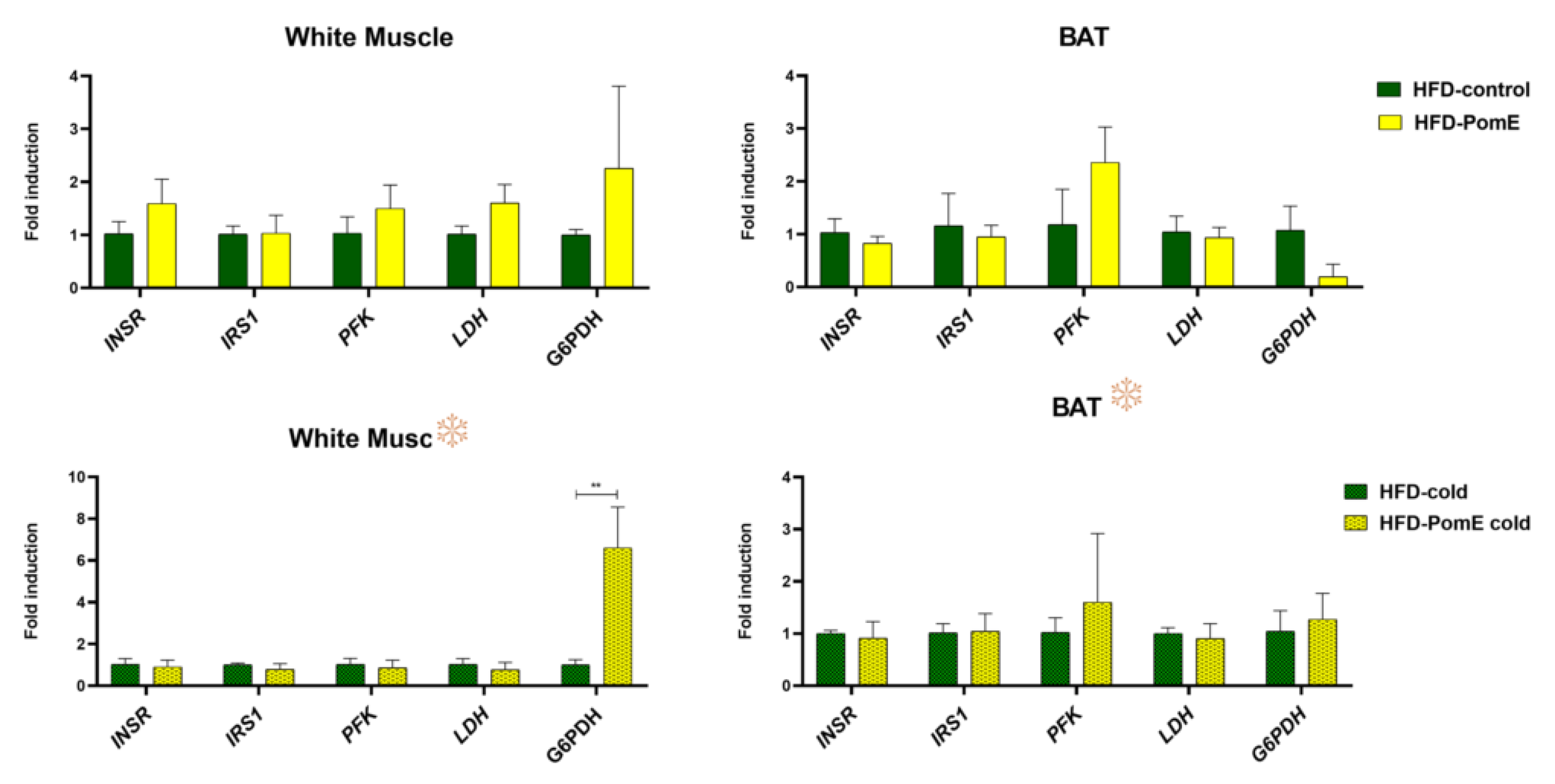
2.7. PomE Affects the Expression of Molecular Targets Involved in Glucose and Redox Homeostasis
3. Discussion
4. Materials and Methods
4.1. Pomegranate Extract (PomE)
4.2. Animal Model
4.3. Glucose Tolerance Test (GTT), Insulin Tolerance Test (ITT), the Index of Homeostasis Model Assessment of Insulin Resistance (HOMA/IR)
4.3.1. Glucose Tolerance Test (GTT)
4.3.2. Insulin Tolerance Test (ITT)
4.3.3. The Index of Homeostasis Model Assessment of Insulin Resistance (HOMA/IR)
4.4. Indirect Calorimetry
4.5. Cold Chambers
4.6. Gene Expression Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| AT | adipose tissue |
| AUC | area under the curve |
| B2M | beta-2-microglobulin |
| BAT | brown adipose tissue |
| DNA | deoxyribonucleic acid |
| EE | energy expenditure |
| eWAT | epididymal white adipose tissue |
| G6PDH | glucose-6-phosphate dehydrogenase |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GTT | glucose tolerance test |
| HFD | high-fat diet |
| HOMA/IR | index of homeostasis model assessment of insulin resistance |
| IR | insulin resistance |
| ITT | insulin tolerance test |
| NADH | reduced nicotinamide adenine dinucleotide |
| ND | normal diet |
| P | pomegranate extract |
| PFK | phosphofructokinase |
| qPCR | quantitative polymerase chain reaction |
| RQ | respiration quotient |
| RNA | ribonucleic acid |
| VCO2 | volume of carbon dioxide |
| VO2 | total volume of oxygen |
References
- Aunan, J.R.; Watson, M.M.; Hagland, H.R.; Søreide, K. Molecular and Biological Hallmarks of Ageing. Br. J. Surg. 2016, 103, e29–e46. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and Age-Related Diseases: From Mechanisms to Therapeutic Strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine Dysregulation and Adipose Tissue Inflammation in Human Obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and Inflammation: The Linking Mechanism and the Complications. Arch. Med. Sci. AMS 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, F.; Cereijo, R.; Gavaldà-Navarro, A.; Villarroya, J.; Giralt, M. Inflammation of Brown/Beige Adipose Tissues in Obesity and Metabolic Disease. J. Intern. Med. 2018, 284, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Poher, A.-L.; Altirriba, J.; Veyrat-Durebex, C.; Rohner-Jeanrenaud, F. Brown Adipose Tissue Activity as a Target for the Treatment of Obesity/Insulin Resistance. Front. Physiol. 2015, 6, 4. [Google Scholar] [CrossRef]
- Saad, B.; Ghareeb, B.; Kmail, A. Metabolic and Epigenetics Action Mechanisms of Antiobesity Medicinal Plants and Phytochemicals. Evid.-Based Complementary Altern. Med. ECAM 2021, 2021, 9995903. [Google Scholar] [CrossRef]
- Reguero, M.; Gómez de Cedrón, M.; Reglero, G.; Quintela, J.C.; Ramírez de Molina, A. Natural Extracts to Augment Energy Expenditure as a Complementary Approach to Tackle Obesity and Associated Metabolic Alterations. Biomolecules 2021, 11, 412. [Google Scholar] [CrossRef]
- Whittle, A.J.; Carobbio, S.; Martins, L.; Slawik, M.; Hondares, E.; Vázquez, M.J.; Morgan, D.; Csikasz, R.I.; Gallego, R.; Rodriguez-Cuenca, S.; et al. BMP8B Increases Brown Adipose Tissue Thermogenesis through Both Central and Peripheral Actions. Cell 2012, 149, 871–885. [Google Scholar] [CrossRef]
- Desjardins, E.M.; Steinberg, G.R. Emerging Role of AMPK in Brown and Beige Adipose Tissue (BAT): Implications for Obesity, Insulin Resistance, and Type 2 Diabetes. Curr. Diab. Rep. 2018, 18, 80. [Google Scholar] [CrossRef]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK Regulates Energy Expenditure by Modulating NAD+ Metabolism and SIRT1 Activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Hondares, E.; Rosell, M.; Díaz-Delfín, J.; Olmos, Y.; Monsalve, M.; Iglesias, R.; Villarroya, F.; Giralt, M. Peroxisome Proliferator-Activated Receptor α (PPARα) Induces PPARγ Coactivator 1α (PGC-1α) Gene Expression and Contributes to Thermogenic Activation of Brown Fat: Involvement of PRDM16. J. Biol. Chem. 2011, 286, 43112–43122. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, X.; Sun, X.; Zhang, L.; Fu, X.; Rogers, C.J.; Berim, A.; Zhang, S.; Wang, S.; Wang, B.; et al. AMPK/α-Ketoglutarate Axis Dynamically Mediates DNA Demethylation in the Prdm16 Promoter and Brown Adipogenesis. Cell Metab. 2016, 24, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Colitti, M.; Montanari, T. Brain-Derived Neurotrophic Factor Modulates Mitochondrial Dynamics and Thermogenic Phenotype on 3T3-L1 Adipocytes. Tissue Cell 2020, 66, 101388. [Google Scholar] [CrossRef]
- Wang, P.; Loh, K.H.; Wu, M.; Morgan, D.A.; Schneeberger, M.; Yu, X.; Chi, J.; Kosse, C.; Kim, D.; Rahmouni, K.; et al. A Leptin–BDNF Pathway Regulating Sympathetic Innervation of Adipose Tissue. Nature 2020, 583, 839–844. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Prado Neto, E.V.; De Alvares Goulart, R.; Bechara, M.D.; Baisi Chagas, E.F.; Audi, M.; Guissoni Campos, L.M.; Landgraf Guiger, E.; Buchaim, R.L.; Buchaim, D.V.; et al. Myokines: A Descriptive Review. J. Sports Med. Phys. Fitness 2020, 60, 1583–1590. [Google Scholar] [CrossRef]
- Ukropec, J.; Anunciado, R.P.; Ravussin, Y.; Hulver, M.W.; Kozak, L.P. UCP1-Independent Thermogenesis in White Adipose Tissue of Cold-Acclimated Ucp1-/- Mice. J. Biol. Chem. 2006, 281, 31894–31908. [Google Scholar] [CrossRef]
- Roesler, A.; Kazak, L. UCP1-Independent Thermogenesis. Biochem. J. 2020, 477, 709–725. [Google Scholar] [CrossRef]
- Okamatsu-Ogura, Y.; Kuroda, M.; Tsutsumi, R.; Tsubota, A.; Saito, M.; Kimura, K.; Sakaue, H. UCP1-Dependent and UCP1-Independent Metabolic Changes Induced by Acute Cold Exposure in Brown Adipose Tissue of Mice. Metabolism 2020, 113, 154396. [Google Scholar] [CrossRef]
- Lim, S.; Honek, J.; Xue, Y.; Seki, T.; Cao, Z.; Andersson, P.; Yang, X.; Hosaka, K.; Cao, Y. Cold-Induced Activation of Brown Adipose Tissue and Adipose Angiogenesis in Mice. Nat. Protoc. 2012, 7, 606–615. [Google Scholar] [CrossRef]
- Pellegrinelli, V.; Peirce, V.J.; Howard, L.; Virtue, S.; Türei, D.; Senzacqua, M.; Frontini, A.; Dalley, J.W.; Horton, A.R.; Bidault, G.; et al. Adipocyte-Secreted BMP8b Mediates Adrenergic-Induced Remodeling of the Neuro-Vascular Network in Adipose Tissue. Nat. Commun. 2018, 9, 4974. [Google Scholar] [CrossRef] [PubMed]
- Perrini, S.; Porro, S.; Nigro, P.; Cignarelli, A.; Caccioppoli, C.; Genchi, V.A.; Martines, G.; De Fazio, M.; Capuano, P.; Natalicchio, A.; et al. Reduced SIRT1 and SIRT2 Expression Promotes Adipogenesis of Human Visceral Adipose Stem Cells and Associates with Accumulation of Visceral Fat in Human Obesity. Int. J. Obes. 2020, 44, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Chalkiadaki, A.; Guarente, L. High-Fat Diet Triggers Inflammation-Induced Cleavage of SIRT1 in Adipose Tissue To Promote Metabolic Dysfunction. Cell Metab. 2012, 16, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Huynh, F.K.; Hershberger, K.A.; Hirschey, M.D. Targeting Sirtuins for the Treatment of Diabetes. Diabetes Manag. Lond. Engl. 2013, 3, 245–257. [Google Scholar] [CrossRef]
- Kohlgruber, A.C.; Gal-Oz, S.T.; LaMarche, N.M.; Shimazaki, M.; Duquette, D.; Koay, H.-F.; Nguyen, H.N.; Mina, A.I.; Paras, T.; Tavakkoli, A.; et al. Γδ T Cells Producing Interleukin-17A Regulate Adipose Regulatory T Cell Homeostasis and Thermogenesis. Nat. Immunol. 2018, 19, 464–474. [Google Scholar] [CrossRef]
- Leal, L.G.; Lopes, M.A.; Batista, M.L. Physical Exercise-Induced Myokines and Muscle-Adipose Tissue Crosstalk: A Review of Current Knowledge and the Implications for Health and Metabolic Diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Leopold, J.A.; Loscalzo, J. Cyclic Strain Modulates Resistance to Oxidant Stress by Increasing G6PDH Expression in Smooth Muscle Cells. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H2477–H2485. [Google Scholar] [CrossRef]
- Huang, R.; Chen, H.; Zhou, W.; Ma, C.; Zhang, Y.-H.P. Engineering a Thermostable Highly Active Glucose 6-Phosphate Dehydrogenase and Its Application to Hydrogen Production in Vitro. Appl. Microbiol. Biotechnol. 2018, 102, 3203–3215. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-Induced Oxidative Stress: Friend or Foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Ammar, A.; Bailey, S.J.; Chtourou, H.; Trabelsi, K.; Turki, M.; Hökelmann, A.; Souissi, N. Effects of Pomegranate Supplementation on Exercise Performance and Post-Exercise Recovery in Healthy Adults: A Systematic Review. Br. J. Nutr. 2018, 120, 1201–1216. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Gilson, H.; Jamart, C.; Naslain, D.; Pierre, N.; Deldicque, L.; Francaux, M. Pomegranate and Green Tea Extracts Protect against ER Stress Induced by a High-Fat Diet in Skeletal Muscle of Mice. Eur. J. Nutr. 2015, 54, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yan, C.; Frost, B.; Wang, X.; Hou, C.; Zeng, M.; Gao, H.; Kang, Y.; Liu, J. Pomegranate Extract Decreases Oxidative Stress and Alleviates Mitochondrial Impairment by Activating AMPK-Nrf2 in Hypothalamic Paraventricular Nucleus of Spontaneously Hypertensive Rats. Sci. Rep. 2016, 6, 34246. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Khorramabadi, R.M.; Assadollahi, V.; Khosravi, P.; Cheraghi Venol, A.; Veiskerami, S.; Ahmadvand, H. The Effects of Pomegranate Peel Extract on the Gene Expressions of Antioxidant Enzymes in a Rat Model of Alloxan-Induced Diabetes. Arch. Physiol. Biochem. 2021, 1, 1–9. [Google Scholar] [CrossRef]
- Aksu, D.S.; Sağlam, Y.S.; Yildirim, S.; Aksu, T. Effect of Pomegranate (Punica granatum L.) Juice on Kidney, Liver, Heart and Testis Histopathological Changes, and the Tissues Lipid Peroxidation and Antioxidant Status in Lead Acetate-Treated Rats. Cell. Mol. Biol. Noisy—Gd. Fr. 2017, 63, 33–42. [Google Scholar] [CrossRef]
- Yu, L.-M.; Dong, X.; Xue, X.-D.; Zhang, J.; Li, Z.; Wu, H.-J.; Yang, Z.-L.; Yang, Y.; Wang, H.-S. Protection of the Myocardium against Ischemia/Reperfusion Injury by Punicalagin through an SIRT1-NRF-2-HO-1-Dependent Mechanism. Chem. Biol. Interact. 2019, 306, 152–162. [Google Scholar] [CrossRef]
- Watanabe, J.; Kagami, N.; Kawazoe, M.; Arata, S. A Simplified Enriched Environment Increases Body Temperature and Suppresses Cancer Progression in Mice. Exp. Anim. 2020, 69, 207–218. [Google Scholar] [CrossRef]
- Lodhi, I.J.; Semenkovich, C.F. Why We Should Put Clothes on Mice. Cell Metab. 2009, 9, 111–112. [Google Scholar] [CrossRef]
- Martin, A.R.; Chung, S.; Koehler, K. Is Exercise a Match for Cold Exposure? Common Molecular Framework for Adipose Tissue Browning. Int. J. Sports Med. 2020, 41, 427–442. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, A.S.; Peijs, L.; Beaudry, J.L.; Jespersen, N.Z.; Nielsen, C.H.; Ma, T.; Brunner, A.D.; Larsen, T.J.; Bayarri-Olmos, R.; Prabhakar, B.S.; et al. Proteomics-Based Comparative Mapping of the Secretomes of Human Brown and White Adipocytes Reveals EPDR1 as a Novel Batokine. Cell Metab. 2019, 30, 963.e7–975.e7. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Lee, S.K.; Jang, Y.J.; Park, H.S.; Kim, J.-H.; Lee, Y.J.; Heo, Y.-S. Association between Low SIRT1 Expression in Visceral and Subcutaneous Adipose Tissues and Metabolic Abnormalities in Women with Obesity and Type 2 Diabetes. Diabetes Res. Clin. Pract. 2013, 101, 341–348. [Google Scholar] [CrossRef]
- Liu, H.; Zhan, Q.; Miao, X.; Xia, X.; Yang, G.; Peng, X.; Yan, C. Punicalagin Prevents Hepatic Steatosis through Improving Lipid Homeostasis and Inflammation in Liver and Adipose Tissue and Modulating Gut Microbiota in Western Diet-Fed Mice. Mol. Nutr. Food Res. 2021, 65, e2001031. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Strohacker, K.A.; Kueht, M.L. Pomegranate Seed Oil Consumption during a Period of High-Fat Feeding Reduces Weight Gain and Reduces Type 2 Diabetes Risk in CD-1 Mice. Br. J. Nutr. 2009, 102, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Pfohl, M.; DaSilva, N.A.; Marques, E.; Agudelo, J.; Liu, C.; Goedken, M.; Slitt, A.L.; Seeram, N.P.; Ma, H. Hepatoprotective and Anti-Inflammatory Effects of a Standardized Pomegranate (Punica granatum) Fruit Extract in High Fat Diet-Induced Obese C57BL/6 Mice. Int. J. Food Sci. Nutr. 2021, 72, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Vroegrijk, I.O.C.M.; van Diepen, J.A.; van den Berg, S.; Westbroek, I.; Keizer, H.; Gambelli, L.; Hontecillas, R.; Bassaganya-Riera, J.; Zondag, G.C.M.; Romijn, J.A.; et al. Pomegranate Seed Oil, a Rich Source of Punicic Acid, Prevents Diet-Induced Obesity and Insulin Resistance in Mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 1426–1430. [Google Scholar] [CrossRef]
- Xia, B.; Shi, X.C.; Xie, B.C.; Zhu, M.Q.; Chen, Y.; Chu, X.Y.; Cai, G.H.; Liu, M.; Yang, S.Z.; Mitchell, G.A.; et al. Urolithin A Exerts Antiobesity Effects through Enhancing Adipose Tissue Thermogenesis in Mice. PLoS Biol. 2020, 18, e3000688. [Google Scholar] [CrossRef]
- Cao, K.; Xu, J.; Pu, W.; Dong, Z.; Sun, L.; Zang, W.; Gao, F.; Zhang, Y.; Feng, Z.; Liu, J. Punicalagin, an Active Component in Pomegranate, Ameliorates Cardiac Mitochondrial Impairment in Obese Rats via AMPK Activation. Sci. Rep. 2015, 5, 14014. [Google Scholar] [CrossRef]
- Ok, E.; Do, G.-M.; Lim, Y.; Park, J.-E.; Park, Y.-J.; Kwon, O. Pomegranate Vinegar Attenuates Adiposity in Obese Rats through Coordinated Control of AMPK Signaling in the Liver and Adipose Tissue. Lipids Health Dis. 2013, 12, 163. [Google Scholar] [CrossRef]
- Gharib, E.; Montasser Kouhsari, S. Study of the Antidiabetic Activity of Punica Granatum L. Fruits Aqueous Extract on the Alloxan-Diabetic Wistar Rats. Iran. J. Pharm. Res. IJPR 2019, 18, 358–368. [Google Scholar]
- Estrada-Camarena, E.M.; López-Rubalcava, C.; Ramírez-Rodríguez, G.B.; Pulido, D.; Cervantes-Anaya, N.; Azpilcueta-Morales, G.; Granados-Juárez, A.; Vega-Rivera, N.M.; Islas-Preciado, D.; Treviño, S.; et al. Aqueous Extract of Pomegranate Enriched in Ellagitannins Prevents Anxiety-like Behavior and Metabolic Changes Induced by Cafeteria Diet in an Animal Model of Menopause. Neurochem. Int. 2020, 141, 104876. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.; Einwallner, E. Study of In Vivo Glucose Metabolism in High-Fat Diet-Fed Mice Using Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT). J. Vis. Exp. 2018, 131, 56672. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reguero, M.; Gómez de Cedrón, M.; Sierra-Ramírez, A.; Fernández-Marcos, P.J.; Reglero, G.; Quintela, J.C.; Ramírez de Molina, A. Pomegranate Extract Augments Energy Expenditure Counteracting the Metabolic Stress Associated with High-Fat-Diet-Induced Obesity. Int. J. Mol. Sci. 2022, 23, 10460. https://doi.org/10.3390/ijms231810460
Reguero M, Gómez de Cedrón M, Sierra-Ramírez A, Fernández-Marcos PJ, Reglero G, Quintela JC, Ramírez de Molina A. Pomegranate Extract Augments Energy Expenditure Counteracting the Metabolic Stress Associated with High-Fat-Diet-Induced Obesity. International Journal of Molecular Sciences. 2022; 23(18):10460. https://doi.org/10.3390/ijms231810460
Chicago/Turabian StyleReguero, Marina, Marta Gómez de Cedrón, Aranzazu Sierra-Ramírez, Pablo José Fernández-Marcos, Guillermo Reglero, José Carlos Quintela, and Ana Ramírez de Molina. 2022. "Pomegranate Extract Augments Energy Expenditure Counteracting the Metabolic Stress Associated with High-Fat-Diet-Induced Obesity" International Journal of Molecular Sciences 23, no. 18: 10460. https://doi.org/10.3390/ijms231810460




