Anti-Obesity Effects of Isorhamnetin and Isorhamnetin Conjugates
Abstract
:1. Introduction
2. Literature Search
3. Studies in Cultured Adipocytes
3.1. Summary
4. Studies in Rodents
4.1. Summary
5. Studies in Other Animal Models
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kawser Hossain, M.; Abdal Dayem, A.; Han, J.; Yin, Y.; Kim, K.; Kumar Saha, S.; Yang, G.M.; Choi, H.Y.; Cho, S.G. Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids. Int. J. Mol. Sci. 2016, 17, 569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-López, I.; Lobo-Rodrigo, G.; Portillo, M.P.; Cano, M.P. Characterization, Stability, and Bioaccessibility of Betalain and Phenolic Compounds from. Foods 2021, 10, 1593. [Google Scholar] [CrossRef] [PubMed]
- Matboli, M.; Saad, M.; Hasanin, A.H.; A Saleh, L.; Baher, W.; Bekhet, M.M.; Eissa, S. New insight into the role of isorhamnetin as a regulator of insulin signaling pathway in type 2 diabetes mellitus rat model: Molecular and computational approach. Biomed. Pharmacother. 2021, 135, 111176. [Google Scholar] [CrossRef] [PubMed]
- Kalai, F.Z.; Boulaaba, M.; Ferdousi, F.; Isoda, H. Effects of Isorhamnetin on Diabetes and Its Associated Complications: A Review of In Vitro and In Vivo Studies and a Post Hoc Transcriptome Analysis of Involved Molecular Pathways. Int. J. Mol. Sci. 2022, 23, 704. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An Online Comprehensive Database on Polyphenol Contents in Foods. Database 2010, 2010, bap024. Available online: https://academic.oup.com/database/article/doi/10.1093/database/bap024/401207?login=false (accessed on 21 September 2022). [CrossRef]
- Deng, Y.; Huang, H.; Lei, F.; Fu, S.; Zou, K.; Zhang, S.; Liu, X.; Jiang, L.; Liu, H.; Miao, B.; et al. Endophytic Bacterial Communities of Ginkgo biloba Leaves During Leaf Developmental Period. Front. Microbiol. 2021, 12, 698703. [Google Scholar] [CrossRef]
- Schieber, A.; Keller, P.; Streker, P.; Klaiber, I.; Carle, R. Detection of isorhamnetin glycosides in extracts of apples (Malus domestica cv. “Brettacher”) by HPLC-PDA and HPLC-APCI-MS/MS. Phytochem. Anal. 2002, 13, 87–94. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC-MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012, 135, 2138–2146. [Google Scholar] [CrossRef]
- Olas, B.; Kontek, B.; Malinowska, P.; Żuchowski, J.; Stochmal, A. Hippophae rhamnoides L. Fruits Reduce the Oxidative Stress in Human Blood Platelets and Plasma. Oxid. Med. Cell. Longev. 2016, 2016, 4692486. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.O.; Heo, H.J.; Kim, Y.J.; Yang, H.S.; Lee, C.Y. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef]
- Micek, A.; Godos, J.; Brzostek, T.; Gniadek, A.; Favari, C.; Mena, P.; Libra, M.; Del Rio, D.; Galvano, F.; Grosso, G. Dietary phytoestrogens and biomarkers of their intake in relation to cancer survival and recurrence: A comprehensive systematic review with meta-analysis. Nutr. Rev. 2021, 79, 42–65. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.; Ramos, S. Dietary Flavonoids and Insulin Signaling in Diabetes and Obesity. Cells 2021, 10, 1474. [Google Scholar] [CrossRef] [PubMed]
- Ponte, L.G.S.; Pavan, I.C.B.; Mancini, M.C.S.; da Silva, L.G.S.; Morelli, A.P.; Severino, M.B.; Bezerra, R.M.N.; Simabuco, F.M. The Hallmarks of Flavonoids in Cancer. Molecules 2021, 26, 2029. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Lee, J.; Jung, E.; Kim, S.; Huh, S.; Kim, Y.; Byun, S.Y.; Kim, Y.S.; Park, D. Isorhamnetin represses adipogenesis in 3T3-L1 cells. Obesity 2009, 17, 226–232. [Google Scholar] [CrossRef]
- Lee, J.; Jung, E.; Hwang, W.; Kim, Y.S.; Park, D. Isorhamnetin-induced anti-adipogenesis is mediated by stabilization of beta-catenin protein. Life Sci. 2010, 86, 416–423. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, B.; Oh, M.J.; Yoon, J.; Kim, H.Y.; Lee, K.J.; Lee, J.D.; Choi, K.Y. Persicaria hydropiper (L.) spach and its flavonoid components, isoquercitrin and isorhamnetin, activate the Wnt/β-catenin pathway and inhibit adipocyte differentiation of 3T3-L1 cells. Phytother. Res. 2011, 25, 1629–1635. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, M.; Cai, W.; Yu, L.; Feng, L.; Zhang, L.; Zang, Q.; Wang, Y.; Wang, D.; Chen, H.; et al. Dietary component isorhamnetin is a PPARγ antagonist and ameliorates metabolic disorders induced by diet or leptin deficiency. Sci. Rep. 2016, 6, 19288. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.S.; Kim, Y. Effects of Isorhamnetin on Adipocyte Mitochondrial Biogenesis and AMPK Activation. Molecules 2018, 23, 1853. [Google Scholar] [CrossRef]
- Ganbold, M.; Owada, Y.; Ozawa, Y.; Shimamoto, Y.; Ferdousi, F.; Tominaga, K.; Zheng, Y.W.; Ohkohchi, N.; Isoda, H. Isorhamnetin Alleviates Steatosis and Fibrosis in Mice with Nonalcoholic Steatohepatitis. Sci. Rep. 2019, 9, 16210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eseberri, I.; Miranda, J.; Lasa, A.; Mosqueda-Solís, A.; González-Manzano, S.; Santos-Buelga, C.; Portillo, M.P. Effects of Quercetin Metabolites on Triglyceride Metabolism of 3T3-L1 Preadipocytes and Mature Adipocytes. Int. J. Mol. Sci. 2019, 20, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, C.S.; Seo, Y. Antiadipogenic activity of isohamnetin 3-O-β-d-glucopyranoside from Salicornia herbacea. Immunopharmacol. Immunotoxicol. 2012, 34, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Im, S.H.; Wang, Z.; Lim, S.S.; Lee, O.H.; Kang, I.J. Bioactivity-guided isolation and identification of anti-adipogenic compounds from Sanguisorba officinalis. Pharm. Biol. 2017, 55, 2057–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Ross, S.E. Inhibition of adipogenesis by Wnt signaling. Science 2000, 289, 950–953. [Google Scholar] [CrossRef]
- Kanazawa, A.; Tsukada, S.; Kamiyama, M.; Yanagimoto, T.; Nakajima, M.; Maeda, S. Wnt5b partially inhibits canonical Wnt/beta-catenin signaling pathway and promotes adipogenesis in 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 2005, 330, 505–510. [Google Scholar] [CrossRef]
- Bennett, C.N.; Ross, S.E.; Longo, K.A.; Bajnok, L.; Hemati, N.; Johnson, K.W.; Harrison, S.D.; MacDougald, O.A. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 2002, 277, 30998–31004. [Google Scholar] [CrossRef]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heller, R.S.; Klein, T.; Ling, Z.; Heimberg, H.; Katoh, M.; Madsen, O.D.; Serup, P. Expression of Wnt, Frizzled, sFRP, and DKK genes in adult human pancreas. Gene Expr. 2003, 11, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Kalisz, J. The Impact of Dietary Flavonols on Central Obesity Parameters in Polish Adults. Nutrients 2022, 14, 5051. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, C.; Torres, N.; Gutiérrez-Uribe, J.A.; Noriega, L.G.; Torre-Villalvazo, I.; Leal-Díaz, A.M.; Antunes-Ricardo, M.; Márquez-Mota, C.; Ordaz, G.; Chavez-Santoscoy, R.A.; et al. The effect of isorhamnetin glycosides extracted from Opuntia ficus-indica in a mouse model of diet induced obesity. Food Funct. 2015, 6, 805–815. [Google Scholar] [CrossRef]
- Farias-Pereira, R.; Savarese, J.; Yue, Y.; Lee, S.H.; Park, Y. Fat-lowering effects of isorhamnetin are via NHR-49-dependent pathway in Caenorhabditis elegans. Curr. Res. Food Sci. 2020, 2, 70–76. [Google Scholar] [CrossRef]
- Jiang, H.; Yamashita, Y.; Nakamura, A.; Croft, K.; Ashida, H. Quercetin and its metabolite isorhamnetin promote glucose uptake through different signalling pathways in myotubes. Sci. Rep. 2019, 9, 2690. [Google Scholar] [CrossRef] [PubMed]
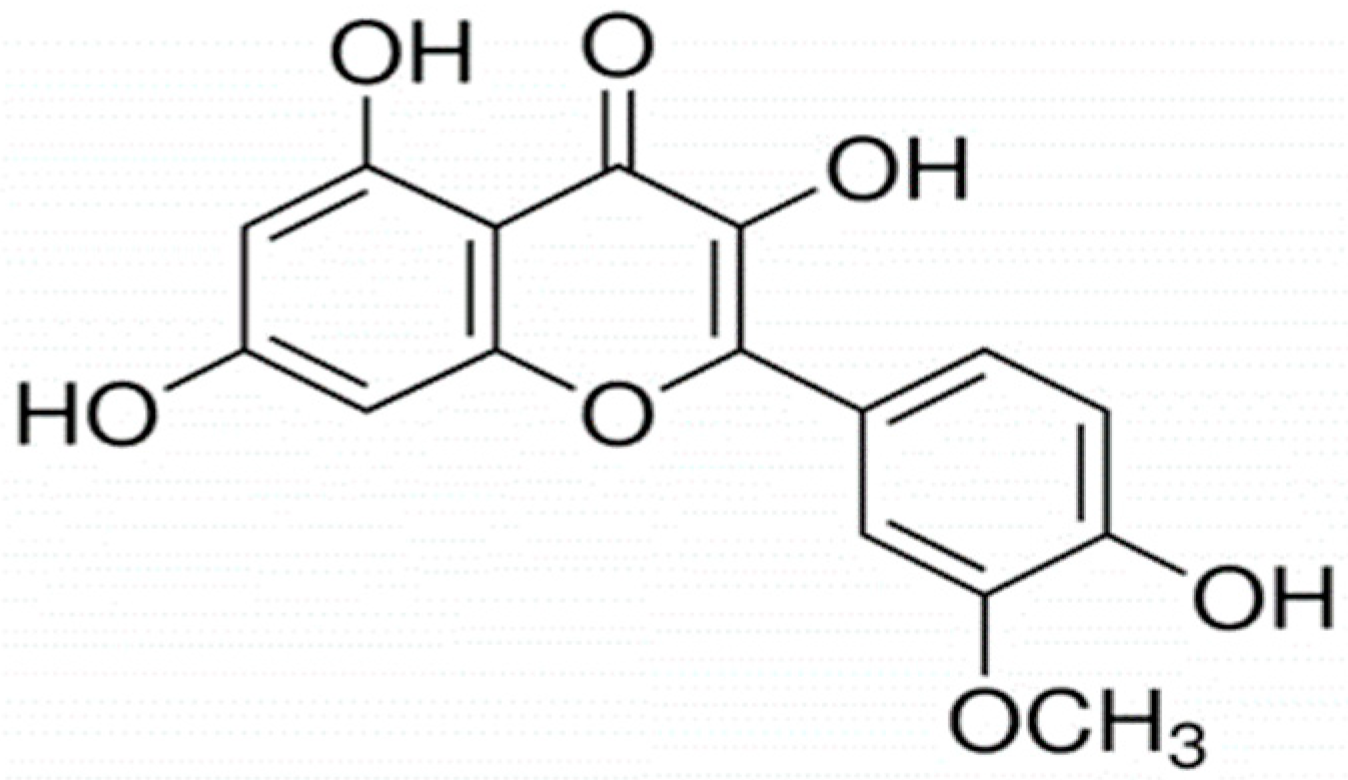
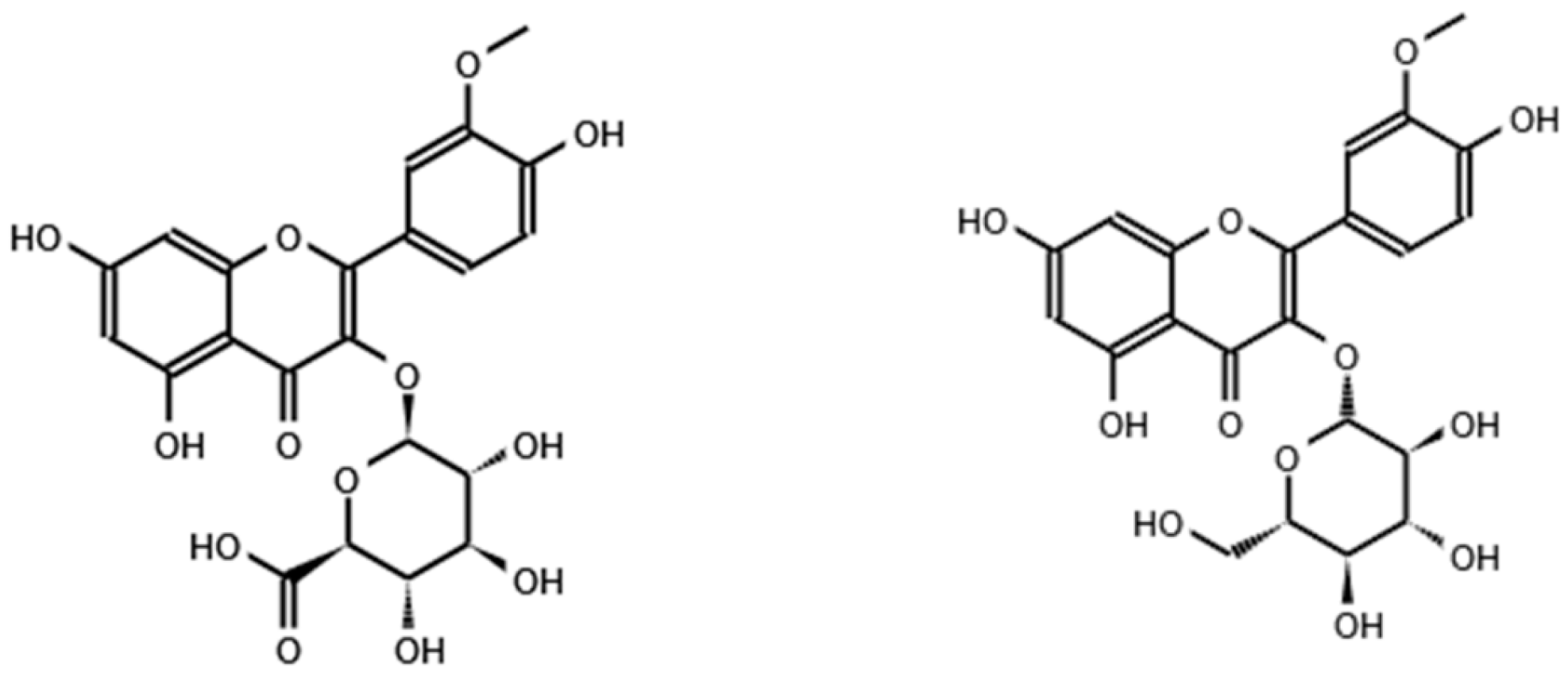

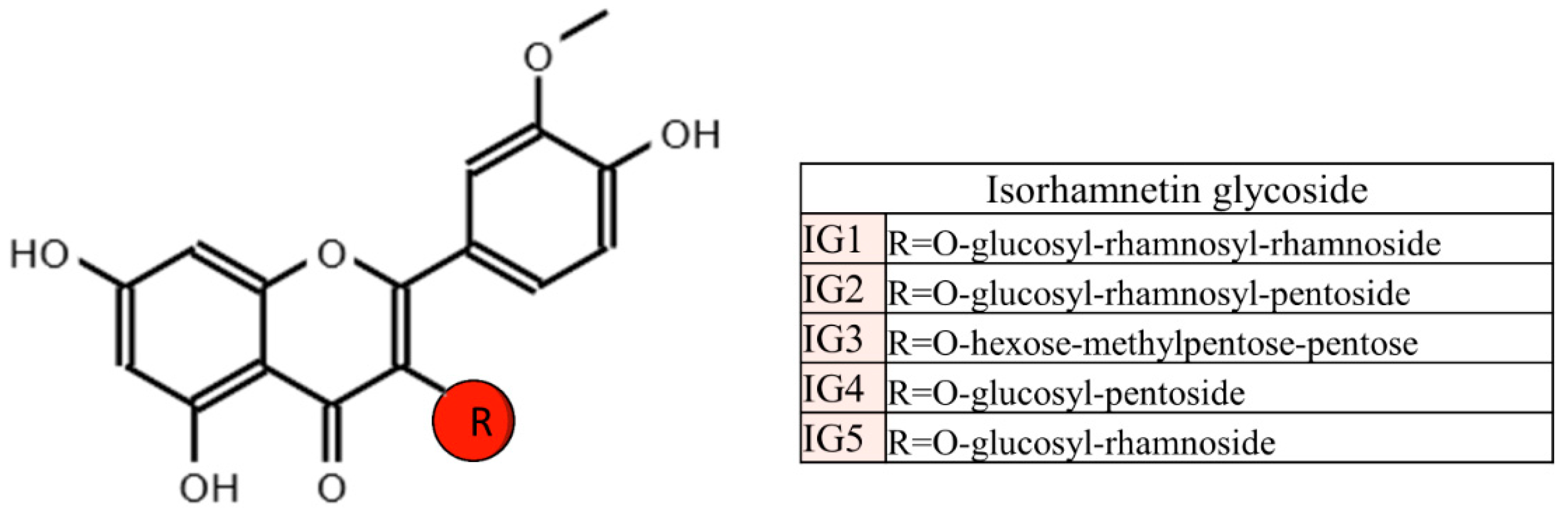
| Food Source or Plant | Mean Content | SD | Ref. |
|---|---|---|---|
| Isorhamnetin | |||
| Yellow onion | 9.31 mg/100 g | 21.65 | [5] |
| Red onion | 1.51 mg/100 g | 2.37 | [5] |
| Red wine | 0.33 mg/100 mL | 0.14 | [5] |
| Almond | 0.14 mg/100 g | 0.13 | [5] |
| Sea-buckthornberry juice | 0.09 mg/100 mL | 0.07 | [5] |
| Ginkgo biloba leaves (seasonal variation) | 23–693 mg/100 g | - | [6] |
| Isorhamnetin 3-O-glucoside | |||
| Pear | 0.45 mg/100 g | 0.42 | [5] |
| Green grapes | 0.17 mg/100 g | 0.19 | [5] |
| Apple | NQ | [7] | |
| Wild strawberry | 1.14 mg/100 mL | 0.12 | [8] |
| Isorhamnetin 3-O-galactoside | |||
| Almonds | 0.58 mg/100 g | 0.19 | [5] |
| Apple | NQ | [7] | |
| Wild strawberry | 0.44 mg/100 mL | 0.05 | [8] |
| Wild blackberry | 1.25 mg/100 mL | 0.1 | [8] |
| Isorhamnetin 3-O-glucoside 7-O-rhamnoside | |||
| Sea-buckthornberry juice | 7.05 mg/100 mL | 0.64 | [5] |
| Isorhamnetin glucoxyl-rhamnosyl-pentoside | |||
| Prickly pear from Opuntia stricta var. dilleni | 5.01 mg/100 g | 0.0 | [2] |
| Isorhamnetin glucoxyl-rhamnosyl-rhamnoside | |||
| Prickly pear from Opuntia dillenii | 0.4 mg/100 g | 0.02 | [2] |
| Isorhamnetin 3-O-hexoside | |||
| Hippophae rhamnoides | 56.25 mg/100 g | 0.1 | [9] |
| Isorhamnetin 3-O-hexoside-deoxyhexoside | |||
| Hippophae rhamnoides | 60.82 mg/100 g | 0.6 | [9] |
| Sweet cherries | 0.09 mg/100 g | 0.03 | [10] |
| Sour cherries | 1.57 mg/100 g | 0.48 | [10] |
| Reference | Compound and Dose | Cell Type and Experimental Design | Effects | Mechanisms of Action |
|---|---|---|---|---|
| Isorhamnetin | ||||
| Lee et al. (2009) [16] | Isorhamnetin 1, 10, 25, 50 μM 25, 50 μM | 3T3-L1 pre-adipocytes The 9 days of the differentiation period Mature adipocytes Treatment of 20 h on day 9 after differentiation | ↓ TG content ↓ Adipocyte differentiation (25 and 50 μM) NS TG content | ↓ Pparγ, C/ebpα, Pgc-1, Lpl, Cd36, Ap2 and Lxr-α gene expression NS C/ebpβ, C/ebpδ and Krox gene expression ↓ GPDH activity ↓ Adiponectin gene and protein expressions ↓ Il-6, Mcp-1 and Pai-1 gene expressions NS Pparγ gene expression |
| Lee et al. (2010) [17] | Isorhamnetin 1, 10, 25, 50 μM | Human mesenchymal stem cells The 21 days of the differentiation period | ↓ Adipocyte differentiation (10, 25 and 50 μM) | ↓ Dkk1, Sfrp1, C/ebpα, Pparγ gene expression NS Sfrp2, Sfrp3, Sfrp4 and Dkk3 gene expression ↓ Lrp5, Lrp6, Fzd4, Fzd6 and Fzd7 gene expression ↑ Total and nuclear β-catenin protein expression ↑ GSK 3β phosphorylation |
| Lee et al. (2011) [18] | Isorhamnetin 50 μM | 3T3-L1 pre-adipocytes The 6 days of the differentiation period | ↓ TG content ↓ Adipocyte differentiation | ↑ Nuclear β-catenin protein expression ↑Wnt/β-catenin signalling activity |
| Zhang et al. (2016) [19] | Isorhamnetin 10, 25 and 50 μM 50 μM | 3T3-L1 pre-adipocytes The 9 days of the differentiation period Treatment at different stages; from day 0, 3 or 6 of differentiation | ↓ TG content ↓Adipocyte differentiation (all doses) ↓ TG content (treatment at day 3 or later) | Treatment at day 0: ↓ C/ebpα, Pparγ and Ap2 gene expression NS Pparγ and C/ebpβ Cd36, Acc, Ucp2, Lpl gene expression Treatment at day 6: ↓ Pparγ, Ap2, Cd36, Fas, Acc, Ucp2 and Lpl gene expression |
| Lee and Kim (2018) [20] | Isorhamnetin 0.1, 0.5, 1, 10, 20, 50 μM for cell viability 0.1, 0.5, 1, 10, 20 μM for TG accumulation | 3T3-L1 pre-adipocytes The 7 days of the differentiation period | ↓ TG content ↓ Adipocyte differentiation (20 μM) | ↓ GPDH activity ↓ Pparγ and Ap2 gene expression |
| Ganbold et al. (2019) [21] | Isorhamnetin 10 and 20 μM | 3T3-L1 pre-adipocytes The 14 days of the differentiation period | ↓ TG content (20 μM) | Not analysed |
| Eseberri et al. (2019) [22] | Isorhamnetin 0.1, 1 and 10 μM | 3T3-L1 pre-adipocytes The 8 days of the differentiation period Mature adipocytes treated on day 12 after differentiation for 24 h | ↓ TG content ↓ Adipocyte differentiation (10 μM) NS TG content | NS C/ebpβ, Srebf1, C/ebpα, Dgat1, Dgat2 ↓ Bcl2 gene expression ↑ Trp3 gene expression NS Cas3 gene expression |
| Conjugated Isorhamnetin | ||||
| Kong and Seo (2012) [23] | Isorhamnetin 3-O-β-d-glucopyranoside 1, 10 and 20 μM | 3T3-L1 pre-adipocytes The 6 days of the differentiation period | ↓ TG content ↓ Adipocyte differentiation (all doses) | ↓ PPARγ, SREBP1, C/EBPα protein expression ↓ FAS, GLUT4, RXRα and leptin protein expression ↑ AMPK activity |
| Im et al. (2017) [24] | Isorhamnetin-3-O-d- glucuronide 5, 10 and 20 μM | 3T3-L1 pre-adipocytes From day 4 to day 8 after differentiation | ↓ TG content | Not analysed |
| Reference | Compound | Experimental Design | Effects | Mechanisms of Action |
|---|---|---|---|---|
| Isorhamnetin | ||||
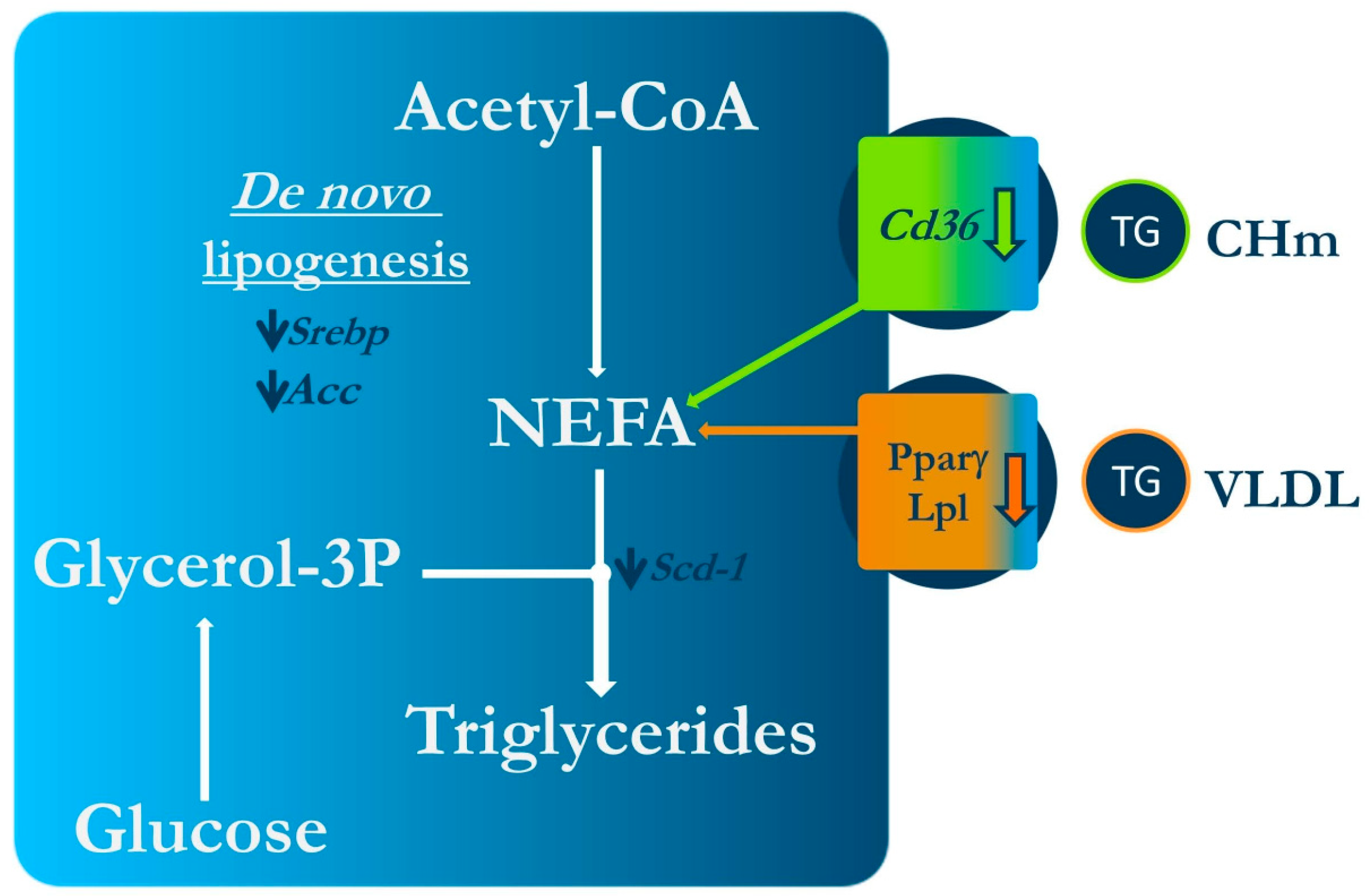
| Zhang et al. (2016) [19] | Isorhamnetin 1% in the diet Isorhamnetin 100 mg/kg BW/day by gavage | Female 7-week-old C57/BL6 mice Standard or high-fat diet (60% energy from fat) Experimental period length: 4 weeks Female 12-week-old C57BL/KsJ-ob/ob mice Standard diet Experimental period length: 2 weeks | ↓ Body weight ↓ Size of visceral adipocytes ↓ Fasting serum glucose and insulin levels and insulin resistance ↓ Leptin resistance ↓ Serum TG, total-cholesterol and FFA NS LDL- and HDL-cholesterol NS Body weight ↑ Glucose tolerance ↓ Serum TG NS serum total-, LDL- and HDL-cholesterol | NS Pparγ, Ap2 and Lpl gene expression ↓ Cd36, Scd1 and Srebp1c gene expression NS FAS gene expression ↓ Pparγ, Ap2 Cd36, Lpl, Acc gene expression |
| Ganbold et al. (2019) [21] | Isorhamnetin 50 mg/kg BW/day | Male 7-week-old C57BL/6J mice with NASH Standard or high-fat diet (60% of energy from fat) Experimental period length: 2 weeks | NS body weight, adipose tissue and adipocyte size NS serum total and HDL-cholesterol | |
| Isorhamnetin glycosides | ||||
| Rodríguez-Rodríguez et al. (2015) [34] | Opuntia fucus-indica extract with isorhamnetin glycosides 0.3% or 0.6% in the diet | Male 8-week-old C57BL/6NCrl mice Standard or high-fat diet (45% of energy from fat) Experimental period length: 12 weeks | Body weight ↑ Energy expenditure ↓ Adipocyte size in visceral and subcutaneous adipose tissues ↓ Serum total cholesterol, LDL-cholesterol and HDL-cholesterol (0.6%) NS Serum TG ↓ Serum glucose and insulin levels and HOMA-IR ↓ Serum leptin | |
| Lacking Gene | Related Alteration | Effect |
|---|---|---|
| Sbp-1 | Lipogenesis | ↓TG |
| Tub-1 | Lipogenesis | ↓TG |
| Nhr-49 | b-oxidation, lipolysis | NS |
| Aak-2 | Energy metabolism | ↓TG |
| Aak-1 and Aak-2 | Energy metabolism | ↓TG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Arceo, M.; Gomez-Lopez, I.; Carr-Ugarte, H.; Eseberri, I.; González, M.; Cano, M.P.; Portillo, M.P.; Gómez-Zorita, S. Anti-Obesity Effects of Isorhamnetin and Isorhamnetin Conjugates. Int. J. Mol. Sci. 2023, 24, 299. https://doi.org/10.3390/ijms24010299
González-Arceo M, Gomez-Lopez I, Carr-Ugarte H, Eseberri I, González M, Cano MP, Portillo MP, Gómez-Zorita S. Anti-Obesity Effects of Isorhamnetin and Isorhamnetin Conjugates. International Journal of Molecular Sciences. 2023; 24(1):299. https://doi.org/10.3390/ijms24010299
Chicago/Turabian StyleGonzález-Arceo, Maitane, Iván Gomez-Lopez, Helen Carr-Ugarte, Itziar Eseberri, Marcela González, M. Pilar Cano, María P. Portillo, and Saioa Gómez-Zorita. 2023. "Anti-Obesity Effects of Isorhamnetin and Isorhamnetin Conjugates" International Journal of Molecular Sciences 24, no. 1: 299. https://doi.org/10.3390/ijms24010299





