Analysis of the Influence of Both the Average Molecular Weight and the Content of Crosslinking Agent on Physicochemical Properties of PVP-Based Hydrogels Developed as Innovative Dressings
Abstract
:1. Introduction
2. Results and Discussion
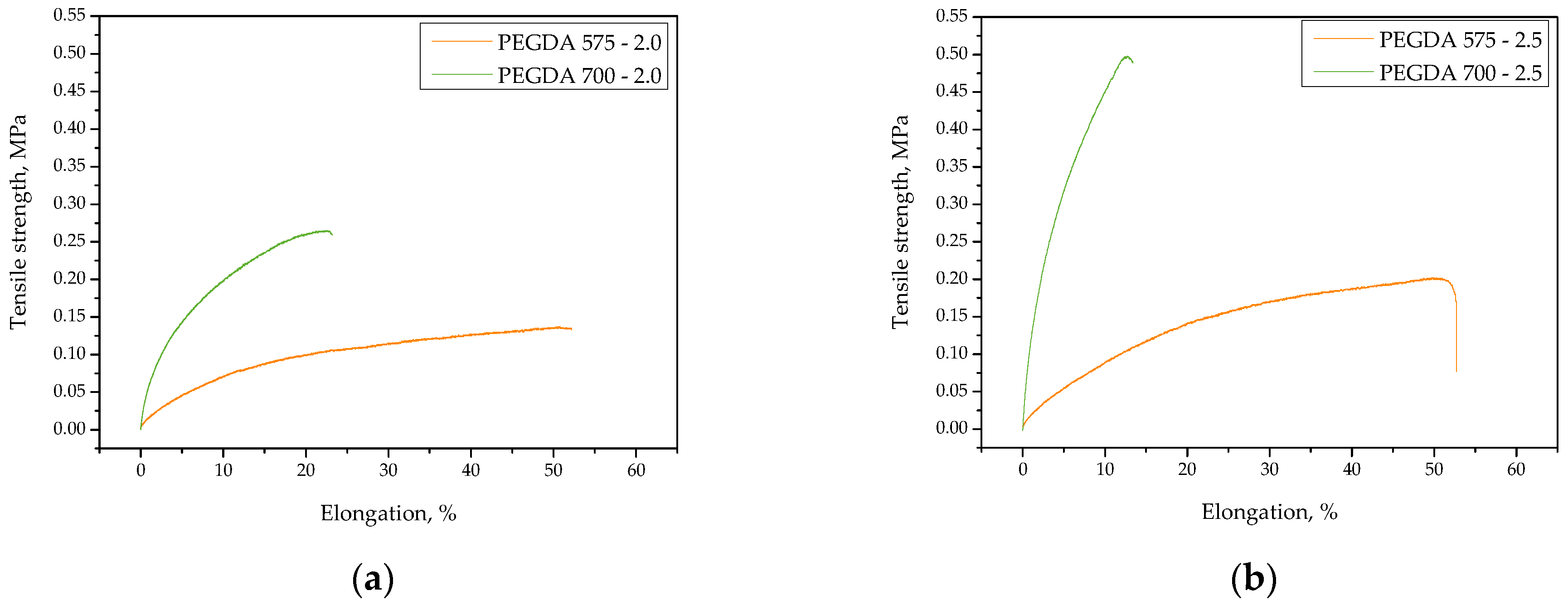
2.1. Studies on the Tensile Strength and Percentage Elongation of the Hydrogels
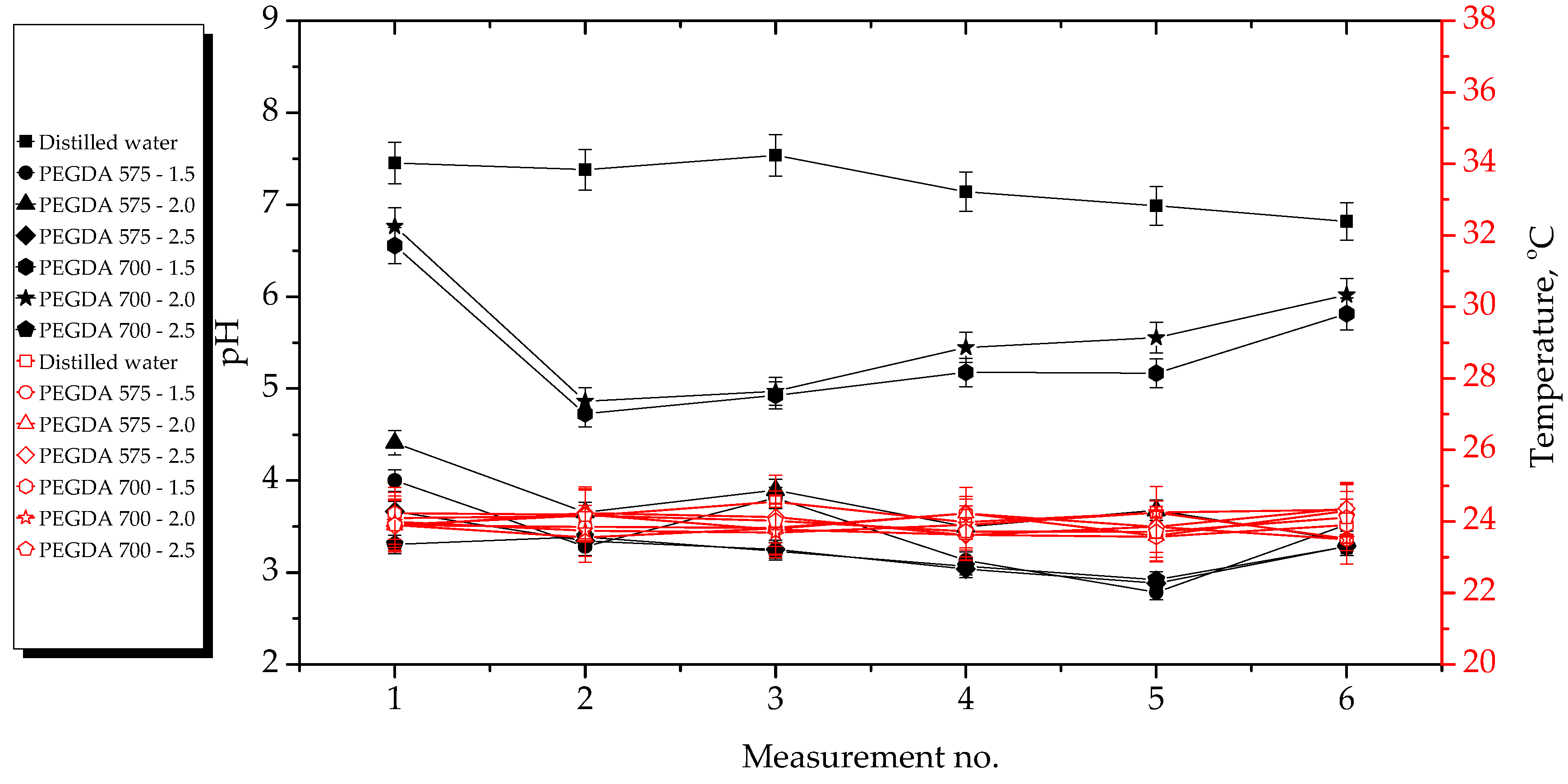
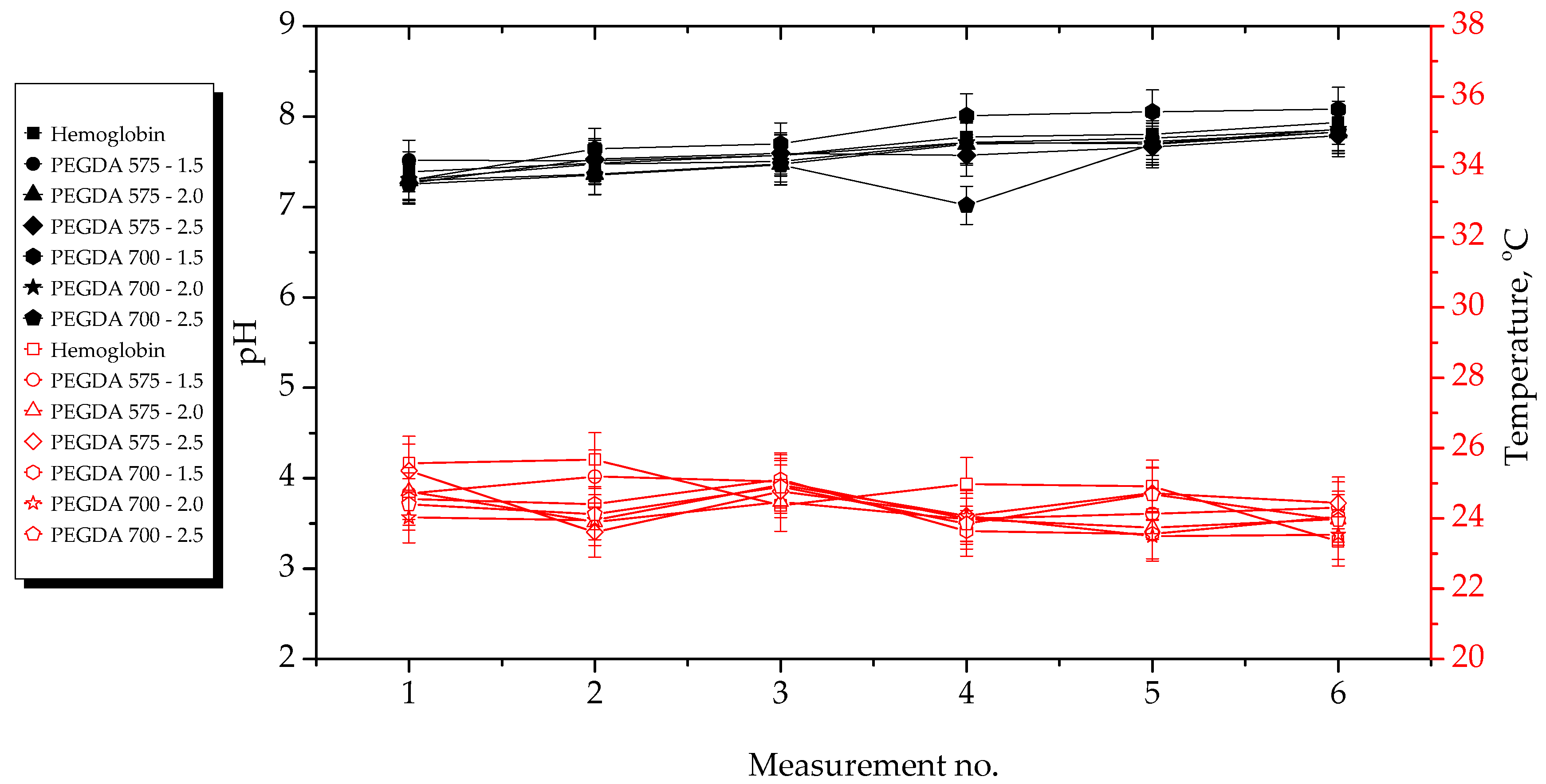
2.2. Results of Incubation Studies
2.3. Analysis of Hydrogel Structure Using FT-IR Spectroscopy
2.4. Sorption Properties of Hydrogels
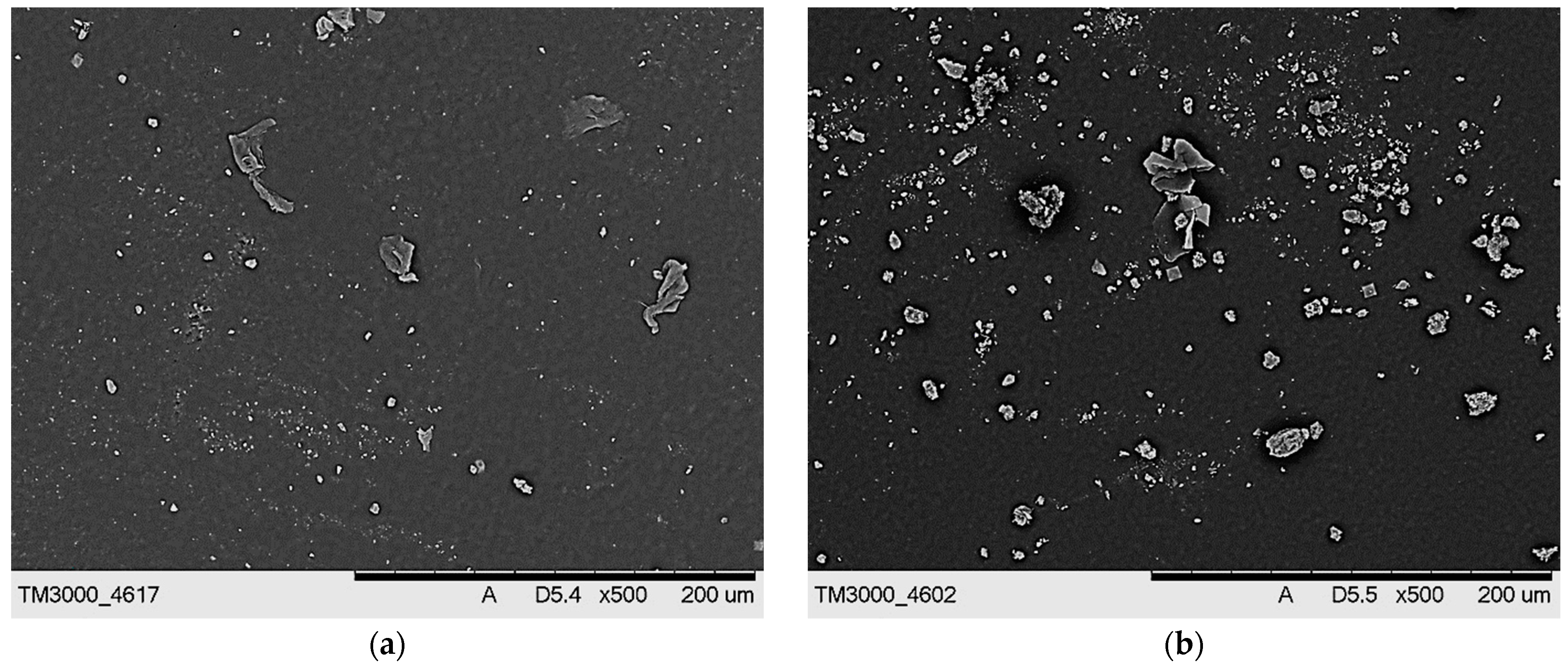
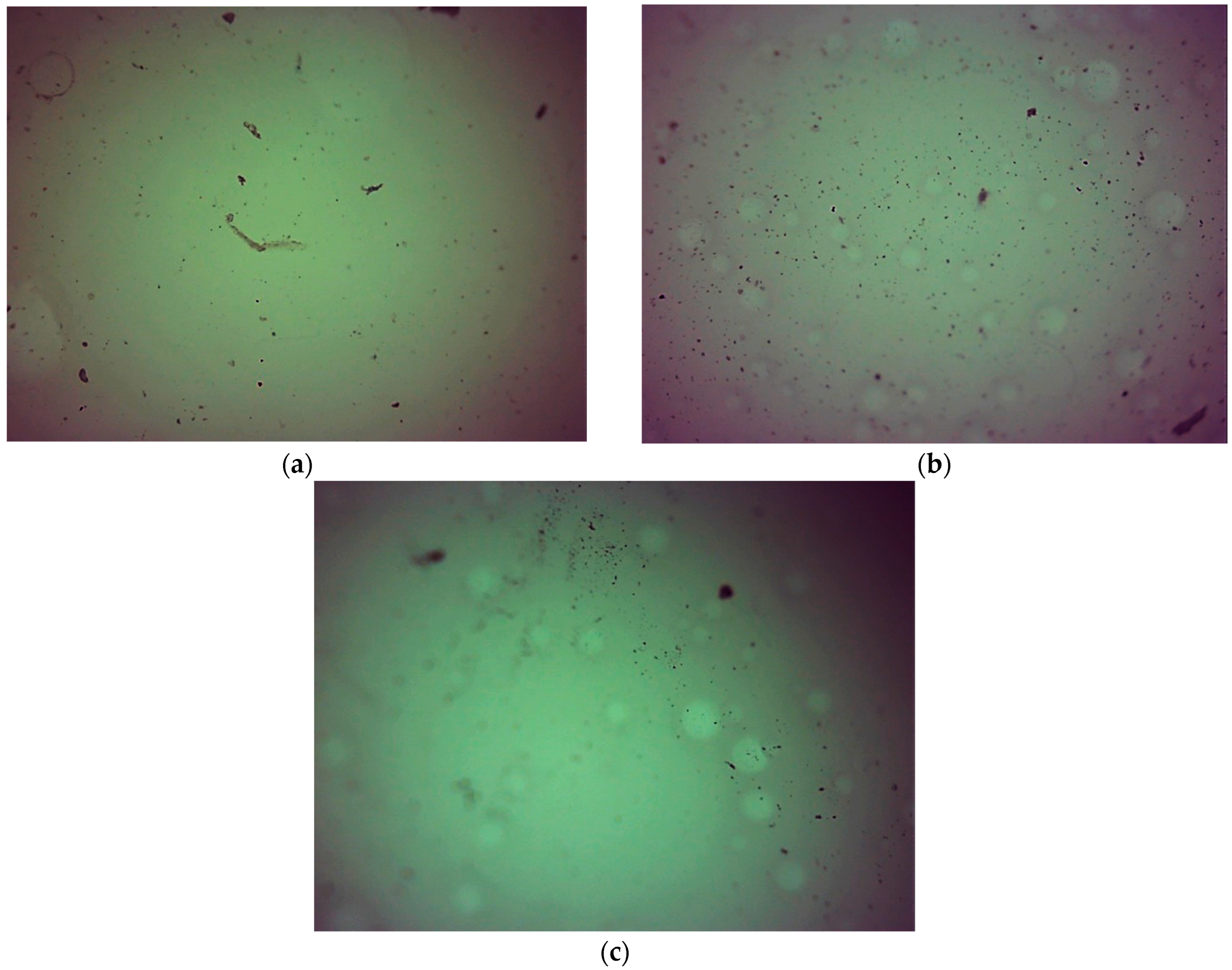
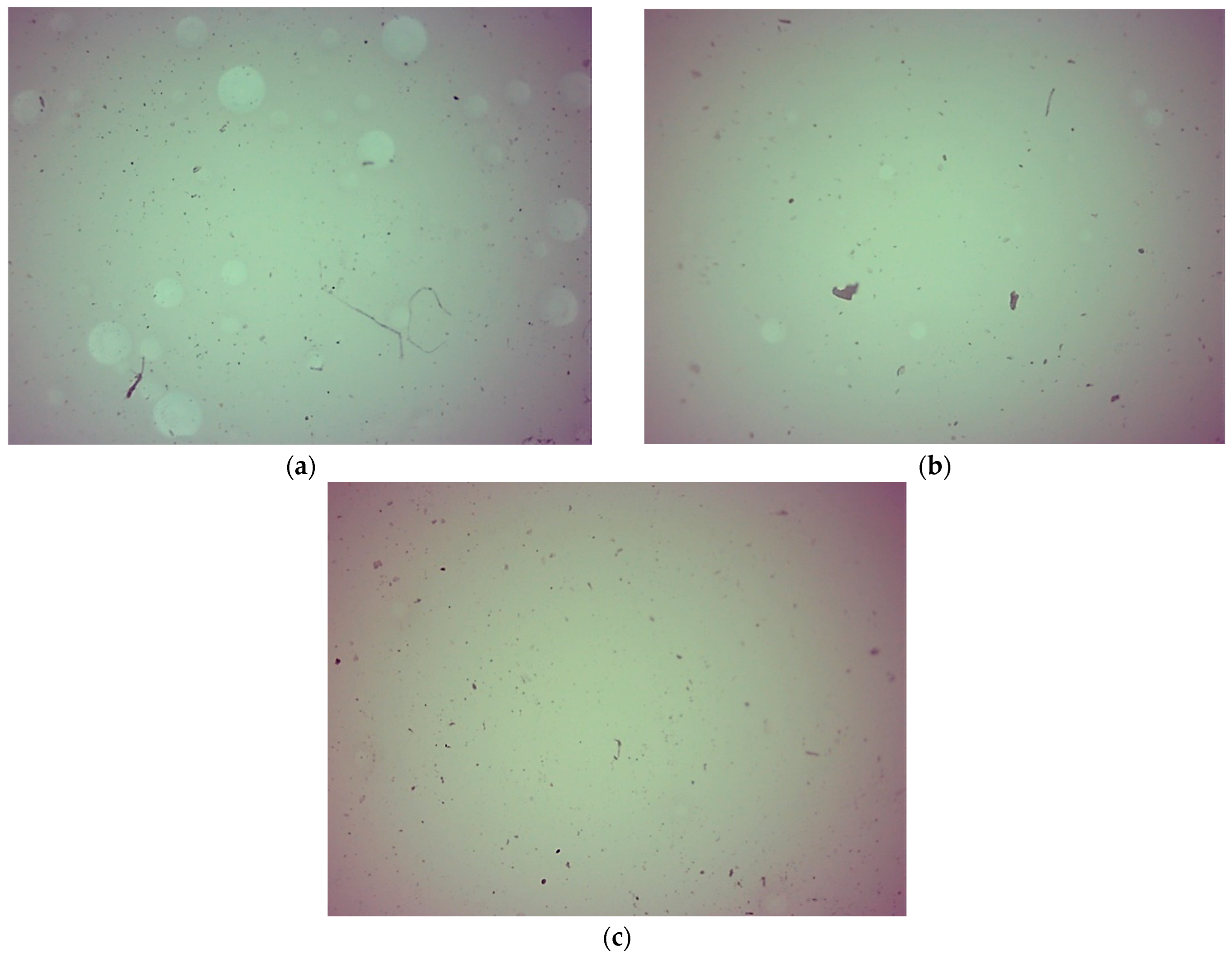
2.5. Characterization of Hydrogels via Microscopic Techniques
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Hydrogel Dressings via UV-Induced Polymerization
3.3. Investigations on the Elongation and Tensile Strength of Hydrogels
3.4. Incubation Studies of Hydrogels in Selected Simulated Physiological Liquids
3.5. Analysis of Hydrogels Using Fourier Transform Infrared (FT-IR) Spectroscopy
3.6. Sorption Properties of Hydrogels
3.7. Microscopic Analysis of Hydrogels
4. Conclusions
- Both the amount of crosslinking agent used during the photopolymerization process and its average molecular weight affected both the tensile strength and percentage elongation of the hydrogels. The more crosslinking agent in the hydrogel matrix, the higher its tensile strength, which was related to the increase in the crosslinking density of the hydrogels. On the other hand, the more crosslinking agent in the hydrogel sample, the less elongation.
- Hydrogels showed the highest stability during incubation in SBF and 2% hemoglobin solution. The most considerable changes in pH values during hydrogel incubation were observed in the case of distilled water. The significant observed pH decrease was probably due to the release of Aloe vera juice from the hydrogel matrices to the incubation medium. In the case of Ringer solution, the changes in pH demonstrated the buffering properties of the hydrogels.
- The absorption band characteristic of the polysaccharides included in Aloe vera juice was disappeared from the FT-IR spectra of the hydrogels after incubation, possibly indicating the release of this modifier from the hydrogel matrix and confirming our assumptions about the reason for the pH decrease in distilled water.
- All hydrogels showed swelling ability. The highest sorption capacity was observed in distilled water due to the lack of divalent ions or proteins that could have increase the crosslinking degree of the hydrogels, thus decreasing their swelling ability, as observed in the case of other tested solutions. The higher the content of the crosslinking agent in the hydrogel, the lower its swelling ability.
- Hydrogels showed similar surface morphology, irrespective of the content of the crosslinking agent and its average molecular weight, probably due to the presence of modifiers (Aloe vera juice and vitamin C) in hydrogel matrices, which filled up the hydrogels’ pores and made their surfaces smooth.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Bercea, M. Poly(vinylpyrrolidone)—A versatile polymer for biomedical and beyond medical applications. Polym. Plast. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Abdelrazek, E.M.; Elashmawi, I.S.; El-Khodary, A.; Yassin, A. Structural, optical, thermal and electrical studies on PVA/PVP blends filled with lithium bromide. Curr. Appl. Phys. 2010, 10, 607–613. [Google Scholar] [CrossRef]
- Mishra, R.; Varshney, R.; Das, N.; Sircar, D.; Roy, P. Synthesis and characterization of gelatin-PVP polymer composite scaffold for potential application in bone tissue engineering. Eur. Polym. J. 2019, 119, 155–168. [Google Scholar] [CrossRef]
- Smaida, R.; Pijnenburg, L.; Irusta, S.; Himawan, E.; Mendoza, G.; Harmouch, E.; Idoux-Gillet, Y.; Kuchler-Bopp, S.; Benkirane-Jessel, N.; Hua, G. Potential Implantable Nanofibrous Biomaterials Combined with Stem Cells for Subchondral Bone Regeneration. Materials 2020, 13, 3087. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, D.; Zhao, K.; Song, K.; Liang, J. Electrohydrodynamic jet 3D printing of PCL/PVP composite scaffold for cell culture. Talanta 2020, 211, 120750. [Google Scholar] [CrossRef] [PubMed]
- Adamo, G.; Grimaldi, N.; Campora, S.; Bulone, D.; Bondì, M.L.; Al-Sheikhly, M.; Sabatino, M.A.; Dispenza, C.; Ghersi, G. Multi-Functional Nanogels for Tumor Targeting and Redox-Sensitive Drug and siRNA Delivery. Molecules 2016, 21, 1594. [Google Scholar] [CrossRef]
- Wójcik-Pastuszka, D.; Potempa, A.; Musiał, W. Bipolymeric Pectin Millibeads Doped with Functional Polymers as Matrices for the Controlled and Targeted Release of Mesalazine. Molecules 2020, 25, 5711. [Google Scholar] [CrossRef]
- Kurakula, M.; Koteswara Rao, G.S.N. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug. Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. The Use of Poly(N-vinyl pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef]
- Hu, Y.; Yan, P.; Fu, L.; Zheng, Y.; Kong, W.; Wu, H.; Yu, X. Polyvinyl alcohol/polyvinyl pyrrolidone crosslinked hydrogels induce wound healing through cell proliferation and migration. J. Biomater. Tissue Eng. 2017, 7, 310–318. [Google Scholar] [CrossRef]
- Pagano, C.; Luzi, F.; Ricci, M.; Michele, A.D.; Puglia, D.; Ceccarini, M.R.; Beccari, T.; Blasi, F.; Cossignani, L.; Schoubben, A.; et al. Wound Dressing: Combination of Acacia Gum/PVP/Cyclic Dextrin in Bioadhesive Patches Loaded with Grape Seed Extract. Pharmaceutics 2022, 14, 485. [Google Scholar] [CrossRef] [PubMed]
- Biazar, E.; Roveimiab, Z.; Shahhosseini, G.; Khataminezhad, M.; Zafari, M.; Majdi, A. Biocompatibility evaluation of a new hydrogel dressing based on polyvinylpyrrolidone/polyethylene glycol. J. Biomed. Biotechnol. 2012, 2012, 343989. [Google Scholar] [CrossRef] [PubMed]
- Contardi, M.; Kossyvaki, D.; Picone, P.; Summa, M.; Guo, X.; Heredia-Guerrero, J.A.; Giacomazza, D.; Carzino, R.; Goldoni, L.; Scoponi, G.; et al. Electrospun polyvinylpyrrolidone (PVP) hydrogels containing hydroxycinnamic acid derivatives as potential wound dressings. Chem. Eng. J. 2021, 409, 128144. [Google Scholar] [CrossRef]
- Sun, S.; Hao, M.; Ding, C.; Zhang, J.; Ding, Q.; Zhang, Y.; Zhao, Y.; Liu, W. SF/PVP nanofiber wound dressings loaded with phlorizin: Preparation, characterization, in vivo and in vitro evaluation. Colloids Surf. B Biointerfaces 2022, 217, 112692. [Google Scholar] [CrossRef]
- Alipour, R.; Khorshidi, A.; Shojaei, A.F.; Mashayekhi, F.; Moghaddam, M.J.M. Skin wound healing acceleration by Ag nanoparticles embedded in PVA/PVP/Pectin/Mafenide acetate composite nanofibers. Polym. Test. 2019, 79, 106022. [Google Scholar] [CrossRef]
- Aytimur, A.; Uslu, I. Promising Materials for Wound Dressing: PVA/PAA/PVP Electrospun Nanofibers. Polym. Plast. Technol. Eng. 2014, 53, 655–660. [Google Scholar] [CrossRef]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. Chitosan-PVP-nano silver oxide wound dressing: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2015, 72, 49–57. [Google Scholar] [CrossRef]
- Poonguzhali, R.; Basha, S.K.; Kumari, S. Novel asymmetric chitosan/PVP/nanocellulose wound dressing: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2018, 112, 1300–1309. [Google Scholar] [CrossRef]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. In vivo evaluation of chitosan–PVP–titanium dioxide nanocomposite as wound dressing material. Carbohydr. Polym. 2013, 95, 530–539. [Google Scholar] [CrossRef]
- Singh, B.; Pal, L. Radiation crosslinking polymerization of sterculia polysaccharide–PVA–PVP for making hydrogel wound dressings. Int. J. Biol. Macromol. 2011, 48, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, L.; Hu, H.; Zhai, N.; Peng, J.; Nho, Y.; Li, J.; Wei, G. Radiation synthesis of PVP/CMC hydrogels as wound dressing. Nucl. Instrum. Methods Phys. Res. B Beam Interact. Mater. At. 2007, 265, 385–389. [Google Scholar] [CrossRef]
- de Lima, G.G.; de Lima, D.W.F.; de Oliveira, M.J.A.; Lugão, A.B.; Alcântara, M.T.S.; Devine, D.M.; de Sá, M.J.C. Synthesis and in vivo Behavior of PVP/CMC/Agar Hydrogel Membranes Impregnated with Silver Nanoparticles for Wound Healing Applications. ACS Appl. Bio Mater. 2018, 1, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, K.; Ding, C.; Sun, S.; Zheng, Y.; Ding, Q.; Hong, B.; Liu, W. Fabrication of chitosan/PVP/dihydroquercetin nanocomposite film for in vitro and in vivo evaluation of wound healing. Int. J. Biol. Macromol. 2022, 206, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Varsei, M.; Tanha, N.R.; Gorji, M.; Mazinani, S. Fabrication and optimization of PCL/PVP nanofibers with Lawsonia inermis for antibacterial wound dressings. Polym. Polym. Compos. 2021, 29, S1403–S1413. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Chen, J.; Garcia, E.S.; Zimmerman, S.C. Intramolecularly Cross-Linked Polymers: From Structure to Function with Applications as Artificial Antibodies and Artificial Enzymes. Acc. Chem. Res. 2020, 53, 1244–1256. [Google Scholar] [CrossRef]
- Ninciuleanu, C.M.; Ianchiş, R.; Alexandrescu, E.; Mihăescu, C.I.; Scomoroşcenco, C.; Nistor, C.L.; Preda, S.; Petcu, C.; Teodorescu, M. The Effects of Monomer, Crosslinking Agent, and Filler Concentrations on the Viscoelastic and Swelling Properties of Poly(methacrylic acid) Hydrogels: A Comparison. Materials 2021, 14, 2305. [Google Scholar] [CrossRef]
- Chavda, H.V.; Patel, C.N. Effect of crosslinker concentration on characteristics of superporous hydrogel. Int. J. Pharm. Investig. 2011, 1, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Huang, X.; Jiang, J.; Shang, S.; Songa, Z. Hydrogels with high mechanical strength cross-linked by a rosin-based crosslinking agent. RSC Adv. 2017, 7, 42541–42548. [Google Scholar] [CrossRef] [Green Version]
- Kopač, T.; Ručigaj, A.; Krajnc, M. The mutual effect of the crosslinker and biopolymer concentration on the desired hydrogel properties. Int. J. Biol. Macromol. 2020, 159, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.H.; Ashton, M.; Dodou, K. Effect of Crosslinking Agent Concentration on the Properties of Unmedicated Hydrogels. Pharmaceutics 2015, 7, 305–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoti, G.; Caldera, F.; Cecone, C.; Rubin Pedrazzo, A.; Anceschi, A.; Appleton, S.L.; Khazaei Monfared, Y.; Trotta, F. Effect of the Cross-Linking Density on the Swelling and Rheological Behavior of Ester-Bridged β-Cyclodextrin Nanosponges. Materials 2021, 14, 478. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ranjha, N.M. Effect of degree of cross-linking on swelling and on drug release of low viscous chitosan/poly(vinyl alcohol) hydrogels. Polym. Bull. 2014, 71, 2133–2158. [Google Scholar] [CrossRef]
- Bai, J.; Wang, R.; Wang, X.; Liu, S.; Wang, X.; Ma, J.; Qin, Z.; Jiao, T. Biomineral calcium-ion-mediated conductive hydrogels with high stretchability and self-adhesiveness for sensitive iontronic sensors. Cell Rep. Phys. Sci. 2021, 2, 100623. [Google Scholar] [CrossRef]
- Rahman, S.; Islam, M.; Islam, S.; Zaman, A.; Ahmed, T.; Biswas, S.; Sharmeen, S.; Rashid, T.U.; Rahman, M.M. Morphological characterization of hydrogels. In Cellulose-Based Superabsorbent Hydrogels. Polymers and Polymeric Composites: A; Mondal, M.I., Ed.; Springer: Cham, Switzerland; New York, NY, USA, 2019; pp. 819–863. [Google Scholar]



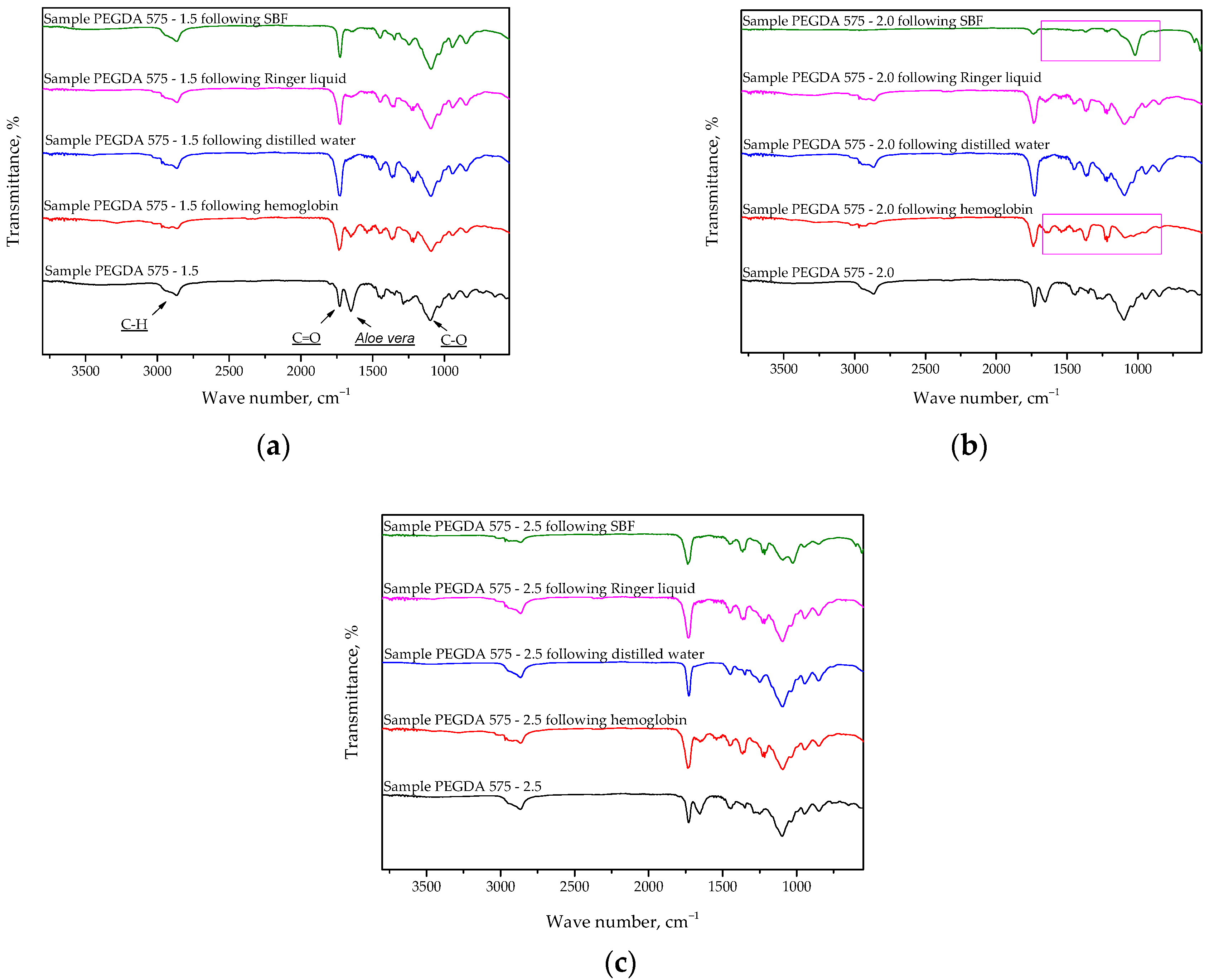





| Wavenumber, cm−1 | Chemical Bond | Type of Vibration |
|---|---|---|
| 2850 | C-H | Stretching |
| 1750 | C=O | Stretching |
| 1100 | C-O | Stretching |
| Sample * | 15% PVP Solution, mL | Aloe vera Juice, mL | 5% Vitamin C Solution, mL | PEGDA 575, mL | PEGDA 700, mL | Photoinitiator, mL |
|---|---|---|---|---|---|---|
| PEGDA 575—1.5 | 7.0 | 3.0 | 2.0 | 1.5 | - | 0.25 |
| PEGDA 575—2.0 | 2.0 | |||||
| PEGDA 575—2.5 | 2.5 | |||||
| PEGDA 700—1.5 | - | 1.5 | ||||
| PEGDA 700—2.0 | 2.0 | |||||
| PEGDA 700—2.5 | 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kędzierska, M.; Jamroży, M.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Bańkosz, M.; Gruca, M.; Potemski, P.; Tyliszczak, B. Analysis of the Influence of Both the Average Molecular Weight and the Content of Crosslinking Agent on Physicochemical Properties of PVP-Based Hydrogels Developed as Innovative Dressings. Int. J. Mol. Sci. 2022, 23, 11618. https://doi.org/10.3390/ijms231911618
Kędzierska M, Jamroży M, Drabczyk A, Kudłacik-Kramarczyk S, Bańkosz M, Gruca M, Potemski P, Tyliszczak B. Analysis of the Influence of Both the Average Molecular Weight and the Content of Crosslinking Agent on Physicochemical Properties of PVP-Based Hydrogels Developed as Innovative Dressings. International Journal of Molecular Sciences. 2022; 23(19):11618. https://doi.org/10.3390/ijms231911618
Chicago/Turabian StyleKędzierska, Magdalena, Mateusz Jamroży, Anna Drabczyk, Sonia Kudłacik-Kramarczyk, Magdalena Bańkosz, Mateusz Gruca, Piotr Potemski, and Bożena Tyliszczak. 2022. "Analysis of the Influence of Both the Average Molecular Weight and the Content of Crosslinking Agent on Physicochemical Properties of PVP-Based Hydrogels Developed as Innovative Dressings" International Journal of Molecular Sciences 23, no. 19: 11618. https://doi.org/10.3390/ijms231911618





