Investigation of Intestinal Absorption and Excretion of Paracetamol in Streptozotocin-Induced Hyperglycemia
Abstract
1. Introduction
2. Results
2.1. Validation of a Chromatographic Method to Determine the Metabolites in Perfusate
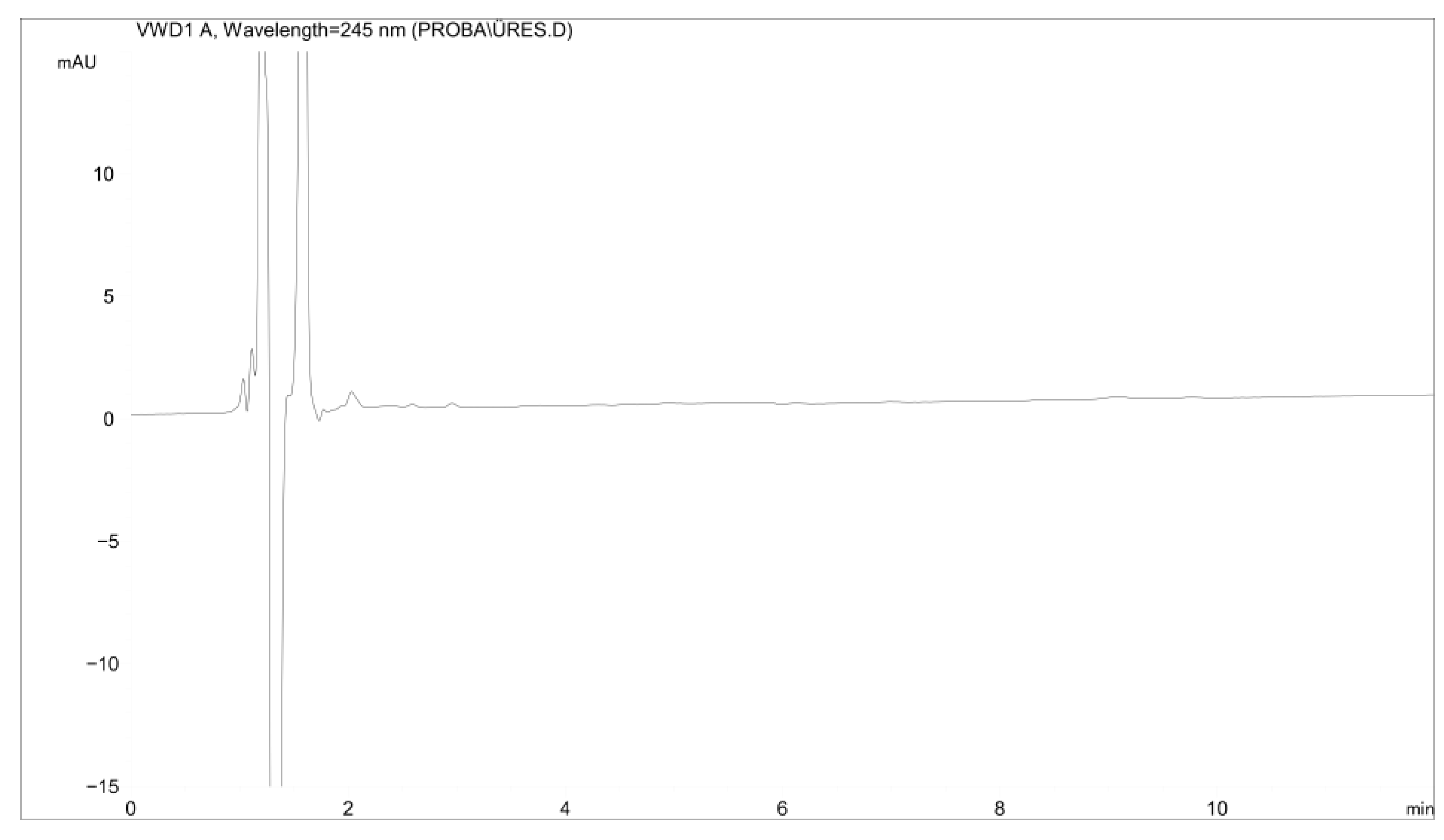
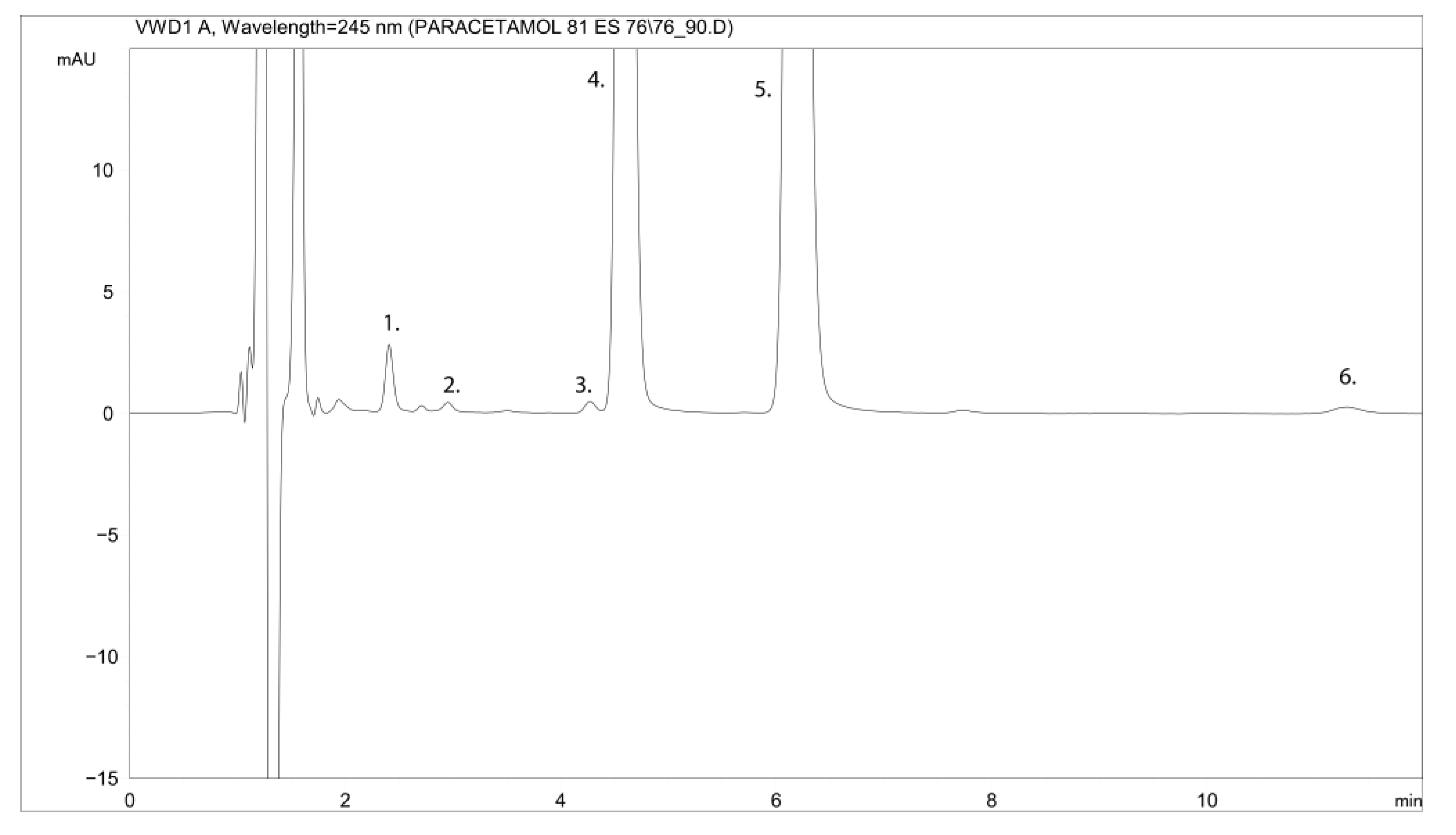
2.1.1. Specificity
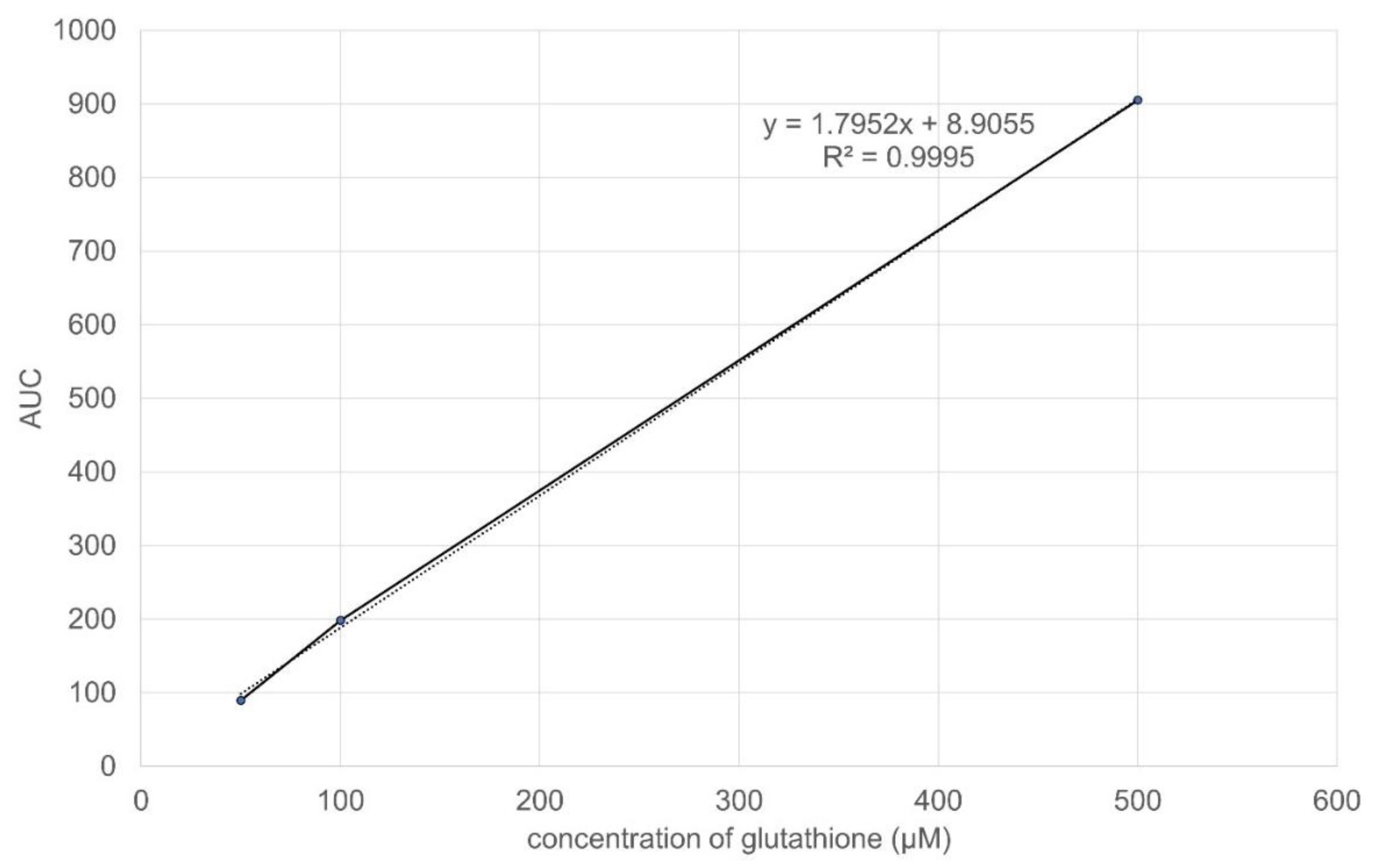
2.1.2. Linearity
2.1.3. Precision
2.1.4. Determination of the Limits of Detection and Quantification
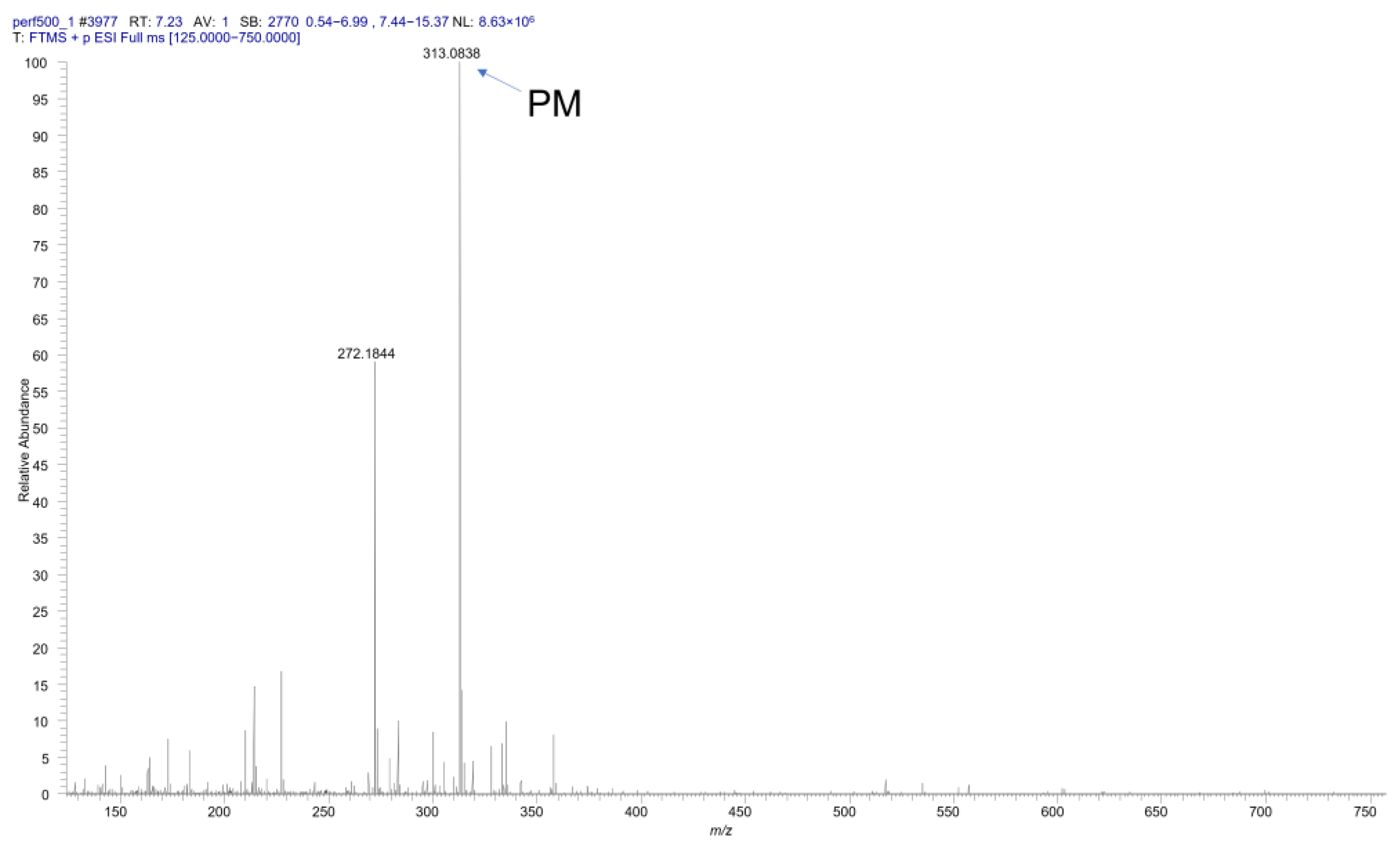
2.1.5. Identification of the Metabolites by HRMS Measurement
2.1.6. Calculations and Statistical Analysis
2.2. Figures
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals and Experimental Procedure
4.3. HPLC and MS Measurements to Determine the Metabolites in Perfusate
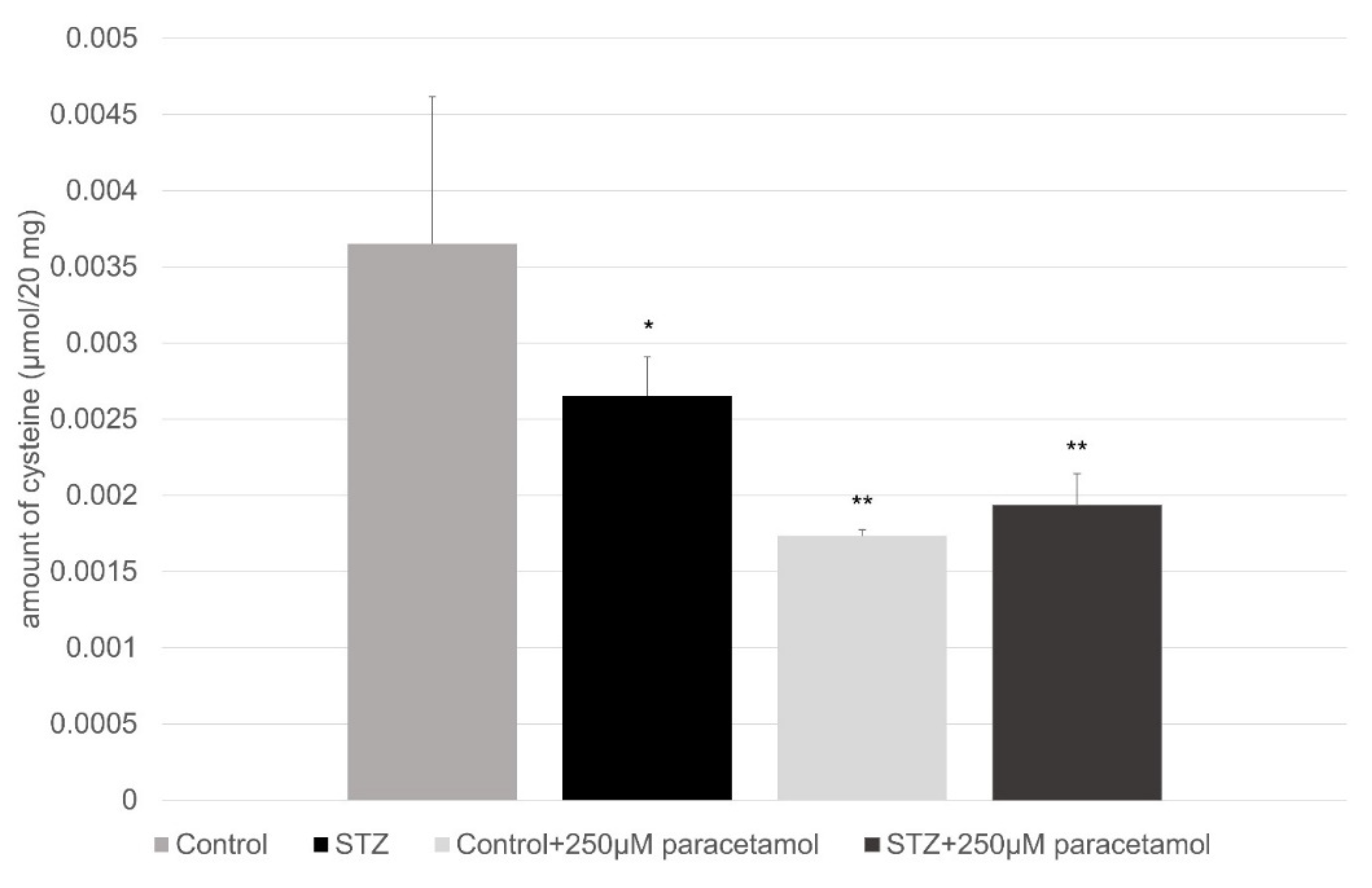
4.4. HPLC Measurement to Determine the Amount of Cysteine and Glutathione
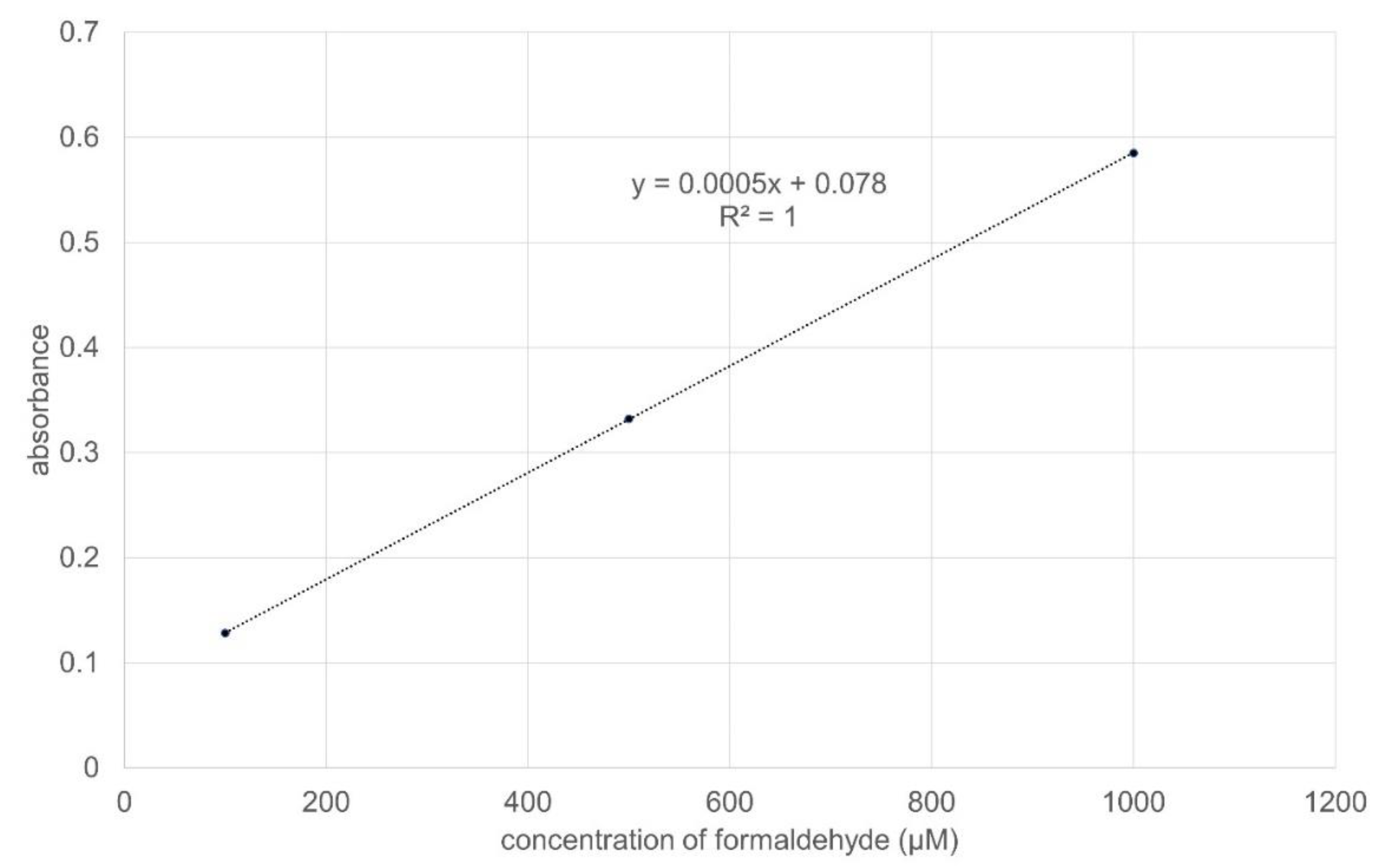
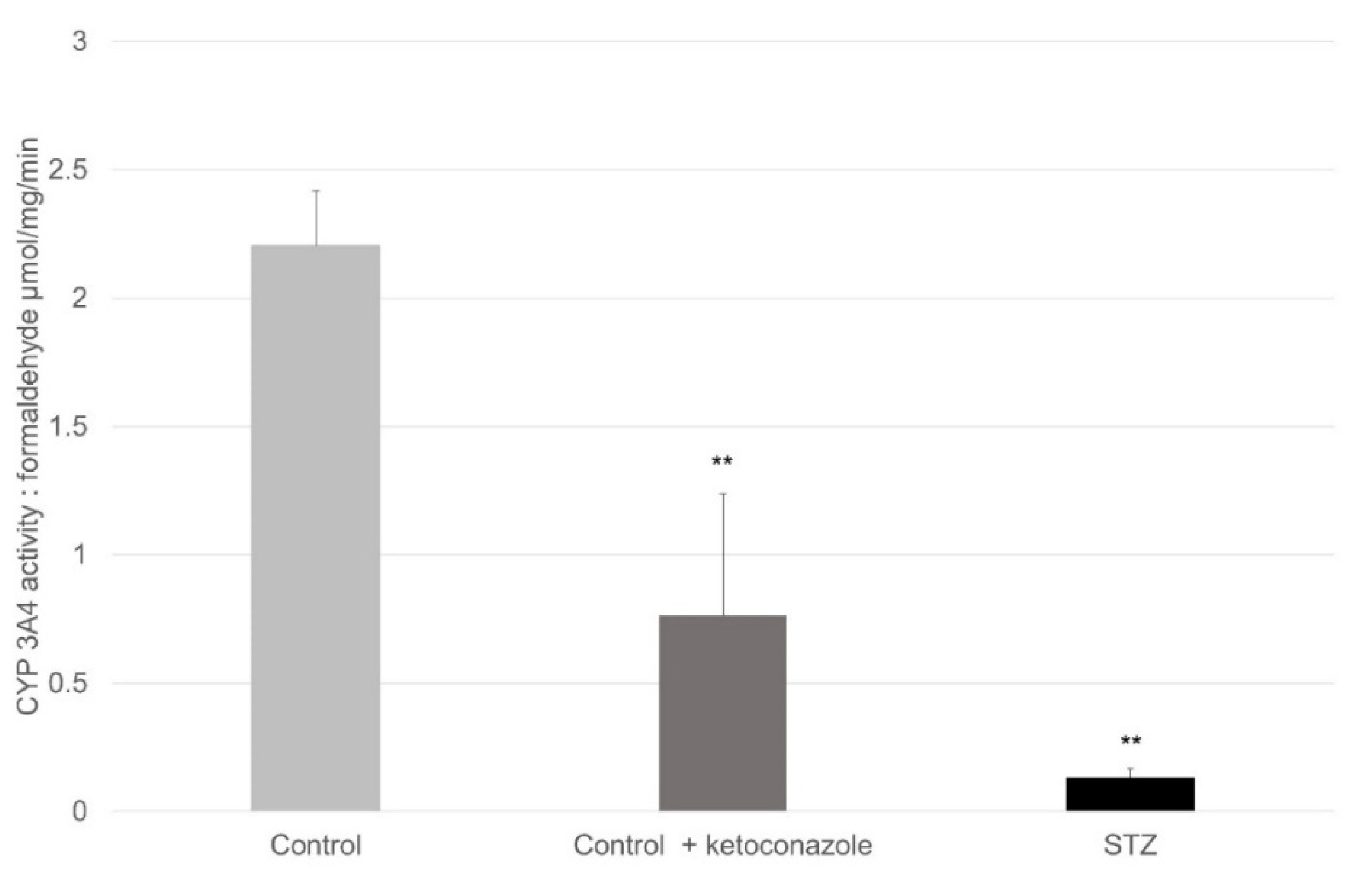
4.5. Spectrophotometric Method to Determine CYP 3A4 Activity in the Small Intestine
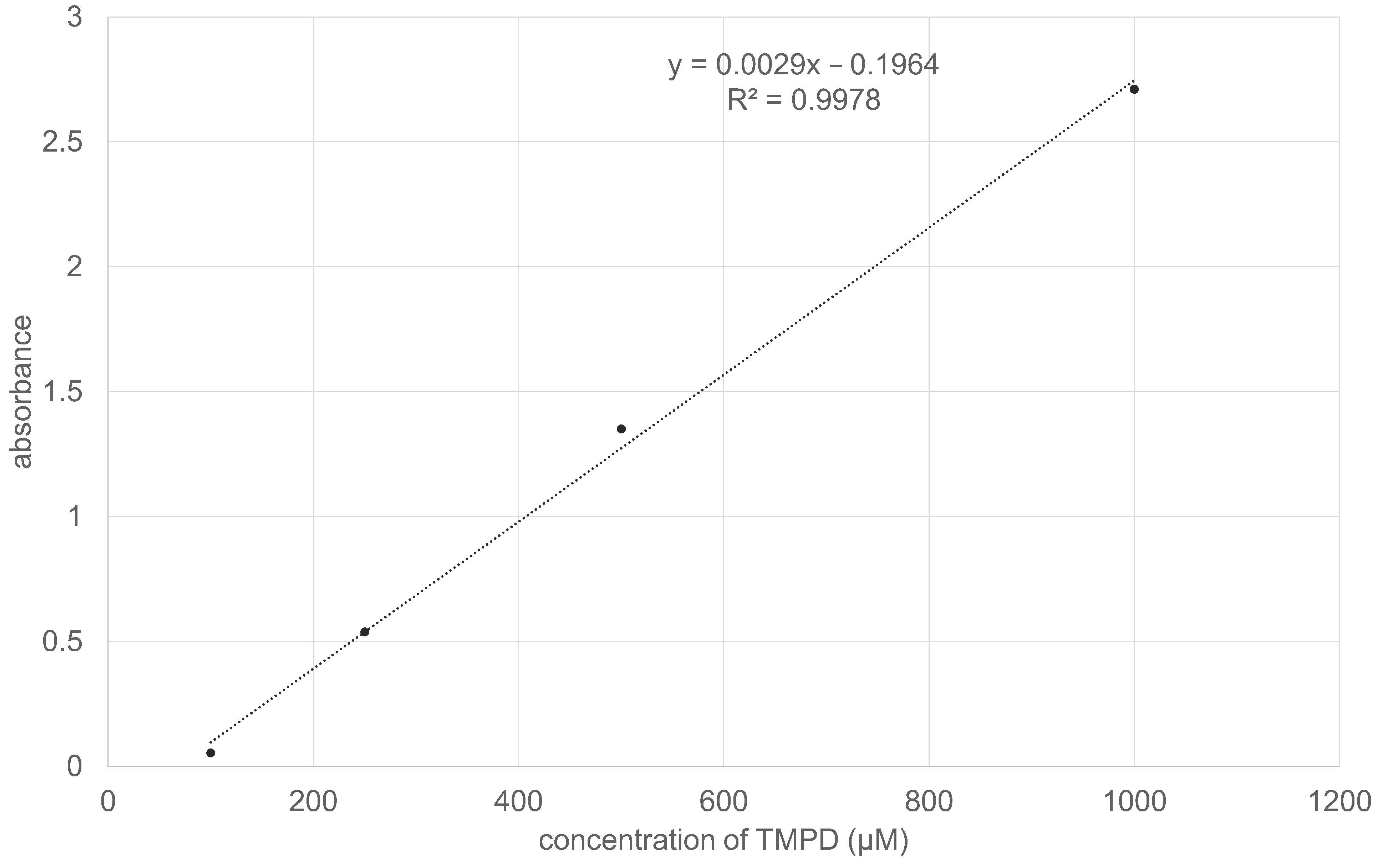
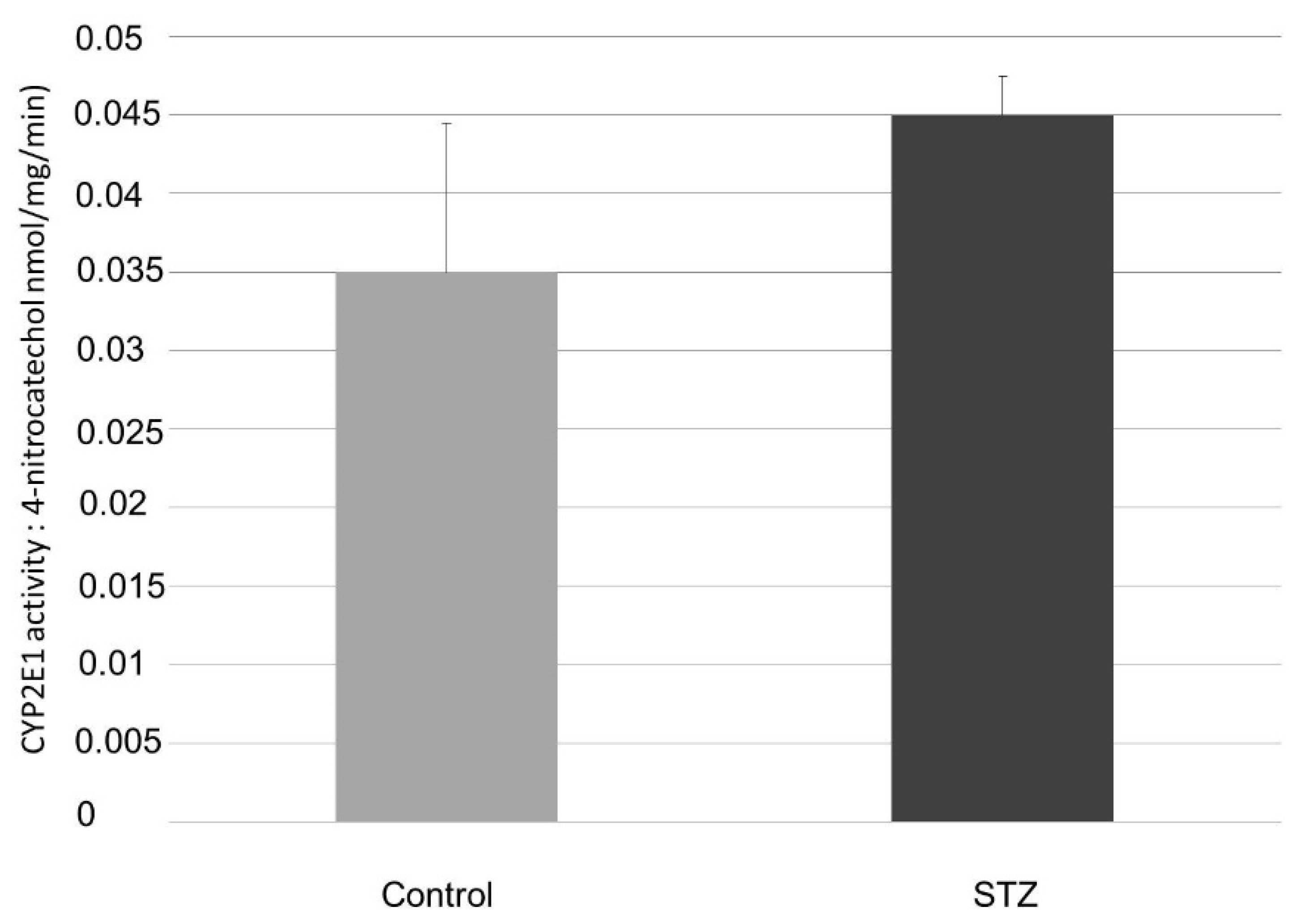
4.6. HPLC Measurement to Determine CYP2E1 Activity in the Small Intestine
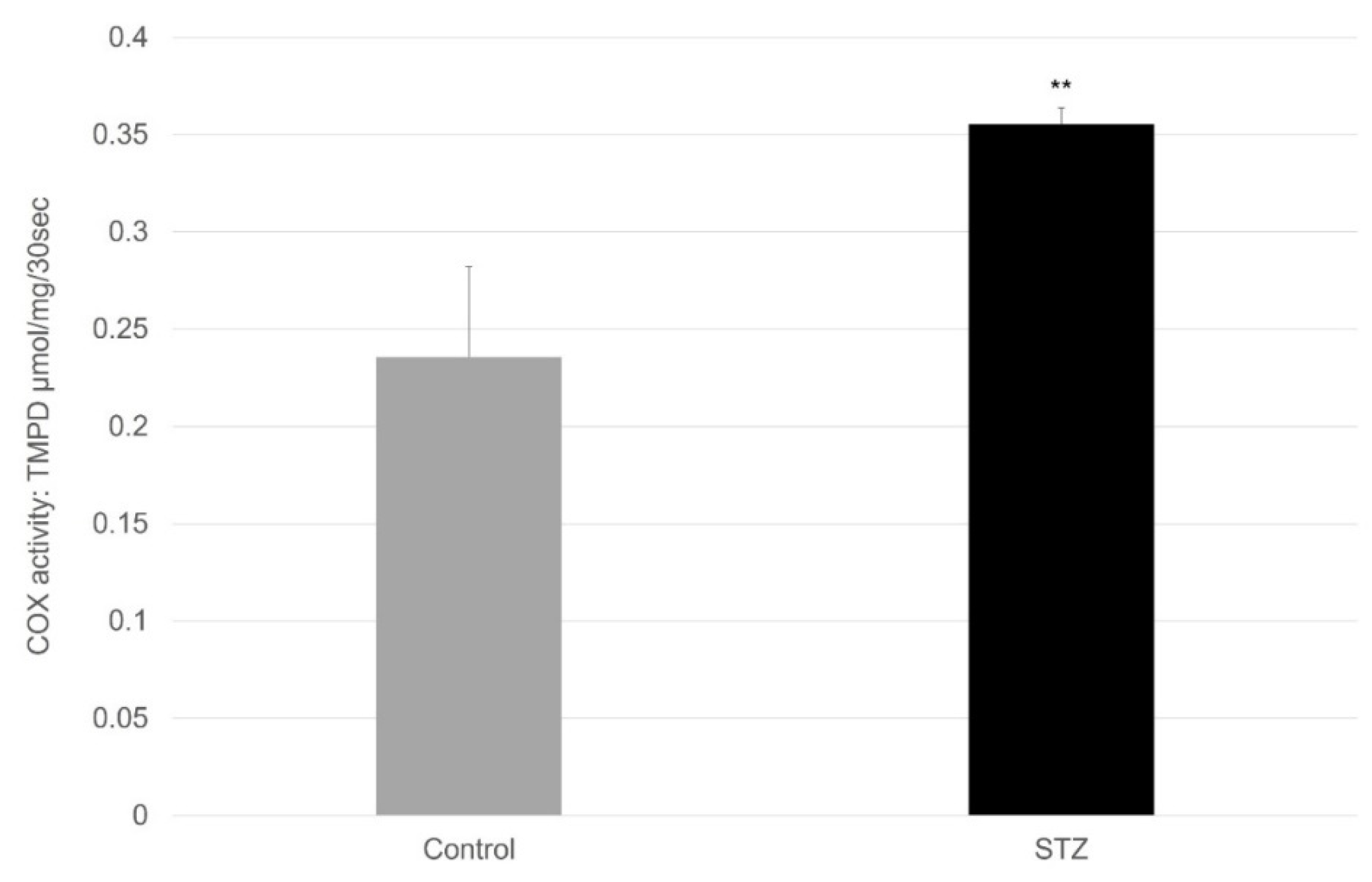
4.7. Spectrophotometric Method to Determine COX Activity in the Small Intestine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A





| Concentration of Standard Solution (µM) Paracetamol | Intra-Day Precision of Retention Time (RSD %) (n = 5) | Intra-Day Precision of Peak Areas (RSD %) (n = 5) | Day-to-Day Precision of Retention Time (RSD %) (n = 5) | Day-to-Day Precision of Peak Areas (RSD %) (n = 5) |
|---|---|---|---|---|
| 10 | 0.79 | 1.47 | 1.06 | 1.63 |
| 50 | 0.09 | 0.22 | 1.55 | 4.24 |
| 100 | 0.48 | 0.05 | 1.65 | 2.39 |
| 500 | 0.15 | 0.14 | 1.57 | 3.64 |
| 1000 | 0.25 | 0.1 | 1.28 | 7.30 |
| Concentration of Standard Solution (µM) Paracetamol β-D-glucuronide | Intra-Day Precision of Retention Time (RSD %) (n = 5) | Intra-Day Precision of Peak Areas (RSD %) (n = 5) | Day-to-Day Precision of Retention Time (RSD %) (n = 5) | Day-to-Day Precision of Peak Areas (RSD %) (n = 5) |
|---|---|---|---|---|
| 2 | 0.64 | 1.69 | 0.81 | 1.20 |
| 5 | 0.12 | 0.78 | 1.11 | 4.83 |
| 10 | 0.36 | 0.14 | 1.21 | 2.73 |
| 50 | 0.16 | 0.23 | 1.03 | 3.98 |
| 100 | 0.18 | 0.15 | 0.87 | 8.06 |
| Concentration of Standard Solution (µM) Paracetamol Sulphate | Intra-Day Precision of Retention Time (RSD %) (n = 5) | Intra-Day Precision of Peak Areas (RSD %) (n = 5) | Day-to-Day Precision of Retention Time (RSD %) (n = 5) | Day-to-Day Precision of Peak Areas (RSD %) (n = 5) |
|---|---|---|---|---|
| 2 | 0.80 | 2.62 | 1.29 | 2.31 |
| 4 | 0.19 | 1.38 | 2.03 | 4.05 |
| 6 | 0.60 | 0.75 | 2.13 | 2.80 |
| 8 | 0.15 | 0.60 | 1.70 | 3.90 |
| 10 | 0.30 | 0.22 | 1.60 | 8.35 |
| Concentration of Standard Solution (µM) Paracetamol Cysteine | Intra-Day Precision of Retention Time (RSD %) (n = 5) | Intra-Day Precision of Peak Areas (RSD %) (n = 5) | Day-to-Day Precision of Retention Time (RSD %) (n = 5) | Day-to-Day Precision of Peak Areas (RSD %) (n = 5) |
|---|---|---|---|---|
| 2 | 0.92 | 1.81 | 1.27 | 1.69 |
| 4 | 0.11 | 0.39 | 1.77 | 4.65 |
| 6 | 0.59 | 0.64 | 1.92 | 3.01 |
| 8 | 0.16 | 1.28 | 1.64 | 4.40 |
| 10 | 0.29 | 0.66 | 1.47 | 8.12 |
| Concentration of Standard Solution (µM) Paracetamol Mercapturate | Intra-Day Precision of Retention Time (RSD %) (n = 5) | Intra-Day Precision of Peak Areas (RSD %) (n = 5) | Day-to-Day Precision of Retention Time (RSD %) (n = 5) | Day-to-Day Precision of Peak Areas (RSD %) (n = 5) |
|---|---|---|---|---|
| 2 | 0.89 | 0.75 | 1.80 | 3.13 |
| 4 | 0.11 | 1.21 | 2.54 | 5.62 |
| 6 | 0.69 | 0.17 | 2.72 | 2.81 |
| 8 | 0.25 | 0.13 | 1.97 | 4.10 |
| 10 | 0.37 | 0.11 | 1.73 | 8.30 |
References
- Almási, A.; Fischer, E.; Perjési, P. A simple and rapid ion-pair HPLC method for simultaneous quantitation of 4-nitrophenol and its glucuronide and sulfate conjugates. J. Biochem. Biophys. Methods 2006, 69, 43–50. [Google Scholar] [CrossRef]
- Chen, W.; Koenigs, L.L.; Thompson, S.J.; Peter, R.M.; Rettie, A.E.; Trager, W.F.; Nelson, S.D. Oxidation of acetaminophen to its toxic quinone imine and nontoxic catechol metabolites by baculovirus-expressed and purified human cytochromes P450 2E1 and 2A6. Chem. Res Toxicol. 1998, 11, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.; Almási, A.; Bojcsev, S.; Fischer, T.; Kovács, N.P.; Perjési, P. Effect of experimental diabetes and insulin replacement on intestinal metabolism and excretion of 4-nitrophenol in rats. Can. J. Physiol. Pharmacol. 2015, 93, 459–464. [Google Scholar] [CrossRef]
- Kovács, N.P.; Almási, A.; Garai, K.; Kuzma, M.; Vancea, S.; Fischer, E.; Perjési, P. Investigation of intestinal elimination and biliary excretion of ibuprofen in hyperglycemic rats. Can. J. Physiol. Pharmacol. 2019, 97, 1080–1089. [Google Scholar] [CrossRef]
- Mohammed, H.O.; Almási, A.; Molnár, S.; Perjési, P. The Intestinal and Biliary Metabolites of Ibuprofen in the Rat with Experimental Hyperglycemia. Molecules 2022, 27, 4000. [Google Scholar] [CrossRef]
- Fritz, A.; Busch, D.; Lapczuk, J.; Ostrowski, M.; Drozdzik, M.; Oswald, S. Expression of clinically relevant drug-metabolizing enzymes along the human intestine and their correlation to drug transporters and nuclear receptors: An intra-subject analysis. Basic Clin. Pharmacol. Toxicol. 2019, 124, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.S.; Zhang, Q.Y. The small intestine as a xenobiotic-metabolizing organ. Drug Metab. Dispos. 2003, 31, 1520–1525. [Google Scholar] [CrossRef]
- Aminoshariae, A.; Khan, A. Acetaminophen: Old Drug, New Issues. J. Endod. 2015, 41, 588–593. [Google Scholar] [CrossRef]
- Bertolini, A.; Ferrari, A.; Ottani, A.; Guerzoni, S.; Tacchi, R.; Leone, S. Paracetamol: New Vistas of an Old Drug. CNS Drug Rev. 2006, 12, 250–275. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, S.S. Paracetamol (acetaminophen): A familiar drug with an unexplained mechanism of action. Temperature 2021, 8, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Przybyła, G.W.; Szychowski, K.A.; Gmiński, J. Paracetamol—An old drug with new mechanisms of action. Clin. Exp. Pharmacol. Physiol. 2020, 48, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.V.; Mehta, V. Paracetamol: Mechanisms and updates. Contin. Educ. Anaesth. Crit. Care Pain 2014, 14, 153–158. [Google Scholar] [CrossRef]
- Toussaint, K.; Yang, X.C.; Zielinski, M.A.; Reigle, K.L.; Sacavage, S.D.; Nagar, S.; Raffa, R.B. What do we (not) know about how paracetamol (acetaminophen) works? J. Clin. Pharm. Ther. 2010, 35, 617–638. [Google Scholar] [CrossRef]
- Prescott, L. Kinetics and metabolism of paracetamol and phenacetin. Br. J. Clin. Pharmacol. 1980, 10, 291S–298S. [Google Scholar] [CrossRef]
- Raffa, R.B.; Pergolizzi, J.V.; Taylor, R.; Decker, J.F.; Patrick, J.T. Acetaminophen (Paracetamol) Oral Absorption and Clinical Influences. Pain Pract. 2014, 14, 668–677. [Google Scholar] [CrossRef]
- Forrest, J.A.H.; Clements, J.A.; Prescott, L.F. Clinical Pharmacokinetics of Paracetamol. Clin. Pharmacokinet. 1982, 7, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Mazaleuskaya, L.L.; Sangkuhl, K.; Thorn, C.F.; FitzGerald, G.A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Pathways of acetaminophen metabolism at the therapeutic versus toxic doses. Pharmacogenet. Genom. 2015, 25, 416–426. [Google Scholar] [CrossRef]
- McGill, M.R.; Jaeschke, H. Metabolism and Disposition of Acetaminophen: Recent Advances in Relation to Hepatotoxicity and Diagnosis. Pharm. Res. 2013, 30, 2174–2187. [Google Scholar] [CrossRef]
- Graham, G.G.; Scott, K.F.; Day, R.O. Tolerability of Paracetamol. Drug Saf. 2005, 28, 227–240. [Google Scholar] [CrossRef]
- Lauterburg, B.H. Analgesics and Glutathione. Am. J. Ther. 2002, 9, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.G.; Davies, M.J.; Day, R.O.; Mohamudally, A.; Scott, K.F. The modern pharmacology of paracetamol: Therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology 2013, 21, 201–232. [Google Scholar] [CrossRef] [PubMed]
- Poltorack, C.D.; Dixon, S.J. Understanding the role of cysteine in ferroptosis: Progress & paradoxes. FEBS J. 2022, 289, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- Callaghan, M.J.; Ceradini, D.J.; Gurtner, G.C. Hyperglycemia-induced reactive oxygen species and impaired endothelial progenitor cell function. Antioxid. Redox. Signal. 2005, 7, 1476–1482. [Google Scholar] [CrossRef]
- Tesauro, M.; Nisticò, S.; Noce, A.; Tarantino, A.; Marrone, G.; Costa, A.; Rovella, V.; Di Cola, G.; Campia, U.; Lauro, D.; et al. The possible role of glutathione-S-transferase activity in diabetic nephropathy. Int. J. Immunopathol. Pharmacol. 2015, 28, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.J.; Pimpão, A.B.; Fernandes, D.G.F.; Morello, J.; Sequeira, C.O.; Calado, J.; Antunes, A.M.M.; Almeida, M.S.; Branco, P.; Monteiro, E.C.; et al. Cysteine as a Multifaceted Player in Kidney, the Cysteine-Related Thiolome and Its Implications for Precision Medicine. Molecules 2022, 27, 1416. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmac. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar] [PubMed]
- Brunner, L.J.; Bai, S. Simple and rapid assay for acetaminophen and conjugated metabolites in low-volume serum samples. J. Chromatogr. B Biomed. Sci. Appl. 1999, 732, 323–329. [Google Scholar] [CrossRef]
- Jensen, L.S.; Valentine, J.; Milne, R.W.; Evans, A.M. The quantification of paracetamol, paracetamol glucuronide and paracetamol sulphate in plasma and urine using a single high-performance liquid chromatography assay. J. Pharm. Biomed. Anal. 2004, 34, 585–593. [Google Scholar] [CrossRef]
- Vertzoni, M.V.; Archontaki, H.A.; Galanopoulou, P. Development and optimization of a reversed-phase high-performance liquid chromatographic method for the determination of acetaminophen and its major metabolites in rabbit plasma and urine after a toxic dose. J. Pharm. Biomed. Anal. 2003, 32, 487–493. [Google Scholar] [CrossRef]
- Alkhawaja, B.; Arafat, T.; Mallah, E.; Qinna, N.; Idkaidek, N.; Dayyih, W.A.; Al-Hroub, H.; Badwan, A.A. Simultaneous determination of paracetamol and its metabolites in rat serum by HPLC method and its application supplement-drug pharmacokinetic interaction. Int. J. Pharm. Anal. 2014, 39, 2051–2740. [Google Scholar]
- Lau, G.S.N.; Critchley, J.A.J.H. The estimation of paracetamol and its major metabolites in both plasma and urine by a single high-performance liquid chromatography assay. J. Pharm. Biomed. Anal. 1994, 12, 1563–1572. [Google Scholar] [CrossRef]
- Esteban, A.; Graells, M.; Satorre, J.; Pérez-Mateo, M. Determination of paracetamol and its four major metabolites in mouse plasma by reversed-phase ion-pair high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1992, 573, 121–126. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Grosheva, I.; Zheng, D.; Soffer, E.; Blacher, E.; Braverman, S.; Tengeler, A.C.; Barak, O.; Elazar, M.; et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 2018, 359, 1376–1383. [Google Scholar] [CrossRef]
- Schäfer, C.; Schröder, K.R.; Höglinger, O.; Tollabimazraehno, S.; Lornejad-Schäfer, M.R. Acetaminophen changes intestinal epithelial cell membrane properties, subsequently affecting absorption processes. Cell. Physiol. Biochem. 2013, 32, 431–447. [Google Scholar] [CrossRef]
- Kulo, A.; Peeters, M.Y.; Allegaert, K.; Smits, A.; de Hoon, J.; Verbesselt, R.; Lewi, L.; van de Velde, M.; Knibbe, C.A. Pharmacokinetics of paracetamol and its metabolites in women at delivery and post-partum. Br. J. Clin. Pharmacol. 2013, 75, 850–860. [Google Scholar] [CrossRef]
- Siegers, C.P.; Rozman, K.; Klaassen, C.D. Biliary excretion and enterohepatic circulation of paracetamol in the rat. Xenobiotica 1983, 13, 591–596. [Google Scholar] [CrossRef]
- Hart, S.; Calder, I.; Ross, B.; Tange, J. Renal metabolism of paracetamol: Studies in the isolated perfused rat kidney. Clin. Sci. 1980, 58, 379–384. [Google Scholar] [CrossRef]
- Almási, A.; Bojcsev, S.; Fischer, T.; Simon, H.; Perjési, P.; Fischer, E. Metabolic enzyme activities and drug excretion in the small intestine and in the liver in the rat. Acta Physiol. Hung. 2013, 100, 478–488. [Google Scholar] [CrossRef]
- Dostalek, M.; Court, M.H.; Yan, B.; Akhlaghi, F. Significantly reduced cytochrome P450 3A4 expression and activity in liver from humans with diabetes mellitus. Br. J. Pharmacol. 2011, 163, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Dong, Y.; Lu, R.; Yang, B.; Wang, S.; Xing, G.; Jiang, Y. Susceptibility to the acute toxicity of acrylonitrile in streptozotocin-induced diabetic rats: Protective effect of phenethyl isothiocyanate, a phytochemical CYP2E1 inhibitor. Drug Chem. Toxicol. 2021, 44, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Song, B.J.; Veech, R.L.; Saenger, P. Cytochrome P450IIE1 is elevated in lymphocytes from poorly controlled insulin-dependent diabetics. J. Clin. Endocrinol. Metab. 1990, 71, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Lucas, D.; Farez, C.; Bardou, L.G.; Vaisse, J.; Attali, J.R.; Valensi, P. Cytochrome P450 2E1 activity in diabetic and obese patients as assessed by chlorzoxazone hydroxylation. Fundam. Clin. Pharmacol. 1998, 12, 553–558. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kishimoto, S.; Shibatani, N.; Inotsume, N.; Takeuchi, Y.; Fukushima, S. Effects of insulin on CYP3A activity and nicardipine disposition in streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2010, 62, 883–889. [Google Scholar] [CrossRef]
- Kellogg, A.P.; Wiggin, T.D.; Larkin, D.D.; Hayes, J.M.; Stevens, M.J.; Pop-Busui, R. Protective effects of cyclooxygenase-2 gene inactivation against peripheral nerve dysfunction and intraepidermal nerve fiber loss in experimental diabetes. Diabetes 2007, 56, 2997–3005. [Google Scholar] [CrossRef]
- Manyike, P.T.; Kharasch, E.D.; Kalhorn, T.F.; Slattery, J.T. Contribution of CYP2E1 and CYP3A to acetaminophen reactive metabolite formation. Clin. Pharmacol. Ther. 2000, 67, 275–282. [Google Scholar] [CrossRef]
- Bezirtzoglou, E.E. Intestinal cytochromes P450 regulating the intestinal microbiota and its probiotic profile. Microb. Ecol. Health Dis. 2012, 23, 18370. [Google Scholar] [CrossRef]
- Lindell, M.; Lang, M.; Lennernäs, H. Expression of genes encoding for drug metabolising cytochrome P450 enzymes and P-glycoprotein in the rat small intestine; comparison to the liver. Eur. J. Drug Metab. Pharmacokinet. 2003, 28, 41–48. [Google Scholar] [CrossRef]
- Thelen, K.; Dressman, J.B. Cytochrome P450-mediated metabolism in the human gut wall. J. Pharm. Pharmacol. 2009, 61, 541–558. [Google Scholar] [CrossRef]
- Choi, S.W.; Benzie, I.F.; Ma, S.W.; Strain, J.J.; Hannigan, B.M. Acute hyperglycemia and oxidative stress: Direct cause and effect? Free Radic. Biol. Med. 2008, 44, 1217–1231. [Google Scholar] [CrossRef]
- Qutob, M.; Hussein, M.A.; Alamry, K.A.; Rafatullah, M. A review on the degradation of acetaminophen by advanced oxidation process: Pathway, by-products, biotoxicity, and density functional theory calculation. RSC Adv. 2022, 12, 18373–18396. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.O.; Almási, A.; Kun, B.; Wittmann, I.; Perjési, P. Study on oxidative transformations of endogenous lipids, proteins, and amino acids in hyperglycaemic rats. 2022; Sent for publication. [Google Scholar]
- Giustarini, D.; Dalle-Donne, I.; Milzani, A.; Rossi, R. Oxidative stress induces a reversible flux of cysteine from tissues to blood in vivo in the rat. FEBS J. 2009, 276, 4946–4958. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Long, Y.C. Crosstalk between cystine and glutathione is critical for the regulation of amino acid signaling pathways and ferroptosis. Sci. Rep. 2016, 6, 30033. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.; Sato, H. The oxidative stress-inducible cystine/glutamate antiporter, system x (c) (-): Cystine supplier and beyond. Amino Acids 2012, 42, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Walker, V.; Mills, G.A.; Andreson, M.E.; Ingle, B.L.; Jackson, J.M.; Moss, C.L.; Sharrod-Cole, H.; Skipp, P.J. The acetaminophen metabolite N-acetyl-p-benzoquinone imine (NAPQI) inhibits glutathione synthetase in vitro; a clue to the mechanism of 5-oxoprolinuric acidosis? Xenobiotica 2017, 47, 164–175. [Google Scholar] [CrossRef]
- Bakke, J.E.; Rafter, J.; Larsen, G.L.; Gustafsson, J.A.; Gustafsson, B.E. Enterohepatic circulation of the mercapturic acid and cysteine conjugates of propachlor. Drug Metab. Dispos. 1981, 9, 525–528. [Google Scholar] [PubMed]
- Grafström, R.; Ormstad, K.; Moldéus, P.; Orrenius, S. Paracetamol metabolism in the isolated perfused rat liver with further metabolism of a biliary paracetamol conjugate by the small intestine. Biochem. Pharmacol. 1979, 28, 3573–3579. [Google Scholar] [CrossRef]
- Bessems, J.G.; Vermeulen, N.P. Paracetamol (acetaminophen)-induced toxicity: Molecular and biochemical mechanisms, analogues and protective approaches. Crit. Rev. Toxicol. 2001, 31, 55–138. [Google Scholar] [CrossRef]
- Ware, J.A.; Reilly, T.P.; Svensson, C.K. Cellular distribution of N-acetyltransferase activity in the rat small intestine. Biochem. Pharmacol. 1998, 55, 1475–1479. [Google Scholar] [CrossRef]
- Heard, K.J.; Green, J.L.; James, L.P.; Judge, B.S.; Zolot, L.; Rhyee, S.; Dart, R.C. Acetaminophen-cysteine adducts during therapeutic dosing and following overdose. BMC Gastroenterol. 2011, 11, 20. [Google Scholar] [CrossRef]
- Brundu, S.; Nencioni, L.; Celestino, I.; Coluccio, P.; Palamara, A.T.; Magnani, M.; Fraternale, A. Validation of a Reversed-Phase High Performance Liquid Chromatography Method for the Simultaneous Analysis of Cysteine and Reduced Glutathione in Mouse Organs. Oxid. Med. Cell. Longev. 2016, 2016, 1746985. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, M.; Patel, K.; Pate, S. Screening of some selected plants for CYP3A inhibition and bioavailability enhancement. Adv. Res. Pharm. Biol. 2011, 1, 19–27. [Google Scholar]
- Elbarbry, F.; Wilby, K.; Alcorn, J. Validation of a HPLC method for the determination of p-nitrophenol hydroxylase activity in rat hepatic microsomes. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 834, 199–203. [Google Scholar] [CrossRef]
- Liu, L.; Massey, T.E. Bioactivation of aflatoxin B1 by lipoxygenases, prostaglandin H synthase and cytochrome P450 monooxygenase in guinea-pig tissues. Carcinogenesis 1992, 13, 533–539. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mészáros, P.; Kovács, S.; Kulcsár, G.; Páskuj, M.; Almási, A. Investigation of Intestinal Absorption and Excretion of Paracetamol in Streptozotocin-Induced Hyperglycemia. Int. J. Mol. Sci. 2022, 23, 11913. https://doi.org/10.3390/ijms231911913
Mészáros P, Kovács S, Kulcsár G, Páskuj M, Almási A. Investigation of Intestinal Absorption and Excretion of Paracetamol in Streptozotocin-Induced Hyperglycemia. International Journal of Molecular Sciences. 2022; 23(19):11913. https://doi.org/10.3390/ijms231911913
Chicago/Turabian StyleMészáros, Petra, Sára Kovács, Győző Kulcsár, Melinda Páskuj, and Attila Almási. 2022. "Investigation of Intestinal Absorption and Excretion of Paracetamol in Streptozotocin-Induced Hyperglycemia" International Journal of Molecular Sciences 23, no. 19: 11913. https://doi.org/10.3390/ijms231911913
APA StyleMészáros, P., Kovács, S., Kulcsár, G., Páskuj, M., & Almási, A. (2022). Investigation of Intestinal Absorption and Excretion of Paracetamol in Streptozotocin-Induced Hyperglycemia. International Journal of Molecular Sciences, 23(19), 11913. https://doi.org/10.3390/ijms231911913





