The Flavonol Quercitrin Hinders GSK3 Activity and Potentiates the Wnt/β-Catenin Signaling Pathway
Abstract
:1. Introduction
2. Results
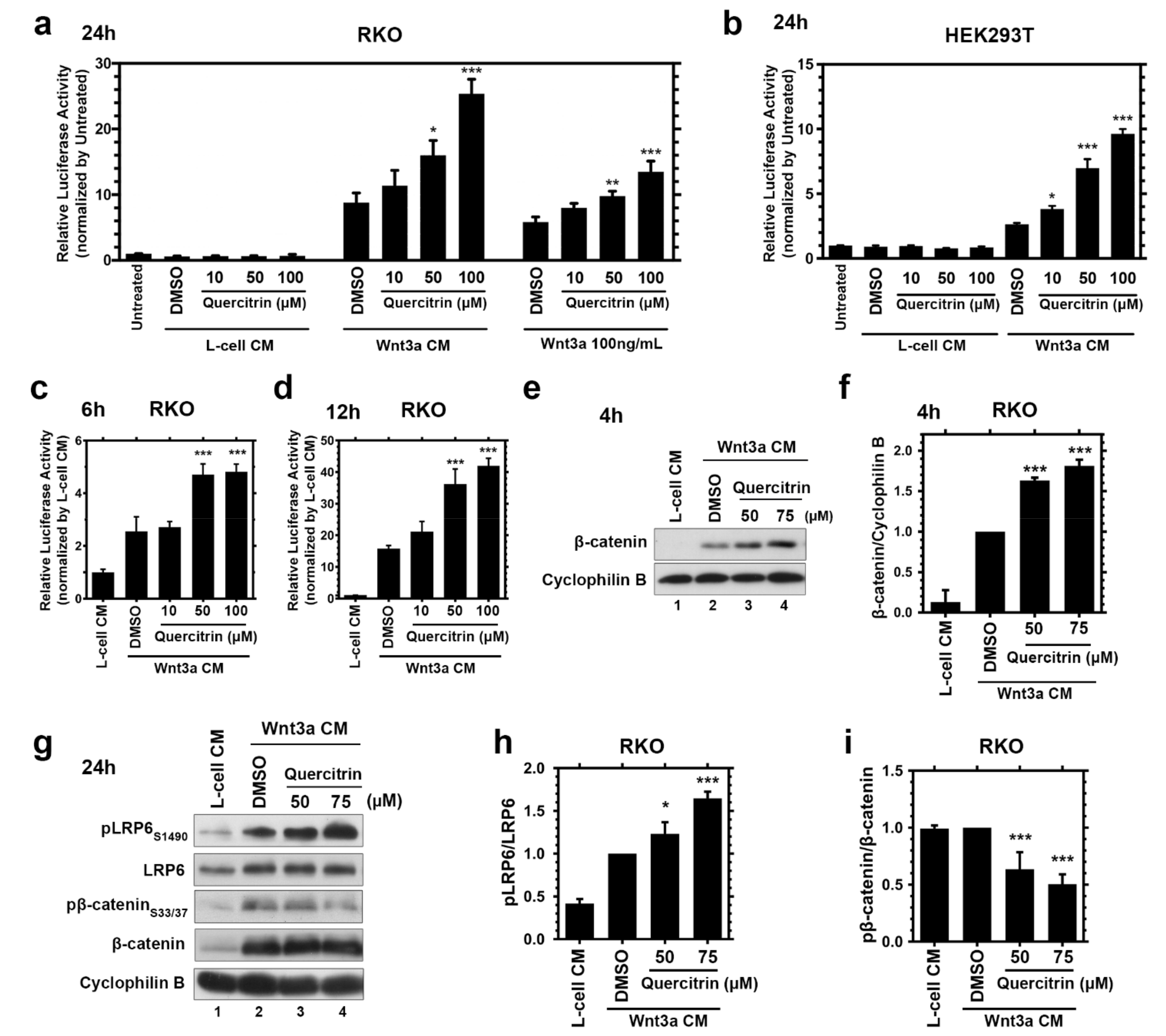
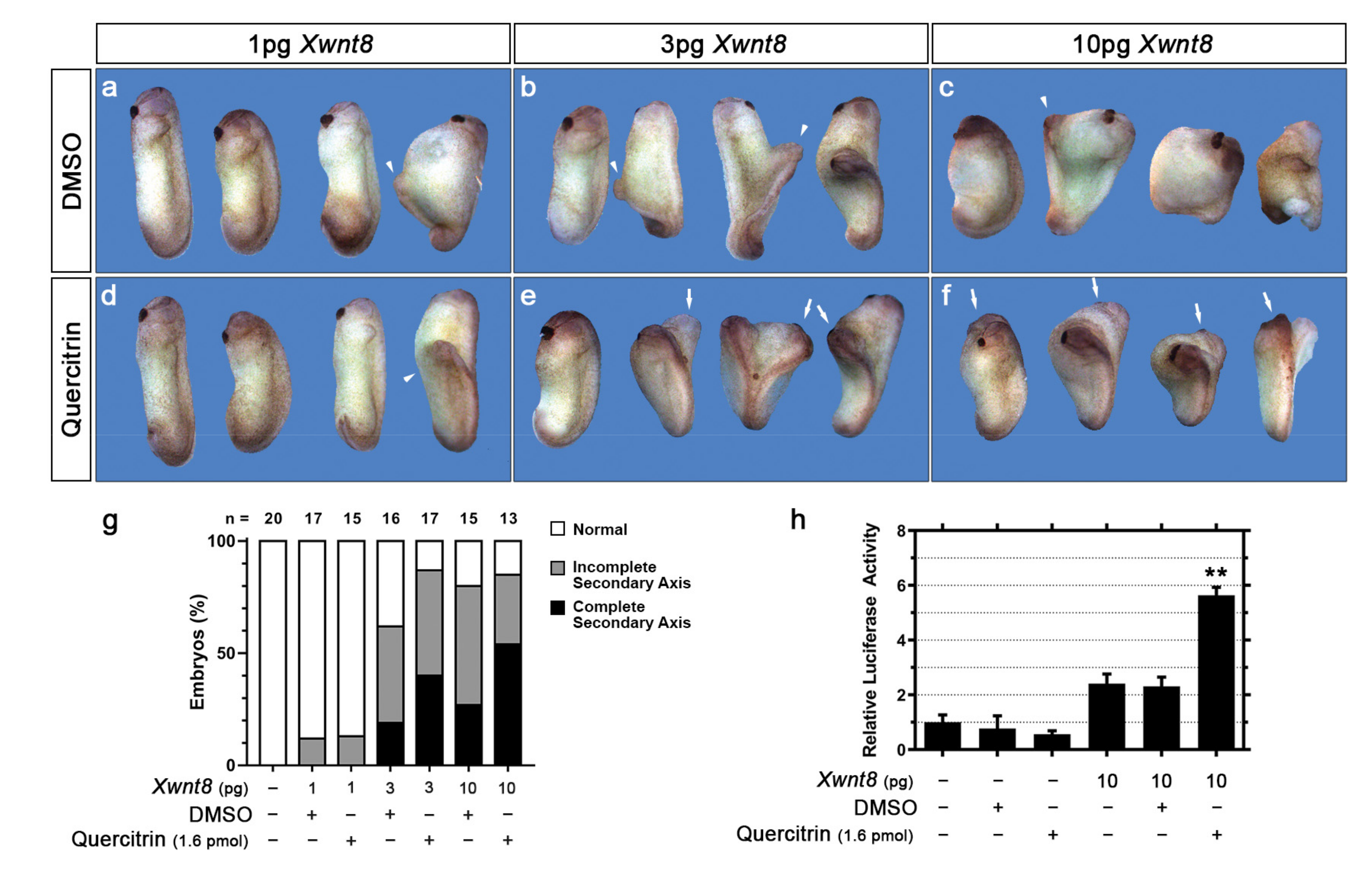
2.1. Quercitrin Potentiates the Wnt/β-Catenin Signaling Pathway
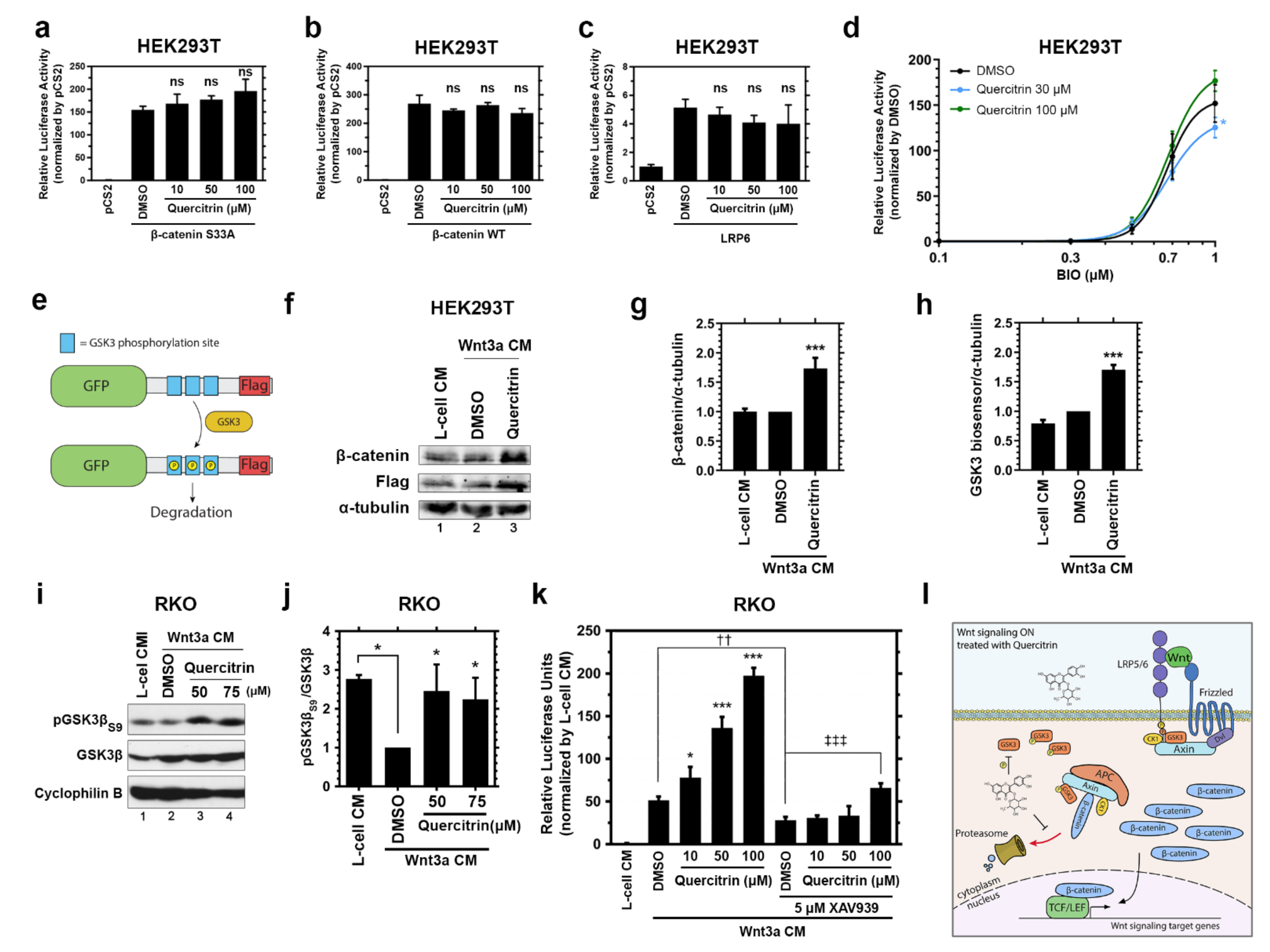
2.2. Quercitrin Facilitates GSK3 S9 Phosphorylation and Hinders GSK3 Activity
2.3. Quercitrin Potentiates the Canonical Wnt Effects on Hippocampal Synapses In Vitro and Rescues the AβO-Induced Memory Impairment in Mice
3. Discussion
4. Materials and Methods
4.1. Reagents and Cell Culture
4.2. Xenopus Laevis Embryo Manipulation
4.3. Mature Hippocampal Neuronal Culture
4.4. Mice Experiments
4.5. AβO Infusion and Pharmacological Treatments
4.6. Behavioral Analysis
4.7. Dual-Luciferase Assay
4.8. Immunoblotting
4.9. Immunocytochemistry and Synaptic Puncta Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macdonald, B.T.; Tamai, K.; He, X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [Green Version]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Shen, K. WNTs in synapse formation and neuronal circuitry. EMBO J. 2012, 31, 2697–2704. [Google Scholar] [CrossRef] [Green Version]
- McLeod, F.; Salinas, P.C. Wnt proteins as modulators of synaptic plasticity. Curr. Opin. Neurobiol. 2018, 53, 90–95. [Google Scholar] [CrossRef]
- Chen, J.; Park, C.S.; Tang, S.-J. Activity-dependent Synaptic Wnt Release Regulates Hippocampal Long Term Potentiation. J. Biol. Chem. 2006, 281, 11910–11916. [Google Scholar] [CrossRef] [Green Version]
- Magdesian, M.H.; Carvalho, M.M.V.F.; Mendes, F.A.; Saraiva, L.M.; Juliano, M.A.; Juliano, L.; Garcia-Abreu, J.; Ferreira, S.T. Amyloid-β Binds to the Extracellular Cysteine-rich Domain of Frizzled and Inhibits Wnt/β-Catenin Signaling. J. Biol. Chem. 2008, 283, 9359–9368. [Google Scholar] [CrossRef] [Green Version]
- Tapia-Rojas, C.; Inestrosa, N.C. Loss of canonical Wnt signaling is involved in the pathogenesis of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 1705. [Google Scholar]
- Tapia-Rojas, C.; Inestrosa, N.C. Wnt signaling loss accelerates the appearance of neuropathological hallmarks of Alzheimer’s disease in J20-APP transgenic and wild-type mice. J. Neurochem. 2018, 144, 443–465. [Google Scholar] [CrossRef] [Green Version]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–186. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Näslund, J.; Haroutunian, V.; Mohs, R.C.; Davis, K.L.; Davies, P.; Greengard, P.; Buxbaum, J. Correlation Between Elevated Levels of Amyloid β-Peptide in the Brain and Cognitive Decline. JAMA 2000, 283, 1571–1577. [Google Scholar] [CrossRef]
- Townsend, M.; Shankar, G.M.; Mehta, T.; Walsh, D.M.; Selkoe, D.J. Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: A potent role for trimers. J. Physiol. 2006, 572, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hartmann, H.; Do, V.M.; Abramowski, D.; Sturchler-Pierrat, C.; Staufenbiel, M.; Sommer, B.; Van De Wetering, M.; Clevers, H.; Saftig, P.; et al. Destabilization of β-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature 1998, 395, 698–702. [Google Scholar] [CrossRef]
- Toledo, E.; Inestrosa, N. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1ΔE9 mouse model of Alzheimer’s disease. Mol. Psychiatry 2010, 15, 272. [Google Scholar] [CrossRef]
- Rivera, D.S.; Lindsay, C.; Codocedo, J.F.; Morel, I.; Pinto, C.; Cisternas, P.; Bozinovic, F.; Inestrosa, N.C. Andrographolide recovers cognitive impairment in a natural model of Alzheimer’s disease (Octodon degus). Neurobiol. Aging 2016, 46, 204–220. [Google Scholar] [CrossRef]
- Vargas, J.Y.; Fuenzalida, M.; Inestrosa, N.C. In vivo activation of Wnt signaling pathway enhances cognitive function of adult mice and reverses cognitive deficits in an Alzheimer’s disease model. J. Neurosci. 2014, 34, 2191–2202. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, A.R.; Godoy, J.A.; Mullendorff, K.; Olivares, G.; Bronfman, M.; Inestrosa, N.C. Wnt-3a overcomes β-amyloid toxicity in rat hippocampal neurons. Exp. Cell Res. 2004, 297, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Parr, C.; Mirzaei, N.; Christian, M.; Sastre, M. Activation of the Wnt/β-catenin pathway represses the transcription of the β-amyloid precursor protein cleaving enzyme (BACE1) via binding of T-cell factor-4 to BACE1 promoter. FASEB J. 2015, 29, 623–635. [Google Scholar] [CrossRef]
- Lie, D.-C.; Colamarino, S.A.; Song, H.-J.; Désiré, L.; Mira, H.; Consiglio, A.; Lein, E.S.; Jessberger, S.; Lansford, H.; Dearie, A.R.; et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature 2005, 437, 1370–1375. [Google Scholar] [CrossRef]
- Ng, Y.P.; Or TC, T.; Ip, N.Y. Plant alkaloids as drug leads for Alzheimer’s disease. Neurochem. Int. 2015, 89, 260–270. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Biodiversity: A continuing source of novel drug leads. Pure Appl. Chem. 2005, 77, 7–24. [Google Scholar] [CrossRef] [Green Version]
- Maher, P. The Potential of Flavonoids for the Treatment of Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3056. [Google Scholar] [CrossRef] [Green Version]
- He, W.-B.; Abe, K.; Akaishi, T. Oral administration of fisetin promotes the induction of hippocampal long-term potentiation in vivo. J. Pharmacol. Sci. 2018, 136, 42–45. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Schroeder, J.P.; Chan, C.-B.; Song, M.; Yu, S.P.; Weinshenker, D.; Ye, K. 7,8-Dihydroxyflavone Prevents Synaptic Loss and Memory Deficits in a Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology 2013, 39, 638–650. [Google Scholar] [CrossRef] [Green Version]
- Unno, K.; Pervin, M.; Taguchi, K.; Konishi, T.; Nakamura, Y. Green Tea Catechins Trigger Immediate-Early Genes in the Hippocampus and Prevent Cognitive Decline and Lifespan Shortening. Molecules 2020, 25, 1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matias, I.; Diniz, L.P.; Buosi, A.; Neves, G.; Stipursky, J.; Gomes, F.C.A. Flavonoid Hesperidin Induces Synapse Formation and Improves Memory Performance through the Astrocytic TGF-β. Front. Aging Neurosci. 2017, 9, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H. Polyphenols and health: Update and perspectives. Arch. Biochem. Biophys. 2010, 501, 2–5. [Google Scholar] [CrossRef]
- Amado, N.G.; Predes, D.; Moreno, M.M.; Carvalho, I.O.; Mendes, F.A.; Abreu, J.G. Flavonoids and Wnt/β-Catenin Signaling: Potential Role in Colorectal Cancer Therapies. Int. J. Mol. Sci. 2014, 15, 12094–12106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, L.F.S.; Predes, D.; Borges, H.L.; Abreu, J.G. Therapeutic Potential of Naturally Occurring Small Molecules to Target the Wnt/β-Catenin Signaling Pathway in Colorectal Cancer. Cancers 2022, 14, 403. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Sun, C.; Peng, F.; Yu, L.; Chen, Y.; Tan, Y.; Cao, X.; Tang, Y.; Xie, X. Chemistry, pharmacokinetics, pharmacological activities, and toxicity of Quercitrin. Phytother. Res. 2022, 36, 1545–1575. [Google Scholar] [CrossRef]
- Comalada, M.; Camuesco, D.; Sierra, S.; Ballester, I.; Xaus, J.; Gálvez, J.; Zarzuelo, A. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-κB pathway. Eur. J. Immunol. 2005, 35, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Muzitano, M.F.; Cruz, E.A.; de Almeida, A.P.; Da Silva, S.A.G.; Kaiser, C.R.; Guette, C.; Rossi-Bergmann, B.; Costa, S.S. Quercitrin: An Antileishmanial Flavonoid Glycoside from Kalanchoe pinnata. Planta Med. 2005, 72, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Satué, M.; Arriero, M.D.M.; Monjo, M.; Ramis, J.M. Quercitrin and Taxifolin stimulate osteoblast differentiation in MC3T3-E1 cells and inhibit osteoclastogenesis in RAW 264.7 cells. Biochem. Pharmacol. 2013, 86, 1476–1486. [Google Scholar] [CrossRef]
- Gadotti, V.M.; Santos, A.R.S.; Meyre-Silva, C.; Schmeling, L.O.; Machado, C.; Liz, F.H.; Filho, V.C. Antinociceptive action of the extract and the flavonoid quercitrin isolated from Bauhinia microstachya leaves. J. Pharm. Pharmacol. 2005, 57, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Amado, N.; Fonseca, B.; Cerqueira, D.; Reis, A.; Simas, A.; Kuster, R.; Mendes, F.; Abreu, J. Effects of Natural Compounds on Xenopus Embryogenesis: A Potential Read Out for Functional Drug Discovery Targeting Wnt/β-catenin Signaling. Curr. Top. Med. Chem. 2013, 12, 2103–2113. [Google Scholar] [CrossRef]
- Park, C.H.; Chang, J.Y.; Hahm, E.R.; Park, S.; Kim, H.-K.; Yang, C.H. Quercetin, a potent inhibitor against β-catenin/Tcf signaling in SW480 colon cancer cells. Biochem. Biophys. Res. Commun. 2005, 328, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Amado, N.G.; Cerqueira, D.M.; Menezes, F.S.; da Silva, J.F.M.; Neto, V.M.; Abreu, J.G. Isoquercitrin isolated from Hyptis fasciculata reduces glioblastoma cell proliferation and changes β-catenin cellular localization. Anti-Cancer Drugs 2009, 20, 543–552. [Google Scholar] [CrossRef]
- Amado, N.G.; Predes, D.; Fonseca, B.F.; Cerqueira, D.M.; Reis, A.H.; Dudenhoeffer, A.C.; Borges, H.; Mendes, F.A.; Abreu, J.G. Isoquercitrin Suppresses Colon Cancer Cell Growth In Vitro by Targeting the Wnt/β-Catenin Signaling Pathway. J. Biol. Chem. 2014, 289, 35456–35467. [Google Scholar] [CrossRef] [Green Version]
- Giannini, A.; Vivanco, M.; Kypta, R. Analysis of β-Catenin Aggregation and Localization Using GFP Fusion Proteins: Nuclear Import of α-Catenin by the β-Catenin/Tcf Complex. Exp. Cell Res. 2000, 255, 207–220. [Google Scholar] [CrossRef]
- Major, M.B.; Camp, N.D.; Berndt, J.D.; Yi, X.; Goldenberg, S.J.; Hubbert, C.; Biechele, T.L.; Gingras, A.-C.; Zheng, N.; MacCoss, M.J.; et al. Wilms Tumor Suppressor WTX Negatively Regulates WNT/ß-Catenin Signaling. Science 2007, 316, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Gammons, M.V.; Renko, M.; Johnson, C.M.; Rutherford, T.J.; Bienz, M. Wnt Signalosome Assembly by DEP Domain Swapping of Dishevelled. Mol. Cell 2016, 64, 92–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-E.; Huang, H.; Zhao, M.; Zhang, X.; Zhang, A.; Semonov, M.V.; MacDonald, B.T.; Zhang, X.; Abreu, J.G.; Peng, L.; et al. Wnt Stabilization of β-Catenin Reveals Principles for Morphogen Receptor-Scaffold Assemblies. Science 2013, 340, 867–870. [Google Scholar] [CrossRef] [Green Version]
- Breen, E.; Clarke, A.; Steele, G.; Mercurio, A.M. Poorly Differentiated Colon Carcinoma Cell Lines Deficient in α-Catenin Expression Express High Levels of Surface E-cadherin but Lack Ca2+-Dependent Cell-Cell Adhesion. Cell Adhes. Commun. 1993, 1, 239–250. [Google Scholar] [CrossRef]
- Maia, L.A.; Velloso, I.; Abreu, J.G. Advances in the use of Xenopus for successful drug screening. Expert Opin. Drug Discov. 2017, 12, 1153–1159. [Google Scholar] [CrossRef]
- Brannon, M.; Gomperts, M.; Sumoy, L.; Moon, R.T.; Kimelman, D. A β-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev. 1997, 11, 2359–2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijer, L.; Skaltsounis, A.-L.; Magiatis, P.; Polychronopoulos, P.; Knockaert, M.; Leost, M.; Ryan, X.P.; Vonica, C.A.; Brivanlou, A.; Dajani, R.; et al. GSK-3-Selective Inhibitors Derived from Tyrian Purple Indirubins. Chem. Biol. 2003, 10, 1255–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korinek, V.; Barker, N.; Morin, P.J.; van Wichen, D.; de Weger, R.; Kinzler, K.W.; Vogelstein, B.; Clevers, H. Constitutive Transcriptional Activation by a β-Catenin-Tcf Complex in APC−/− Colon Carcinoma. Science 1997, 275, 1784–1787. [Google Scholar] [CrossRef] [Green Version]
- Munemitsu, S.; Albert, I.; Rubinfeld, B.; Polakis, P. Deletion of an amino-terminal sequence beta-catenin in vivo and promotes hyperphosporylation of the adenomatous polyposis coli tumor suppressor protein. Mol. Cell. Biol. 1996, 16, 4088–4094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamos, J.L.; Chu, M.L.-H.; Enos, M.D.; Shah, N.; Weis, W.I. Structural basis of GSK-3 inhibition by N-terminal phosphorylation and by the Wnt receptor LRP6. eLife 2014, 3, e01998. [Google Scholar] [CrossRef]
- Taelman, V.F.; Dobrowolski, R.; Plouhinec, J.-L.; Fuentealba, L.C.; Vorwald, P.P.; Gumper, I.; Sabatini, D.D.; De Robertis, E.M. Wnt Signaling Requires Sequestration of Glycogen Synthase Kinase 3 inside Multivesicular Endosomes. Cell 2010, 143, 1136–1148. [Google Scholar] [CrossRef] [PubMed]
- Peterson-Nedry, W.; Erdeniz, N.; Kremer, S.; Yu, J.; Baig-Lewis, S.; Wehrli, M. Unexpectedly robust assembly of the Axin destruction complex regulates Wnt/Wg signaling in Drosophila as revealed by analysis in vivo. Dev. Biol. 2008, 320, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-M.A.; Mishina, Y.M.; Liu, S.; Cheung, A.; Stegmeier, F.; Michaud, G.A.; Charlat, O.; Wiellette, E.; Zhang, Y.; Wiessner, S.; et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 2009, 461, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Matias, I.; Buosi, A.; Gomes, F.C.A. Functions of flavonoids in the central nervous system: Astrocytes as targets for natural compounds. Neurochem. Int. 2016, 95, 85–91. [Google Scholar] [CrossRef]
- Rosso, S.B.; Inestrosa, N.C. WNT signaling in neuronal maturation and synaptogenesis. Front. Cell. Neurosci. 2013, 7, 103. [Google Scholar] [CrossRef] [Green Version]
- Bossy-Wetzel, E.; Schwarzenbacher, R.; Lipton, S.A. Molecular pathways to neurodegeneration. Nat. Med. 2004, 10, S2–S9. [Google Scholar] [CrossRef]
- Kusserow, A.; Pang, K.; Sturm, C.; Hrouda, M.; Lentfer, J.; Schmidt, H.; Technau, U.; Von Haeseler, A.; Hobmayer, B.; Martindale, M.Q.; et al. Unexpected complexity of the Wnt gene family in a sea anemone. Nature 2005, 433, 156–160. [Google Scholar] [CrossRef]
- Dickins, E.M.; Salinas, P.C. Wnts in action: From synapse formation to synaptic maintenance. Front. Cell. Neurosci. 2013, 7, 162. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, M.; Inoue, K.; Iwamura, H.; Terashima, K.; Soya, H.; Asashima, M.; Kuwabara, T. Reduction in paracrine Wnt3 factors during aging causes impaired adult neurogenesis. FASEB J. 2011, 25, 3570–3582. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Arenas, E. Emerging roles of Wnts in the adult nervous system. Nat. Rev. Neurosci. 2009, 11, 77–86. [Google Scholar] [CrossRef]
- Niehrs, C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Green, B.A.; Farr, J.M.; Lopes, F.C.; Van Raay, T.J. Wnt Drug Discovery: Weaving through the Screens, Patents and Clinical Trials. Cancers 2016, 8, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hlasiwetz, H. Über das Quercitrin. J. Prakt. Chem. 1859, 78, 257–277. [Google Scholar] [CrossRef]
- Hlasiwetz, H.; Pfaundler, L. Ueber den Quercitrinzucker. J. Prakt. Chem. 1863, 90, 452–457. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Major, M.B.; Takanashi, S.; Camp, N.D.; Nishiya, N.; Peters, E.C.; Ginsberg, M.H.; Jian, X.; Randazzo, P.A.; Schultz, P.G.; et al. Small-molecule synergist of the Wnt/β-catenin signaling pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 7444–7448. [Google Scholar] [CrossRef] [Green Version]
- Frame, S.; Cohen, P.; Biondi, R.M. A Common Phosphate Binding Site Explains the Unique Substrate Specificity of GSK3 and Its Inactivation by Phosphorylation. Mol. Cell 2001, 7, 1321–1327. [Google Scholar] [CrossRef]
- Park, E.-J.; Choi, S.J.; Kim, Y.-C.; Lee, S.H.; Park, S.W. Novel small molecule activators of β-catenin-mediated signaling pathway: Structure–activity relationships of indirubins. Bioorg. Med. Chem. Lett. 2009, 19, 2282–2284. [Google Scholar] [CrossRef]
- Tapia-Rojas, C.; Schüller, A.; Lindsay, C.B.; Ureta, R.C.; Mejías-Reyes, C.; Hancke, J.; Melo, F.; Inestrosa, N.C. Andrographolide activates the canonical Wnt signalling pathway by a mechanism that implicates the non-ATP competitive inhibition of GSK-3β: Autoregulation of GSK-3β in vivo. Biochem. J. 2015, 466, 415–430. [Google Scholar] [CrossRef]
- Dohare, P.; Cheng, B.; Ahmed, E.; Yadala, V.; Singla, P.; Thomas, S.; Kayton, R.; Ungvari, Z.; Ballabh, P. Glycogen synthase kinase-3β inhibition enhances myelination in preterm newborns with intraventricular hemorrhage, but not recombinant Wnt3A. Neurobiol. Dis. 2018, 118, 22–39. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.; Yu, S.-W.; Kim, E.-K. Wnt3a upregulates brain-derived insulin by increasing NeuroD1 via Wnt/β-catenin signaling in the hypothalamus. Mol. Brain 2016, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Vargas, J.Y.; Ahumada, J.; Arrázola, M.S.; Fuenzalida, M.; Inestrosa, N.C. WASP-1, a canonical Wnt signaling potentiator, rescues hippocampal synaptic impairments induced by Aβ oligomers. Exp. Neurol. 2015, 264, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Torres, V.I.; Vio, C.P.; Inestrosa, N.C. Canonical Wnt Signaling Modulates the Expression of Pre- and Postsynaptic Components in Different Temporal Patterns. Mol. Neurobiol. 2019, 57, 1389–1404. [Google Scholar] [CrossRef]
- Garner, B.; Ooi, L. Wnt is here! Could Wnt signalling be promoted to protect against Alzheimer disease? An Editorial for ‘Wnt signaling loss accelerates the appearance of neuropathological hallmarks of Alzheimer’s disease in J20-APP transgenic and wild-type mice’. J. Neurochem. 2018, 144, 356–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooper, C.; Killick, R.; Lovestone, S. The GSK3 hypothesis of Alzheimer’s disease. J. Neurochem. 2008, 104, 1433–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shruster, A.; Eldar-Finkelman, H.; Melamed, E.; Offen, D. Wnt signaling pathway overcomes the disruption of neuronal differentiation of neural progenitor cells induced by oligomeric amyloid β-peptide. J. Neurochem. 2010, 116, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.-H.; Bonaguidi, M.A.; Kitabatake, Y.; Sun, J.; Song, J.; Kang, E.; Jun, H.; Zhong, C.; Su, Y.; Guo, J.U.; et al. Secreted Frizzled-Related Protein 3 Regulates Activity-Dependent Adult Hippocampal Neurogenesis. Cell Stem Cell 2013, 12, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Seib, D.R.; Corsini, N.S.; Ellwanger, K.; Plaas, C.; Mateos, A.; Pitzer, C.; Niehrs, C.; Celikel, T.; Martin-Villalba, A. Loss of Dickkopf-1 Restores Neurogenesis in Old Age and Counteracts Cognitive Decline. Cell Stem Cell 2013, 12, 204–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, F.; Borrell, J.; Guaza, C.; Avila, J.; Lucas, J.J. Spatial learning deficit in transgenic mice that conditionally over-express GSK-3β in the brain but do not form tau filaments. J. Neurochem. 2002, 83, 1529–1533. [Google Scholar] [CrossRef] [Green Version]
- Lucas, J.J.; Hernández, F.; Gómez-Ramos, P.; Morán, M.A.; Hen, R.; Avila, J. Decreased nuclear β-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3β conditional transgenic mice. EMBO J. 2001, 20, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Pei, J.-J.; Braak, E.; Braak, H.; Grundke-Iqbal, I.; Iqbal, K.; Winblad, B.; Cowburn, R.F. Distribution of Active Glycogen Synthase Kinase 3β (GSK-3β) in Brains Staged for Alzheimer Disease Neurofibrillary Changes. J. Neuropathol. Exp. Neurol. 1999, 58, 1010–1019. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, B.T.; Yokota, C.; Tamai, K.; Zeng, X.; He, X. Wnt Signal Amplification via Activity, Cooperativity, and Regulation of Multiple Intracellular PPPSP Motifs in the Wnt Co-receptor LRP. J. Biol. Chem. 2008, 283, 16115–16123. [Google Scholar] [CrossRef]
- Metcalfe, C.; Bienz, M. Inhibition of GSK3 by Wnt signalling–two contrasting models. J. Cell Sci. 2011, 124, 3537–3544. [Google Scholar] [CrossRef] [Green Version]
- De Ferrari, G.V.; Chacón, M.A.; Barría, M.I.; Garrido, J.L.; Godoy, J.A.; Olivares, G.; Reyes, A.E.; Alvarez, A.; Bronfman, M.; Inestrosa, N.C. Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by β-amyloid fibrils. Mol. Psychiatry 2003, 8, 195–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaral, L.S.; Ferreira, J.M.; Predes, D.; Abreu, J.G.; Noël, F.; Quintas, L.E.M. Telocinobufagin and Marinobufagin Produce Different Effects in LLC-PK1 Cells: A Case of Functional Selectivity of Bufadienolides. Int. J. Mol. Sci. 2018, 19, 2769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastas, J.N.; Moon, R.T. WNT signalling pathways as therapeutic targets in cancer. Nat. Cancer 2012, 13, 11–26. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, G.C.; Oliveira, L.F.S.; Predes, D.; Fokoue, H.H.; Kuster, R.M.; Oliveira, F.L.; Mendes, F.A.; Abreu, J.G. Piperine suppresses the Wnt/β-catenin pathway and has anti-cancer effects on colorectal cancer cells. Sci. Rep. 2020, 10, 11681. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.F.; Predes, D.; Cerqueira, D.M.; Reis, A.H.; Amado, N.G.; Cayres, M.C.L.; Kuster, R.M.; Oliveira, F.L.; Mendes, F.A.; Abreu, J.G. Derricin and Derricidin Inhibit Wnt/β-Catenin Signaling and Suppress Colon Cancer Cell Growth In Vitro. PLoS ONE 2015, 10, e0120919. [Google Scholar] [CrossRef] [Green Version]
- Predes, D.; Oliveira, L.F.S.; Ferreira, L.S.S.; Maia, L.A.; Delou, J.M.A.; Faletti, A.; Oliveira, I.; Amado, N.G.; Reis, A.H.; Fraga, C.A.M.; et al. The Chalcone Lonchocarpin Inhibits Wnt/β-Catenin Signaling and Suppresses Colorectal Cancer Proliferation. Cancers 2019, 11, 1968. [Google Scholar] [CrossRef] [Green Version]
- Kahn, M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014, 13, 513. [Google Scholar] [CrossRef] [Green Version]
- Cha, P.-H.; Shin, W.; Zahoor, M.; Kim, H.-Y.; Min, S.; Choi, K.-Y. Hovenia dulcis Thunb Extract and Its Ingredient Methyl Vanillate Activate Wnt/β-Catenin Pathway and Increase Bone Mass in Growing or Ovariectomized Mice. PLoS ONE 2014, 9, e85546. [Google Scholar] [CrossRef] [PubMed]
- Tosti, A.; Zaiac, M.N.; Canazza, A.; Sanchis-Gomar, F. Topical application of the Wnt/b-catenin activator methyl vanillate increases hair count and hair mass index in women with androgenetic alopecia. J. Cosmet. Dermatol. 2016, 15, 469–474. [Google Scholar] [CrossRef]
- Guo, H.; Xing, Y.; Liu, Y.; Luo, Y.; Deng, F.; Yang, T.; Yang, K.; Li, Y. Wnt/β-catenin signaling pathway activates melanocyte stem cells in vitro and in vivo. J. Dermatol. Sci. 2016, 83, 45–51. [Google Scholar] [CrossRef]
- Shi, Y.; Shu, B.; Yang, R.; Xu, Y.; Xing, B.; Liu, J.; Chen, L.; Qi, S.; Liu, X.; Wang, P. Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell Res. Ther. 2015, 6, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiquet, B.T.; Blanton, S.H.; Burt, A.; Ma, D.; Stal, S.; Mulliken, J.B.; Hecht, J.T. Variation in WNT genes is associated with non-syndromic cleft lip with or without cleft palate. Hum. Mol. Genet. 2008, 17, 2212–2218. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Li, Y.; Wang, K.; Wang, Y.-Z.; Molotkov, A.; Gao, L.; Zhao, T.; Yamagami, T.; Wang, Y.; Gan, Q.; et al. Lrp6-mediated canonical Wnt signaling is required for lip formation and fusion. Development 2009, 136, 3161–3171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Li, M.; Wang, Y.; Wang, Z.; Zhang, W.; Guan, F.; Chen, Q.; Wang, J. GSK-3β as a target for protection against transient cerebral ischemia. Int. J. Med. Sci. 2017, 14, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Kneidinger, N.; Yildirim, A.; Callegari, J.; Takenaka, S.; Stein, M.M.; Dumitrascu, R.; Bohla, A.; Bracke, K.R.; Morty, R.E.; Brusselle, G.G.; et al. Activation of the WNT/β-Catenin Pathway Attenuates Experimental Emphysema. Am. J. Respir. Crit. Care Med. 2011, 183, 723–733. [Google Scholar] [CrossRef] [Green Version]
- Glass, D.A., 2nd; Bialek, P.; Ahn, J.D.; Starbuck, M.; Patel, M.S.; Clevers, H.; Taketo, M.M.; Long, F.; McMahon, A.P.; Lang, R.A.; et al. Canonical Wnt Signaling in Differentiated Osteoblasts Controls Osteoclast Differentiation. Dev. Cell 2005, 8, 751–764. [Google Scholar] [CrossRef] [Green Version]
- Córdoba, A.; Manzanaro-Moreno, N.; Colom, C.; Rønold, H.J.; Lyngstadaas, S.P.; Monjo, M.; Ramis, J.M. Quercitrin Nanocoated Implant Surfaces Reduce Osteoclast Activity In Vitro and In Vivo. Int. J. Mol. Sci. 2018, 19, 3319. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, S.; Cho, E.J. Flavonoids from Acer okamotoanum Inhibit Adipocyte Differentiation and Promote Lipolysis in the 3T3-L1 Cells. Molecules 2020, 25, 1920. [Google Scholar] [CrossRef] [Green Version]
- Gálvez, J.; Crespo, M.E.; Jiménez, J.; Suárez, A.; Zarzuelo, A. Antidiarrhoeic activity of quercitrin in mice and rats. J. Pharm. Pharmacol. 1993, 45, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, J.; de Medina, F.S.; Jiménez, J.; Torres, M.I.; Fernández, M.I.; Núñez, M.C.; Ríos, A.; Gil, A.; Zarzuelo, A. Effect of Quercitrin on Lactose-Induced Chronic Diarrhoea in Rats. Planta Medica 1995, 61, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Kennell, J.A.; MacDougald, O. Wnt Signaling Inhibits Adipogenesis through β-Catenin-dependent and -independent Mechanisms. J. Biol. Chem. 2005, 280, 24004–24010. [Google Scholar] [CrossRef] [Green Version]
- Nones, J.; Costa, A.P.; Leal, R.; Gomes, F.; Trentin, A.G. The flavonoids hesperidin and rutin promote neural crest cell survival. Cell Tissue Res. 2012, 350, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Nones, J.; Spohr, T.C.L.D.S.; Gomes, F.C.A. Effects of the flavonoid hesperidin in cerebral cortical progenitors in vitro: Indirect action through astrocytes. Int. J. Dev. Neurosci. 2012, 30, 303–313. [Google Scholar] [CrossRef] [PubMed]
- de Sampaio e Spohr, T.C.L.; Stipursky, J.; Sasaki, A.C.; Barbosa, P.R.; Martins, V.; Benjamim, C.F.; Roque, N.F.; Costa, S.L.; Gomes, F.C.A. Effects of the flavonoid casticin from Brazilian Croton betulaster in cerebral cortical progenitors in vitro: Direct and indirect action through astrocytes. J. Neurosci. Res. 2010, 88, 530–541. [Google Scholar] [PubMed]
- De Andrade Teles, R.B.; Diniz, T.C.; Costa Pinto, T.C.; de Oliveira, R.G., Jr.; Gama, E.S.M.; de Lavor, E.M.; Fernandes, A.W.C.; de Oliveira, A.P.; de Almeida Ribeiro, F.P.R.; da Silva, A.A.M.; et al. Flavonoids as Therapeutic Agents in Alzheimer’s and Parkinson’s Diseases: A Systematic Review of Preclinical Evidences. Oxid. Med. Cell. Longev. 2018, 2018, 7043213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Ovais, M.; Ullah, I.; Ahmed, J.; Shahid, M. Flavonoids as Prospective Neuroprotectants and Their Therapeutic Propensity in Aging Associated Neurological Disorders. Front. Aging Neurosci. 2019, 11, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brickman, A.M.; Khan, U.A.; Provenzano, F.A.; Yeung, L.-K.; Suzuki, W.; Schroeter, H.; Wall, M.; Sloan, R.P.; Small, S.A. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat. Neurosci. 2014, 17, 1798–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devore, E.E.; Kang, J.H.; Breteler, M.M.; Grodstein, F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Bensalem, J.; Dudonné, S.; Gaudout, D.; Servant, L.; Calon, F.; Desjardins, Y.; Layé, S.; Lafenetre, P.; Pallet, V. Polyphenol-rich extract from grape and blueberry attenuates cognitive decline and improves neuronal function in aged mice. J. Nutr. Sci. 2018, 7, e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Currais, A.; Prior, M.; Dargusch, R.; Armando, A.; Ehren, J.; Schubert, D.; Quehenberger, O.; Maher, P. Modulation of p25 and inflammatory pathways by fisetin maintains cognitive function in A lzheimer’s disease transgenic mice. Aging Cell 2014, 13, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tian, Z.-K.; Yang, H.-X.; Feng, Z.-J.; Sun, J.-M.; Jiang, H.; Cheng, C.; Ming, Q.-L.; Liu, C.-M. Fisetin improves lead-induced neuroinflammation, apoptosis and synaptic dysfunction in mice associated with the AMPK/SIRT1 and autophagy pathway. Food Chem. Toxicol. 2019, 134, 110824. [Google Scholar] [CrossRef] [PubMed]
- Nieuwkoop, P.; Faber, J. (Eds.) Normal Table of Xenopus laevis (Daudin); North Holland Publishing Company: Amsterdam, The Netherlands, 1967. [Google Scholar]
- Diniz, L.P.; Almeida, J.C.; Tortelli, V.; Lopes, C.V.; Setti-Perdigão, P.; Stipursky, J.; Kahn, S.A.; Romão, L.F.; de Miranda, J.; Alves-Leon, S.V.; et al. Astrocyte-induced Synaptogenesis Is Mediated by Transforming Growth Factor β Signaling through Modulation of d-Serine Levels in Cerebral Cortex Neurons. J. Biol. Chem. 2012, 287, 41432–41445. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predes, D.; Maia, L.A.; Matias, I.; Araujo, H.P.M.; Soares, C.; Barros-Aragão, F.G.Q.; Oliveira, L.F.S.; Reis, R.R.; Amado, N.G.; Simas, A.B.C.; et al. The Flavonol Quercitrin Hinders GSK3 Activity and Potentiates the Wnt/β-Catenin Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 12078. https://doi.org/10.3390/ijms232012078
Predes D, Maia LA, Matias I, Araujo HPM, Soares C, Barros-Aragão FGQ, Oliveira LFS, Reis RR, Amado NG, Simas ABC, et al. The Flavonol Quercitrin Hinders GSK3 Activity and Potentiates the Wnt/β-Catenin Signaling Pathway. International Journal of Molecular Sciences. 2022; 23(20):12078. https://doi.org/10.3390/ijms232012078
Chicago/Turabian StylePredes, Danilo, Lorena A. Maia, Isadora Matias, Hannah Paola Mota Araujo, Carolina Soares, Fernanda G. Q. Barros-Aragão, Luiz F. S. Oliveira, Renata R. Reis, Nathalia G. Amado, Alessandro B. C. Simas, and et al. 2022. "The Flavonol Quercitrin Hinders GSK3 Activity and Potentiates the Wnt/β-Catenin Signaling Pathway" International Journal of Molecular Sciences 23, no. 20: 12078. https://doi.org/10.3390/ijms232012078




