Ojeoksan Ameliorates Cisplatin-Induced Acute Kidney Injury in Mice by Downregulating MAPK and NF-κB Pathways
Abstract
:1. Introduction
2. Results
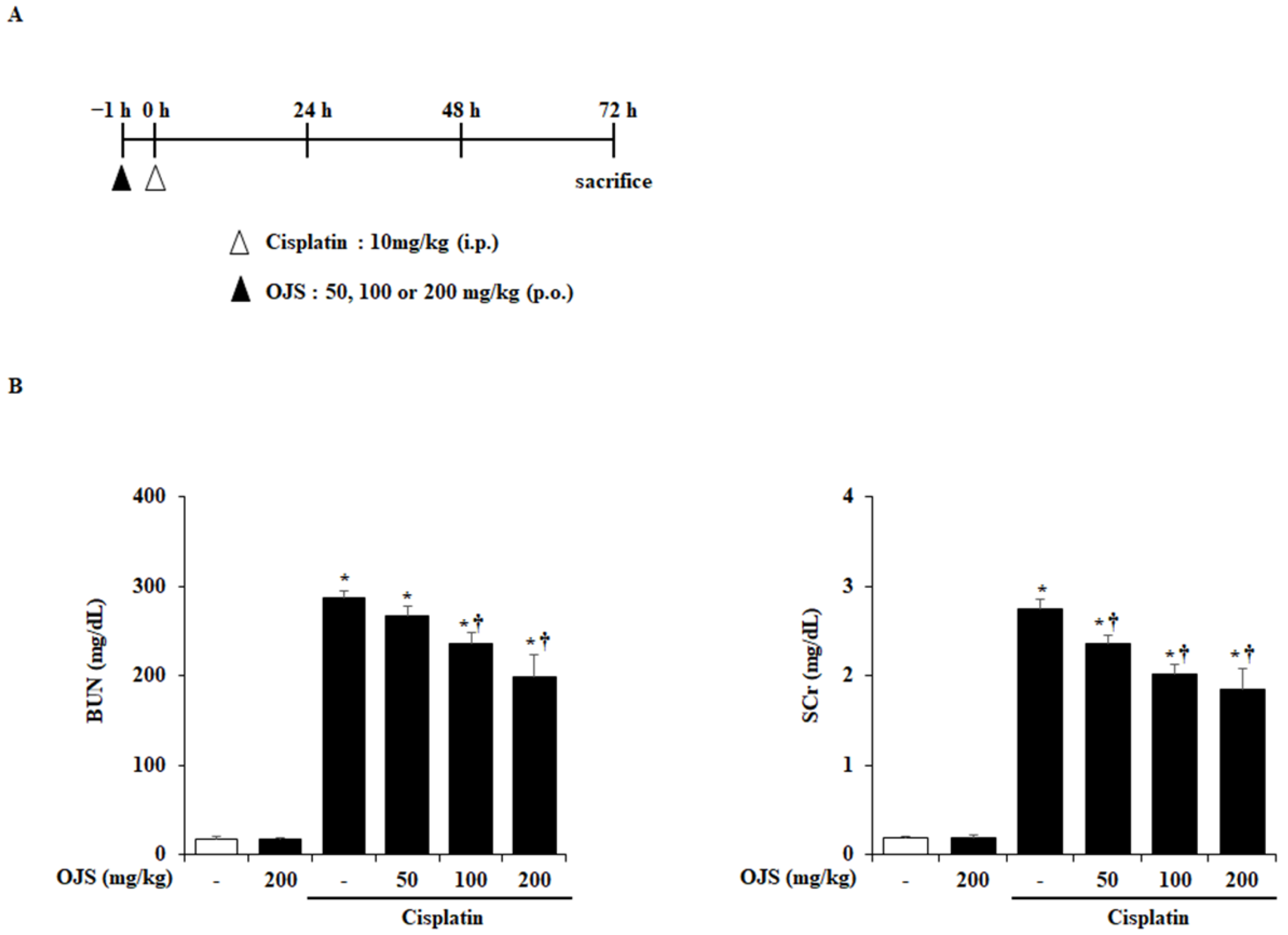
2.1. Effects of OJS on Renal Dysfunction in Cisplatin-Induced AKI
2.2. Effects of OJS on Renal Histopathological Change in Cisplatin-Induced AKI
2.3. Effects of OJS on Renal Cell Death in Cisplatin-Induced AKI
2.4. Effects of OJS on Renal Pro-Inflammatory Cytokine in Cisplatin-Induced AKI
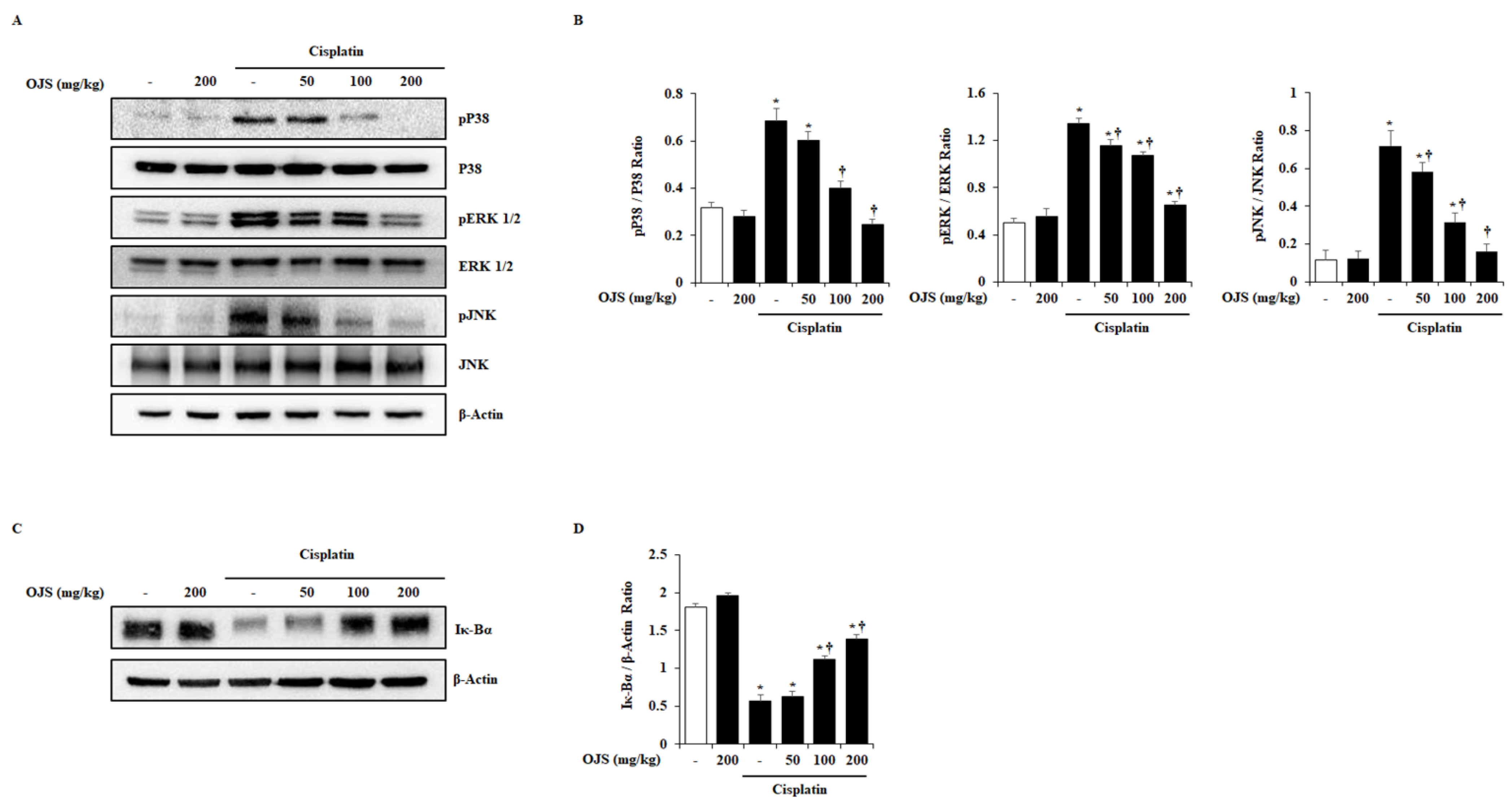
2.5. Effects of OJS on the Activation of MAPK and NF-κB Pathways in Cisplatin-Induced AKI
3. Discussion
4. Materials and Methods
4.1. Preparation of OJS
4.2. Experimental Animal Models
4.3. Measurement of BUN and SCr
4.4. Histological Analysis
4.5. Immunofluorescence Staining
4.6. TUNEL Assay
4.7. RT-PCR
4.8. Western Blot
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makris, K.; Spanou, L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin. Biochem. Rev. 2016, 37, 85–98. [Google Scholar] [PubMed]
- Nash, K.; Hafeez, A.; Hou, S. Hospital-acquired renal insufficiency. Am. J. Kidney Dis. 2002, 39, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.H.; Bushinsky, D.A.; Wish, J.B.; Cohen, J.J.; Harrington, J.T. Hospital-acquired renal insufficiency: A prospective study. Am. J. Med. 1983, 74, 243–248. [Google Scholar] [CrossRef]
- Levy, E.M.; Viscoli, C.M.; Horwitz, R.I. The effect of acute renal failure on mortality. A cohort analysis. JAMA 1996, 275, 1489–1494. [Google Scholar] [CrossRef]
- Mehta, R.L.; Pascual, M.T.; Soroko, S.; Savage, B.R.; Himmelfarb, J.; Ikizler, T.A.; Paganini, E.P.; Chertow, G.M.; Program to Improve Care in Acute Renal Disease (PICARD). Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int. 2004, 66, 1613–1621. [Google Scholar] [CrossRef] [Green Version]
- Hoste, E.A.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef]
- Aldossary, S.A. Review on pharmacology of cisplatin: Clinical use, toxicity and mechanism of resistance of cisplatin. Biomed. Pharmacol. J. 2019, 12, 7–15. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Karasawa, T.; Steyger, P.S. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol. Lett. 2015, 237, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Hanigan, M.H. Role of cysteine S-conjugate β-lyase in the metabolism of cisplatin. J. Pharmacol. Exp. Ther. 2003, 306, 988–994. [Google Scholar] [CrossRef]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. Biomed. Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Yoon, J.; Kim, H.; Ahn, Y.; Jin, S.; Wen, J.; Lee, H.; Lee, Y.; Kang, D. Inhibitory effects of herbal decoction Ojeoksan on proliferation and migration in vascular smooth muscle cells. J. Physiol. Pharmacol. 2019, 70, 287–294. [Google Scholar]
- Kim, J.D.; Son, M.S. 2013 National Health Insurance Statistical Yearbook; Health Insurance Review & Assessment Service: Wonju-si, Korea; National Health Insurance Service: Wonju-si, Korea, 2014. [Google Scholar]
- Lee, M.-J.; Hwang, D.-S.; Lee, J.-M.; Jang, J.-B.; Lee, K.-S.; Lee, C.-H. Activation of Immune System & Antimetastatic Effects of Ojeok-san by Oral Administration. J. Korean Obstet. Gynecol. 2014, 27, 34–45. [Google Scholar]
- Shin, I.S.; Lee, M.Y.; Jeon, W.Y.; Kim, J.C.; Shin, H.K. Ojeok-san, a traditional Korean herbal medicine attenuates airway inflammation and pulmonary fibrosis induced by repeated ovalbumin challenge. J. Ethnopharmacol. 2013, 149, 281–287. [Google Scholar] [CrossRef]
- Yoo, S.-R.; Jeong, S.-J.; Kim, Y.-J.; Lim, H.-S.; Jin, S.-E.; Jeon, W.-Y.; Shin, I.-S.; Shin, N.-R.; Kim, S.-S.; Kim, J.-H. Effects of water and ethanol extracts from Ojeok-san on inflammation and its related diseases. J. Intern. Korean Med. 2012, 33, 418–428. [Google Scholar]
- Han, B.H.; Seo, C.S.; Yoon, J.J.; Kim, H.Y.; Ahn, Y.M.; Eun, S.Y.; Hong, M.H.; Lee, J.G.; Shin, H.K.; Lee, H.S. The inhibitory effect of ojeoksan on early and advanced atherosclerosis. Nutrients 2018, 10, 1256. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.-M.; Moon, S.-O.; Lee, H.-H. Inhibitory effect of by Ojeok-san lipid accumulation in high fat diet-induced obesity mice and 3T3-L1 adipocytes. Korea J. Herbol. 2015, 30, 121–128. [Google Scholar] [CrossRef]
- Cunningham, P.; Sumal, A.; Patton, E.; Helms, H.; Noneman, M.T.; Martinez-Muñiz, G.; Bader, J.E.; Chatzistamou, I.; Aladhami, A.; Unger, C.; et al. Ojeok-san ameliorates visceral and somatic nociception in a mouse model of colitis induced colorectal cancer. PLoS ONE 2022, 17, e0270338. [Google Scholar] [CrossRef]
- Oh, G.S.; Kim, H.J.; Shen, A.; Lee, S.B.; Khadka, D.; Pandit, A.; So, H.S. Cisplatin-induced Kidney Dysfunction and Perspectives on Improving Treatment Strategies. Electrolyte Blood Press. 2014, 12, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Lam, A.Q.; Humphreys, B.D. Onco-nephrology: AKI in the cancer patient. Clin. J. Am. Soc. Nephrol. 2012, 7, 1692–1700. [Google Scholar] [CrossRef] [Green Version]
- Volarevic, V.; Djokovic, B.; Jankovic, M.G.; Harrell, C.R.; Fellabaum, C.; Djonov, V.; Arsenijevic, N. Molecular mechanisms of cisplatin-induced nephrotoxicity: A balance on the knife edge between renoprotection and tumor toxicity. J. Biomed. Sci. 2019, 26, 25. [Google Scholar] [CrossRef] [Green Version]
- McSweeney, K.R.; Gadanec, L.K.; Qaradakhi, T.; Ali, B.A.; Zulli, A.; Apostolopoulos, V. Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations. Cancers 2021, 13, 1572. [Google Scholar] [CrossRef]
- Tan, R.Z.; Wang, C.; Deng, C.; Zhong, X.; Yan, Y.; Luo, Y.; Lan, H.Y.; He, T.; Wang, L. Quercetin protects against cisplatin-induced acute kidney injury by inhibiting Mincle/Syk/NF-κB signaling maintained macrophage inflammation. Phytother. Res. 2020, 34, 139–152. [Google Scholar] [CrossRef]
- Dupre, T.V.; Doll, M.A.; Shah, P.P.; Sharp, C.N.; Siow, D.; Megyesi, J.; Shayman, J.; Bielawska, A.; Bielawski, J.; Beverly, L.J.; et al. Inhibiting glucosylceramide synthase exacerbates cisplatin-induced acute kidney injury. J. Lipid Res. 2017, 58, 1439–1452. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Ravichandran, K.; Ozkok, A.; Wang, Q.; He, Z.; Jani, A.; Ljubanovic, D.; Douglas, I.S.; Edelstein, C.L. The water-soluble triptolide derivative PG490-88 protects against cisplatin-induced acute kidney injury. J. Pharmacol. Exp. Ther. 2014, 349, 518–525. [Google Scholar] [CrossRef] [Green Version]
- Sahu, B.D.; Kumar, J.M.; Sistla, R. Baicalein, a bioflavonoid, prevents cisplatin-induced acute kidney injury by up-regulating antioxidant defenses and down-regulating the MAPKs and NF-κB pathways. PLoS ONE 2015, 10, e0134139. [Google Scholar] [CrossRef] [Green Version]
- Yucetas, C.S.; Ucler, N.; Cakir, T. The Effects of Agomelatine on The Biochemical and Pathological Features of Cisplatin-Induced Peripheral Neuropathy: The First Experimental Study in Rats. Turk. Neurosurg. 2019, 29, 901–908. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ye, Z.W.; Tew, K.D.; Townsend, D.M. Cisplatin chemotherapy and renal function. Adv. Cancer Res. 2021, 152, 305–327. [Google Scholar] [CrossRef]
- Salazar, J.H. Overview of Urea and Creatinine. Labmedicine 2014, 45, E19–E20. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, V.S.; Ferguson, M.A.; Bonventre, J.V. Biomarkers of acute kidney injury. J. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 463–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peres, L.A.; da Cunha, A.D., Jr. Acute nephrotoxicity of cisplatin: Molecular mechanisms. J. Bras. Nefrol. 2013, 35, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-Y.; Lou, D.-Y.; Zhou, L.-Q.; Wang, J.-C.; Yang, B.; He, Q.-J.; Wang, J.-J.; Weng, Q.-J. Natural products: Potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol. Sin. 2021, 42, 1951–1969. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, Y.; Kinoshita, K.; Yano, T.; Asato, K.; Shiga, T.; Hino, S.; Niki, K.; Nagare, Y.; Kishimoto, K.; Shimazu, H. Signaling through the interleukin-18 receptor α attenuates inflammation in cisplatin-induced acute kidney injury. Kidney Int. 2012, 82, 892–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.; Zhang, Z.; He, L.; Wang, Y. CXCL16 regulates cisplatin-induced acute kidney injury. Oncotarget 2016, 7, 31652–31662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humanes, B.; Camano, S.; Lara, J.M.; Sabbisetti, V.; Gonzalez-Nicolas, M.A.; Bonventre, J.V.; Tejedor, A.; Lazaro, A. Cisplatin-induced renal inflammation is ameliorated by cilastatin nephroprotection. Nephrol. Dial. Transplant. 2017, 32, 1645–1655. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Miao, J.; Liu, W.; Peng, L.; Chen, Y.; Zhong, Q. Formononetin protects against cisplatin-induced acute kidney injury through activation of the PPARα/Nrf2/HO-1/NQO1 pathway. Int. J. Mol. Med. 2021, 47, 511–522. [Google Scholar] [CrossRef]
- Miyasato, Y.; Yoshizawa, T.; Sato, Y.; Nakagawa, T.; Miyasato, Y.; Kakizoe, Y.; Kuwabara, T.; Adachi, M.; Ianni, A.; Braun, T.; et al. Sirtuin 7 Deficiency Ameliorates Cisplatin-induced Acute Kidney Injury Through Regulation of the Inflammatory Response. Sci. Rep. 2018, 8, 5927. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.U.; Kim, D.G.; Choi, J.W.; Shin, J.Y.; Kweon, B.; Zhou, Z.; Lee, H.S.; Song, H.J.; Bae, G.S.; Park, S.J. Loganin Attenuates the Severity of Acute Kidney Injury Induced by Cisplatin through the Inhibition of ERK Activation in Mice. Int. J. Mol. Sci. 2021, 22, 1421. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Seger, R.; Krebs, E.G. The MAPK signaling cascade. FASEB J. 1995, 9, 726–735. [Google Scholar] [CrossRef]
- Jo, S.-K.; Cho, W.Y.; Sung, S.A.; Kim, H.K.; Won, N.H. MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int. 2005, 67, 458–466. [Google Scholar] [CrossRef]
- Ramesh, G.; Reeves, W.B. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am. J. Physiol.-Ren. Physiol. 2005, 289, F166–F174. [Google Scholar] [CrossRef] [Green Version]
- Ozkok, A.; Ravichandran, K.; Wang, Q.; Ljubanovic, D.; Edelstein, C.L. NF-κB transcriptional inhibition ameliorates cisplatin-induced acute kidney injury (AKI). Toxicol. Lett. 2016, 240, 105–113. [Google Scholar] [CrossRef]
- Yeh, C.-C.; Kao, S.-J.; Lin, C.-C.; Wang, S.-D.; Liu, C.-J.; Kao, S.-T. The immunomodulation of endotoxin-induced acute lung injury by hesperidin in vivo and in vitro. Life Sci. 2007, 80, 1821–1831. [Google Scholar] [CrossRef]
- Choi, I.-Y.; Kim, S.-J.; Jeong, H.-J.; Park, S.-H.; Song, Y.-S.; Lee, J.-H.; Kang, T.-H.; Park, J.-H.; Hwang, G.-S.; Lee, E.-J.; et al. Hesperidin inhibits experssion of hypoxia inducible factor-1 alpha and inflammatory cytokine production from mast cells. Mol. Cell Biochem. 2007, 305, 153–161. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Gong, J. Naringin Attenuated Acute Lung Injury in Rat Model with Acute Pancreatitis in Pregnancy through Inactivation of p38 MAPK Pathway. Signa Vitae 2020, 16, 189–194. [Google Scholar]
- Yang, Y.; Gong, W.; Jin, C.; Chen, Z.; Zhang, L.; Zou, Y.; Quan, S.; Huang, H. Naringin ameliorates experimental diabetic renal fibrosis by inhibiting the ERK1/2 and JNK MAPK signaling pathways. J. Funct. Foods 2018, 50, 53–62. [Google Scholar] [CrossRef]
- Rim, H.-K.; Cho, W.; Sung, S.H.; Lee, K.-T. Nodakenin suppresses lipopolysaccharide-induced inflammatory responses in macrophage cells by inhibiting tumor necrosis factor receptor-associated factor 6 and nuclear factor-κB pathways and protects mice from lethal endotoxin shock. J. Pharmacol. Exp. Ther. 2012, 342, 654–664. [Google Scholar] [CrossRef]
- Zhang, F.-L.; Zhou, B.-W.; Yan, Z.-Z.; Zhao, J.; Zhao, B.-C.; Liu, W.-F.; Li, C.; Liu, K.-X. 6-Gingerol attenuates macrophages pyroptosis via the inhibition of MAPK signaling pathways and predicts a good prognosis in sepsis. Cytokine 2020, 125, 154854. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Wang, J.; Wang, C.; Yang, Z.; Wang, C.; Zhu, Y.; Zhang, J. Paeoniflorin and albiflorin attenuate neuropathic pain via MAPK pathway in chronic constriction injury rats. Evid.-Based Complement. Alternat. Med. 2016, 2016, 8082753. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Xiao, Z.; Zhao, R.; Lu, C.; Zhang, Y. Paeoniflorin suppressed IL-22 via p38 MAPK pathway and exerts anti-psoriatic effect. Life Sci. 2017, 180, 17–22. [Google Scholar] [CrossRef]
- Serreli, G.; Naitza, M.R.; Zodio, S.; Leoni, V.P.; Spada, M.; Melis, M.P.; Boronat, A.; Deiana, M. Ferulic acid metabolites attenuate LPS-induced inflammatory Response in enterocyte-like cells. Nutrients 2021, 13, 3152. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, Z.; Li, J.; Shi, Y.; Jin, N.; Zou, W.; Gao, Q.; Wang, W.; Liu, F. Ferulic acid inhibits bovine endometrial epithelial cells against LPS-induced inflammation via suppressing NK-κB and MAPK pathway. Res. Vet. Sci. 2019, 126, 164–169. [Google Scholar] [CrossRef]
- Li, W.; Zhi, W.; Zhao, J.; Yao, Q.; Liu, F.; Niu, X. Cinnamaldehyde protects VSMCs against ox-LDL-induced proliferation and migration through S arrest and inhibition of p38, JNK/MAPKs and NF-κB. Vasc. Pharmacol. 2018, 108, 57–66. [Google Scholar] [CrossRef]
- Badger-Emeka, L.I.; Emeka, P.M.; Thirugnanasambantham, K.; Ibrahim, H.I.M. Anti-Allergic Potential of Cinnamaldehyde via the Inhibitory Effect of Histidine Decarboxylase (HDC) Producing Klebsiella pneumonia. Molecules 2020, 25, 5580. [Google Scholar] [CrossRef]
- Ye, S.; Zhu, Y.; Ming, Y.; She, X.; Liu, H.; Ye, Q. Glycyrrhizin protects mice against renal ischemia-reperfusion injury through inhibition of apoptosis and inflammation by downregulating p38 mitogen-activated protein kinase signaling. Exp. Ther. Med. 2014, 7, 1247–1252. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Wang, P.; Lu, S.; Guo, R.; Gao, W.; Tong, H.; Yin, Y.; Han, X.; Liu, T.; Chen, X.; et al. Liquiritin, a novel inhibitor of TRPV1 and TRPA1, protects against LPS-induced acute lung injury. Cell Calcium 2020, 88, 102198. [Google Scholar] [CrossRef]



| Latin Name | Active Ingredient | Bioactivity (Mechanism) |
|---|---|---|
| Citri unshii Percarpium | naringin | Anti-inflammatory (Suppression of p38) [49] Anti-fibrosis (Suppression of ERK and JNK) [50] |
| Aurantii Fructus Immaturus | hesperidin neohesperidin naringin | Anti-inflammatory (Suppression of p38,ERK, JNK and NF-κB) [47,48] |
| Angelicae Gigantis Radix | nodakenin | Anti-inflammatory (Suppression of p38 and NF-κB) [51] |
| Zingiberis Rhizoma | 6-gingerol | Anti-inflammatory (Suppression of p38, ERK, and JNK) [52] |
| Paeoniae Radix | albiflorin paeoniflorin | Analgesic effect (Suppression of p38 and JNK) [53] Anti-psoriatic effect (Suppression of p38) [54] |
| Cnidii Rhizoma | ferulic acid | Anti-inflammatory (Suppression of p38,ERK, JNK, and NF-κB) [55,56] |
| Cinnamomi Cortex | cinnamaldehyde | Anti-atherosclerosis (Suppression of p38, JNK, and NF-κB) [57] Anti-allergic effect (Suppression of p38) [58] |
| Glycyrrhuzae Radix et Rhizoma | glycyrrhizin liquiritin | Anti-apoptosis and anti-inflammatory (Suppression of p38) [59] Anti-asthma (Suppression of NF-κB) [60] |
| Latin Name | Scientific Name | Amount (g) | Origin |
|---|---|---|---|
| Atractylodis Rhizoma | Atractylodes lancea DC | 7.5 | China |
| Citri unshii Percarpium | Citrus reticulata Blanco | 3.7 | Korea |
| Ephedrae Herba | Ephedra sinica Stapf | 3.7 | China |
| Magnoliae Cortex | Magnolia offcinalis Rehder & E.H. Wilson | 3.0 | China |
| Platycodi Radix | Platycodon grandiflorus A. DC | 3.0 | Korea |
| Aurantii Fructus Immaturus | Citrus auratium L. | 3.0 | China |
| Angelicae Gigantis Radix | Angelica gigas Nakai | 3.0 | Korea |
| Zingiberis Rhizoma | Zingiber officinale Roscoe | 3.0 | Korea |
| Paeoniae Radix | Paeonia lactiflora Pall | 3.0 | Korea |
| Poria Sclerotium | Wolfiporia extensa | 3.0 | Korea |
| Angelicae Dahuricae Radix | Angelica dahurica | 2.6 | Korea |
| Cnidii Rhizoma | Ligusticum officinale Kitag | 2.6 | Korea |
| Pinelliae Tuber | Pinellia ternate Ten. Ex Breitenb | 2.6 | China |
| Cinnamomi Cortex | Cinnamomum cassia J. Presl | 2.6 | Vietnam |
| Glycyrrhuzae Radix et Rhizoma | Glycyrrhiza uralensis Fisch | 2.2 | China |
| Zingiberis Rhizoma recens | Zingiber offcinale Roscoe | 3.7 | Korea |
| Allii Fistulosi Bulbus | Alluim fistulosum L. | 3.7 | Korea |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-U.; Kweon, B.; Oh, J.-Y.; Seo, C.-S.; Kim, D.-G.; Kim, H.-Y.; Lee, H.-S.; Park, S.-J.; Bae, G.-S. Ojeoksan Ameliorates Cisplatin-Induced Acute Kidney Injury in Mice by Downregulating MAPK and NF-κB Pathways. Int. J. Mol. Sci. 2022, 23, 12254. https://doi.org/10.3390/ijms232012254
Kim D-U, Kweon B, Oh J-Y, Seo C-S, Kim D-G, Kim H-Y, Lee H-S, Park S-J, Bae G-S. Ojeoksan Ameliorates Cisplatin-Induced Acute Kidney Injury in Mice by Downregulating MAPK and NF-κB Pathways. International Journal of Molecular Sciences. 2022; 23(20):12254. https://doi.org/10.3390/ijms232012254
Chicago/Turabian StyleKim, Dong-Uk, Bitna Kweon, Jin-Young Oh, Chang-Seob Seo, Dong-Gu Kim, Hye-Yoom Kim, Ho-Sub Lee, Sung-Joo Park, and Gi-Sang Bae. 2022. "Ojeoksan Ameliorates Cisplatin-Induced Acute Kidney Injury in Mice by Downregulating MAPK and NF-κB Pathways" International Journal of Molecular Sciences 23, no. 20: 12254. https://doi.org/10.3390/ijms232012254






