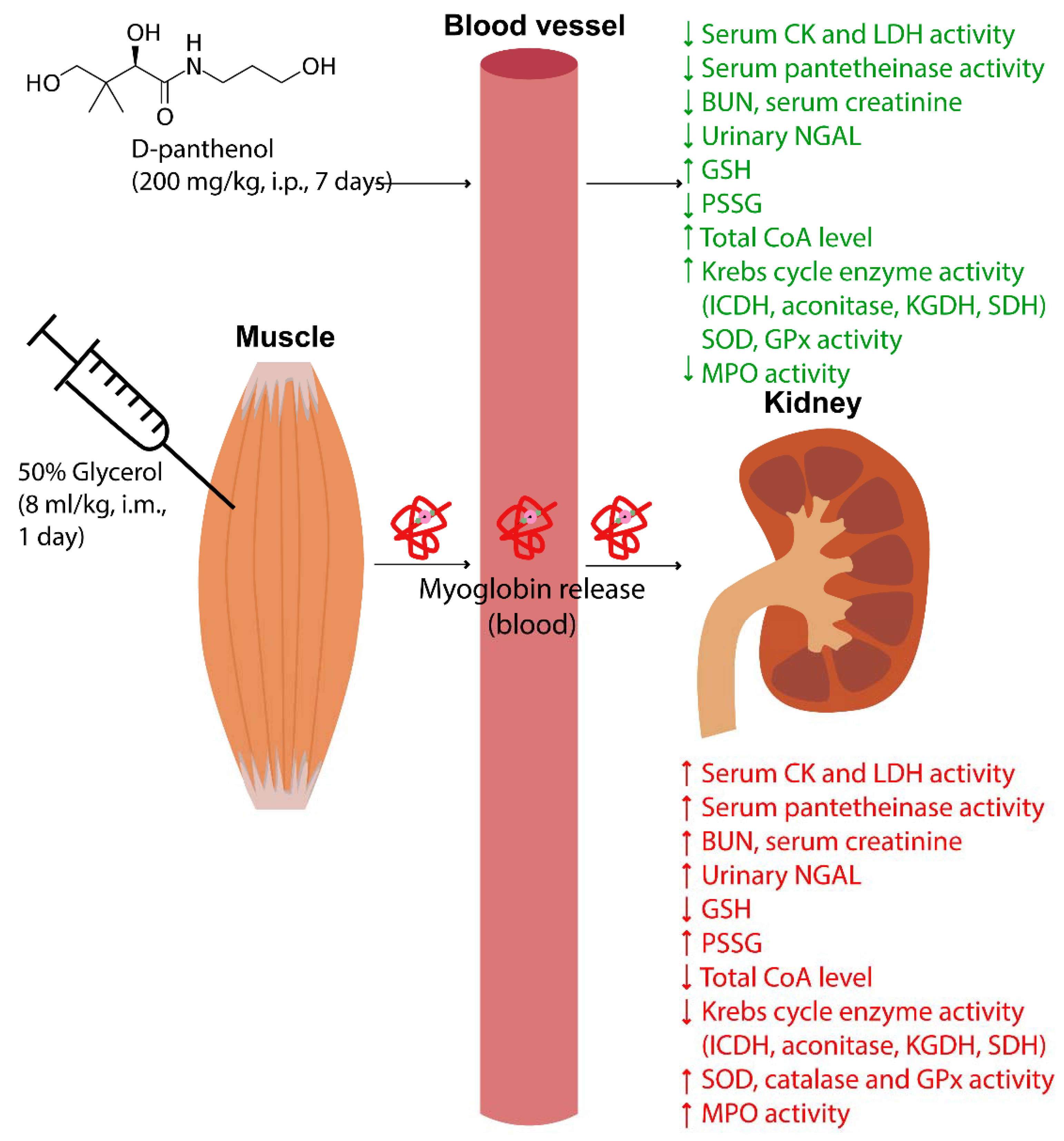
Protective Effect of D-Panthenol in Rhabdomyolysis-Induced Acute Kidney Injury
Abstract
:1. Introduction
2. Results
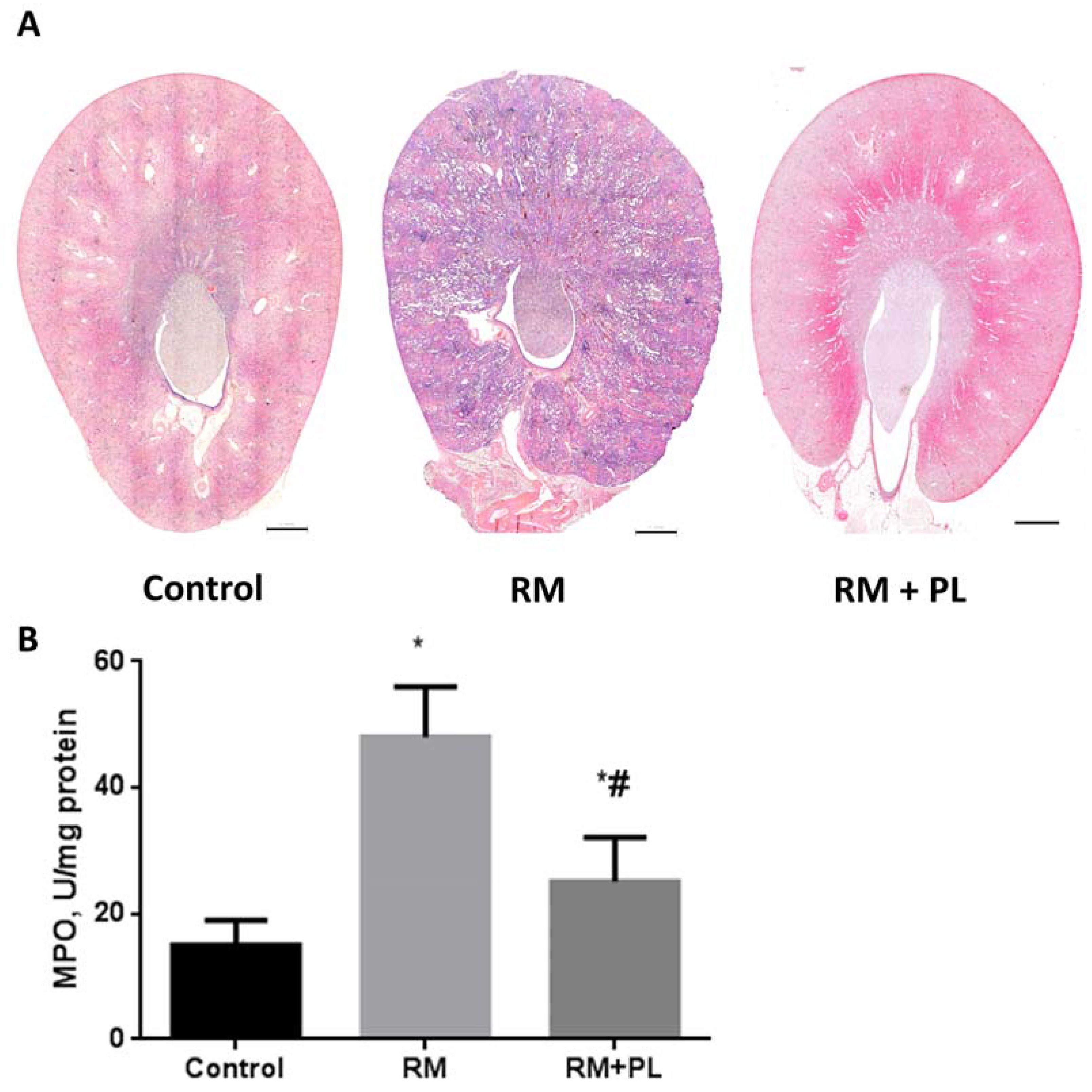
2.1. Protective Effects on Renal Morphology
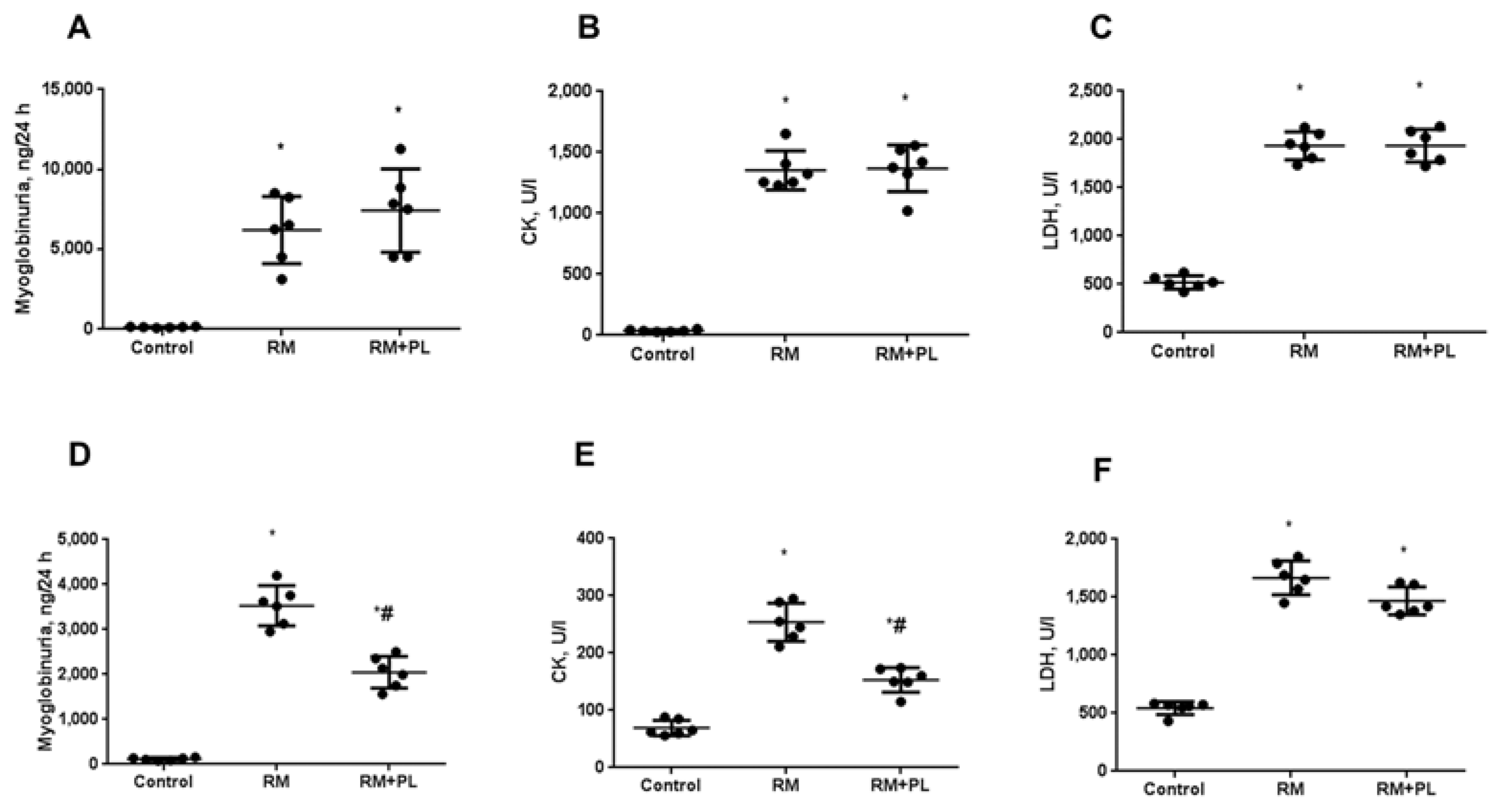
2.2. Changes in the Biochemical Parameters of Blood Serum and Urine after Rhabdomyolysis and PL Administration
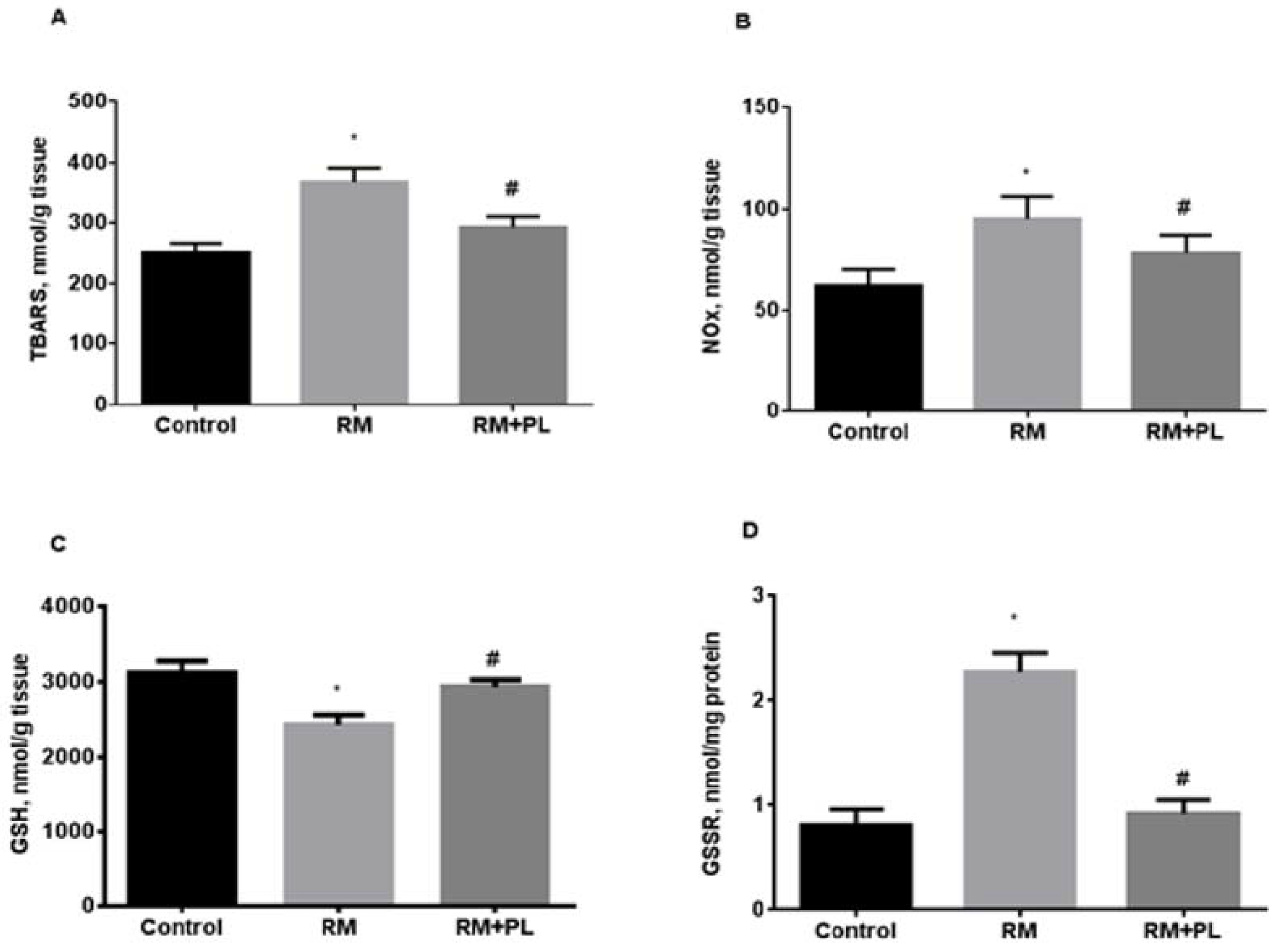
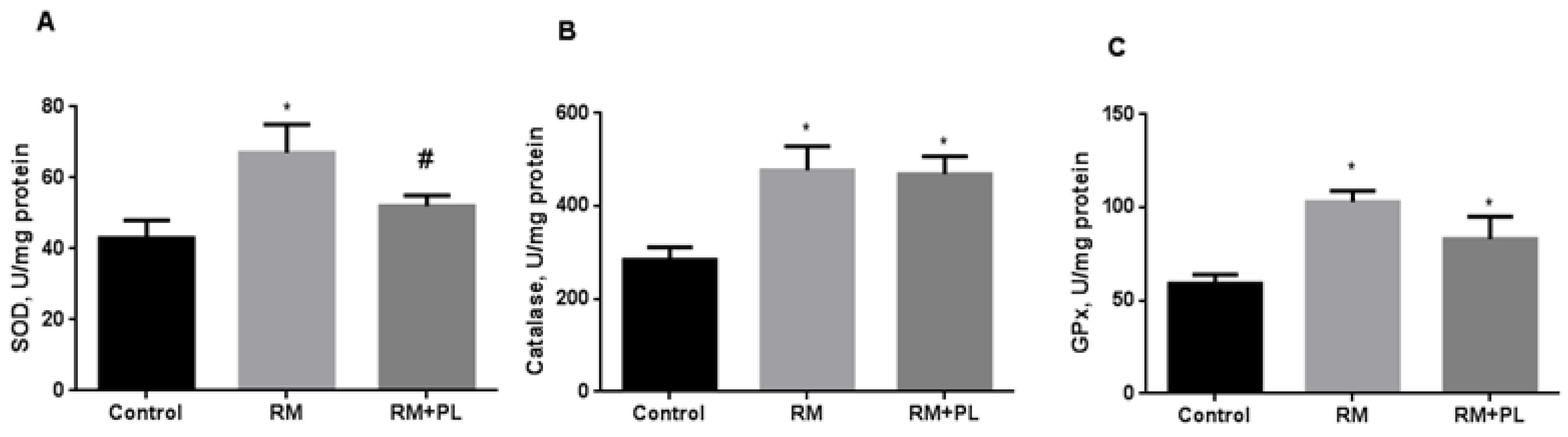
2.3. Oxidative Stress and Activity of Antioxidant Enzymes in Renal Tissue during Rhabdomyolysis and PL Administration
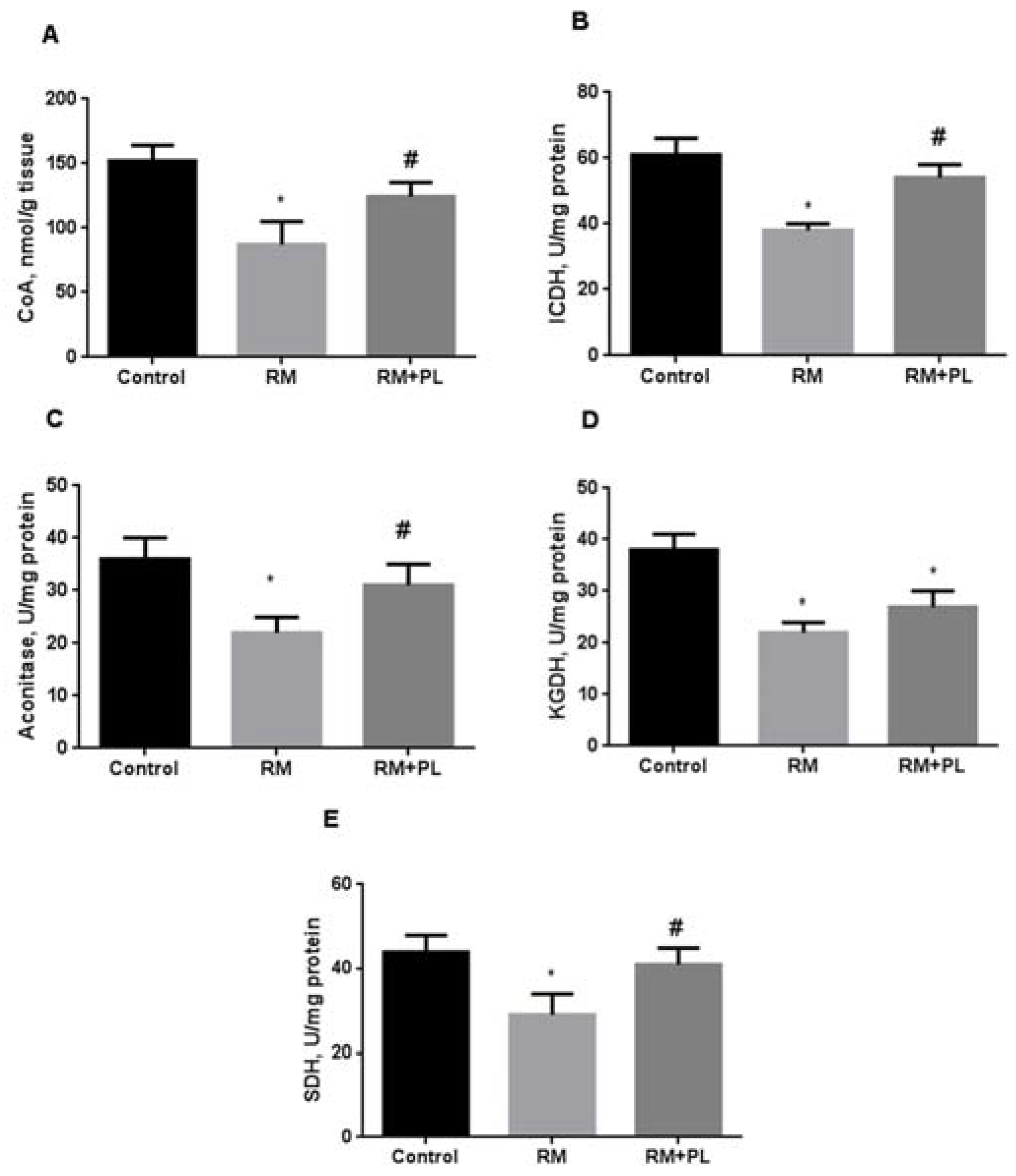
2.4. Determination of Total CoA Levels and Krebs Cycle Enzyme Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Experimental Design
4.4. Morphological Studies of Kidneys
4.5. Biochemical Parameters of Blood Serum and Urine
4.6. Kidney Homogenization and Isolation of Mitochondria
4.7. Oxidative Stress, MPO, and Antioxidant Enzyme Activity Estimation
4.8. Determination of Total CoA and Krebs Cycle Enzyme Activity
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanley, M.; Chippa, V.; Aeddula, N.R.; Quintanilla Rodriguez, B.S.; Adigun, R. Rhabdomyolysis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Efstratiadis, G.; Voulgaridou, A.; Nikiforou, D.; Kyventidis, A.; Kourkouni, E.; Vergoulas, G. Rhabdomyolysis updated. Hippokratia 2007, 11, 129–137. [Google Scholar] [PubMed]
- Giannoglou, G.D.; Chatzizisis, Y.S.; Misirli, G. The syndrome of rhabdomyolysis: Pathophysiology and diagnosis. Eur. J. Intern. Med. 2007, 18, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Thorson, P.; Penmatsa, K.R.; Gupta, P. Rhabdomyolysis: Revisited. Ulst. Med. J. 2021, 90, 61–69. [Google Scholar] [PubMed]
- Nayak, K.; Jindal, A. Myoglobinuria and Acute Kidney Injury. J. Integr. Nephrol. Androl. 2015, 2, 50–54. [Google Scholar] [CrossRef]
- Khan, F.Y. Rhabdomyolysis: A review of the literature. Neth. J. Med. 2009, 67, 272–283. [Google Scholar] [PubMed]
- Petejova, N.; Martinek, A. Acute kidney injury due to rhabdomyolysis and renal replacement therapy: A critical review. Crit. Care 2014, 18, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilgic, Y.; Akbulut, S.; Aksungur, Z.; Erdemli, M.; Ozhan, O.; Parlakpinar, H.; Vardi, N.; Turkoz, Y. Protective effect of dexpanthenol against cisplatin-induced hepatotoxicity. Exp. Ther. Med. 2018, 16, 4049–4057. [Google Scholar] [CrossRef] [Green Version]
- Altintas, R.; Parlakpinar, H.; Beytur, A.; Vardi, N.; Polat, A.; Sagir, M.; Odabas, G.P. Protective Effect of Dexpanthenol on Ischemia-Reperfusion-Induced Renal Injury in Rats. Kidney Blood Press. Res. 2012, 36, 220–230. [Google Scholar] [CrossRef]
- Pınar, N.; Topaloğlu, M.; Seçinti, İ.E.; Büyük, E.; Kaplan, M. Protective effect of dexpanthenol on cisplatin induced nephrotoxicity in rats. Biotech. Histochem. 2021, 97, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Pinar, N.; Ozcan, O.; Dogan, E.; Cakirca, G. Protective effect of dexpanthenol on gentamicin-induced nephrotoxicity in rats. Ann. Med. Res. 2019, 26, 1787–1791. [Google Scholar] [CrossRef]
- Demirci, B.; Yilmaz, M.; Bektas Uysal, H. Protective effect of dexpanthenol (vitamin B5) in a rat model of LPS-induced endotoxic shock. Turk. J. Biochem. 2018, 43, 632–637. [Google Scholar] [CrossRef]
- Boutaud, O.; Roberts, L.J. Mechanism-based therapeutic approaches to rhabdomyolysis-induced renal failure. Free Radic. Biol. Med. 2011, 51, 1062–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plotnikov, E.Y.; Chupyrkina, A.A.; Pevzner, I.B.; Isaev, N.K.; Zorov, D.B. Myoglobin causes oxidative stress, increase of NO production and dysfunction of kidney’s mitochondria. Biochim. Biophys. Acta. 2009, 1792, 796–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenovich, D.S.; Plotnikov, E.Y.; Titko, O.V.; Lukiyenko, E.P.; Kanunnikova, N.P. Effects of Panthenol and N-Acetylcysteine on Changes in the Redox State of Brain Mitochondria under Oxidative Stress In Vitro. Antioxidants 2021, 10, 1699. [Google Scholar] [CrossRef] [PubMed]
- Moiseenok, A.G.; Kanunnikova, N.P.; Gurinovich, V.A. Biotransformation of D-panthenol and its action in protection of activation of oxidizing processes in brain. J. Neurochem. 2003, 85, 30. [Google Scholar] [CrossRef]
- Semenovich, D.S.; Kanunnikova, N.P.; Moiseenok, A.G. Oxidative stress in mitochondria of the brain tissue with aluminum neurotoxycosis and applying of glutathione and modulators of coenzyme A biosynthesis. Dokl. Natl. Acad. Sci. Belarus 2020, 64, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Semenovich, D.S.; Lukiyenko, E.P.; Titko, O.V.; Kanunnikova, N.P. Panthenol and succinate as modulators of changes of redox balance and energy metabolism in the experimental model of Parkinson’s disease. Ind. J. Appl. Res. 2018, 8, 436–438. [Google Scholar]
- Chernikevich, I.P.; Dorofeev, B.F.; Moĭseenok, A.G. Possible ways of regulating detoxifying processes in the alcohol dehydrogenase reaction with pantothenic acid derivatives. Vopr. Med. Khim. 1993, 39, 38–40. [Google Scholar] [PubMed]
- Kelly, G.S. Pantothenic acid. Monograph. Altern. Med. Rev. 2011, 16, 263–274. [Google Scholar] [PubMed]
- Etensel, B.; Ozkisacik, S.; Ozkara, E.; Karul, A.; Oztan, O.; Yazici, M.; Gürsoy, H. Dexpanthenol attenuates lipid peroxidation and testicular damage at experimental ischemia and reperfusion injury. Pediatr. Surg. Int. 2007, 23, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Karahan, G.; Kaya, H.; Eyceyurt, R.S.; Erdogan, M.A.; Yigitturk, G.; Erbas, O. Dexpanthenol reduces fibrosis and aids repair following nerve laceration and neurorrhaphy. Exp. Ther. Med. 2021, 21, 207. [Google Scholar] [CrossRef]
- Bayrak, O.; Seckiner, I.; Solakhan, M.; Karakok, M.; Erturhan, S.M.; Yagci, F. Effects of intravesical dexpanthenol use on lipid peroxidation and bladder histology in a chemical cystitis animal model. Urology 2012, 79, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y.; Chupyrkina, A.A.; Jankauskas, S.S.; Pevzner, I.B.; Silachev, D.N.; Skulachev, V.P.; Zorov, D.B. Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochim. Biophys. Acta. 2011, 1812, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Andrianova, N.V.; Zorov, D.B.; Plotnikov, E.Y. Targeting Inflammation and Oxidative Stress as a Therapy for Ischemic Kidney Injury. Biochemistry 2020, 85, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y.; Morosanova, M.A.; Pevzner, I.B.; Zorova, L.D.; Manskikh, V.N.; Pulkova, N.V.; Galkina, S.I.; Skulachev, V.P.; Zorov, D.B. Protective effect of mitochondria-targeted antioxidants in an acute bacterial infection. Proc. Natl. Acad. Sci. USA 2013, 110, E3100–E3108. [Google Scholar] [CrossRef] [Green Version]
- Slyshenkov, V.S.; Dymkowska, D.; Wojtczak, L. Pantothenic acid and pantothenol increase biosynthesis of glutathione by boosting cell energetics. FEBS Lett. 2004, 569, 169–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gout, I. Coenzyme A, protein CoAlation and redox regulation in mammalian cells. Biochem. Soc. Trans. 2018, 46, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.P.; Muthuraman, A.; Jaggi, A.S.; Singh, N.; Grover, K.; Dhawan, R. Animal models of acute renal failure. Pharmacol. Rep. 2012, 64, 31–44. [Google Scholar] [CrossRef]
- Craig, E.A.; Yan, Z.; Zhao, Q.J. The relationship between chemical-induced kidney weight increases and kidney histopathology in rats. J. Appl. Toxicol. 2014, 35, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K. Creatinine assay by a reaction-kinetic principle. Clin. Chim. Acta 1972, 41, 209–217. [Google Scholar] [CrossRef]
- Rahmatullah, M.; Boyde, T.R. Improvements in the determination of urea using diacetyl monoxime; methods with and without deproteinisation. Clin. Chim. Acta 1980, 107, 3–9. [Google Scholar] [CrossRef]
- Rommelaere, S.; Millet, V.; Gensollen, T.; Bourges, C.; Eeckhoute, J.; Hennuyer, N.; Baugé, E.; Chasson, L.; Cacciatore, I.; Staels, B.; et al. PPARalpha regulates the production of serum Vanin-1 by liver. FEBS Lett. 2013, 587, 3742–3748. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.; Lardy, H. Isolation of Rat Liver and Kidney Mitochondria. Methods Enzymol. 1967, 10, 94–101. [Google Scholar] [CrossRef]
- Peterson, G.L. Determination of total protein. Methods Enzymol. 1983, 91, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Sorte, K.; Basak, A. Development of a modified copper-cadmium reduction method for rapid assay of total nitric oxide. Anal. Methods 2010, 2, 944–947. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Akerboom, T.P.; Sies, H. [48] Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. In Methods in Enzymology; Academic Press: New York, NY, USA, 1981; Volume 77, pp. 373–382. [Google Scholar] [CrossRef]
- Kettle, A.J.; Winterbourn, C.C. Assays for the chlorination activity of myeloperoxidase. Methods Enzymol. 1994, 233, 502–512. [Google Scholar] [CrossRef]
- Kostyuk, V.A.; Potapovich, A.I. Superoxide-driven oxidation of quercetin and a simple sensitive assay for determination of superoxide dismutase. Biochem. Int. 1989, 19, 1117–1124. [Google Scholar] [PubMed]
- Shangari, N.; O’Brien, P.J. Catalase Activity Assays. Curr. Protoc. Toxicol. [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, P.K.; Garland, P.B. Assay of coenzyme A and some acyl derivatives. Methods Enzymol. 1969, 13, 535–551. [Google Scholar] [CrossRef]
- Sanadi, D.R. α-ketoglutarate dehydrogenase from pig heart. Methods Enzymol. 1969, 13, 52–55. [Google Scholar] [CrossRef]
- Popova, T.; Pinheiro de Carvalho, M.A.; Matasova, L.; Medvedeva, L. Regulation of mitochondrial NADP-isocitrate dehydrogenase in rat heart during ischemia. Mol. Cell. Biochem. 2007, 294, 97–105. [Google Scholar] [CrossRef]
- Delaval, E.; Perichon, M.; Friguet, B. Age-related impairment of mitochondrial matrix aconitase and ATP-stimulated protease in rat liver and heart. Eur. J. Biochem. 2004, 271, 4559–4564. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.P. Determination of the activity of succinate, NADH, choline, and alpha-glycerophosphate dehydrogenases. Methods Biochem. Anal. 1974, 22, 123–175. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Dekker, E.E. Evidence for the identity and some comparative properties of alpha-ketoglutarate and 2-keto-4-hydroxyglutarate dehydrogenase activity. J. Biol. Chem. 1980, 255, 1107–1112. [Google Scholar] [CrossRef]


| Groups | Urinary NGAL, ng/24 h | Serum Pantetheinase, U/L | Serum Creatinine, µmol/L | BUN, mmol/L |
|---|---|---|---|---|
| Control | 201 ± 40 | 16 ± 4 | 65 ± 6 | 6.4 ± 1.2 |
| RM | 2220 ± 440 * | 52 ± 8 * | 142 ± 10 * | 18.5 ± 1.5 * |
| RM + PL | 1258 ± 230 *# | 31 ± 6 *# | 106 ± 8 *# | 10.3 ± 1.4 *# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenovich, D.S.; Plotnikov, E.Y.; Lukiyenko, E.P.; Astrowski, A.A.; Kanunnikova, N.P. Protective Effect of D-Panthenol in Rhabdomyolysis-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2022, 23, 12273. https://doi.org/10.3390/ijms232012273
Semenovich DS, Plotnikov EY, Lukiyenko EP, Astrowski AA, Kanunnikova NP. Protective Effect of D-Panthenol in Rhabdomyolysis-Induced Acute Kidney Injury. International Journal of Molecular Sciences. 2022; 23(20):12273. https://doi.org/10.3390/ijms232012273
Chicago/Turabian StyleSemenovich, Dmitry S., Egor Y. Plotnikov, Elena P. Lukiyenko, Alexander A. Astrowski, and Nina P. Kanunnikova. 2022. "Protective Effect of D-Panthenol in Rhabdomyolysis-Induced Acute Kidney Injury" International Journal of Molecular Sciences 23, no. 20: 12273. https://doi.org/10.3390/ijms232012273






