Transcription Factors as Important Regulators of Changes in Behavior through Domestication of Gray Rats: Quantitative Data from RNA Sequencing
Abstract
:1. Introduction
2. Results
2.1. Analysis of RNA-Seq Data from Brain Tissue Samples of Tame and Aggressive Rats
2.2. Gene Expression Analysis in the MT of Tame and Aggressive Rats by qPCR
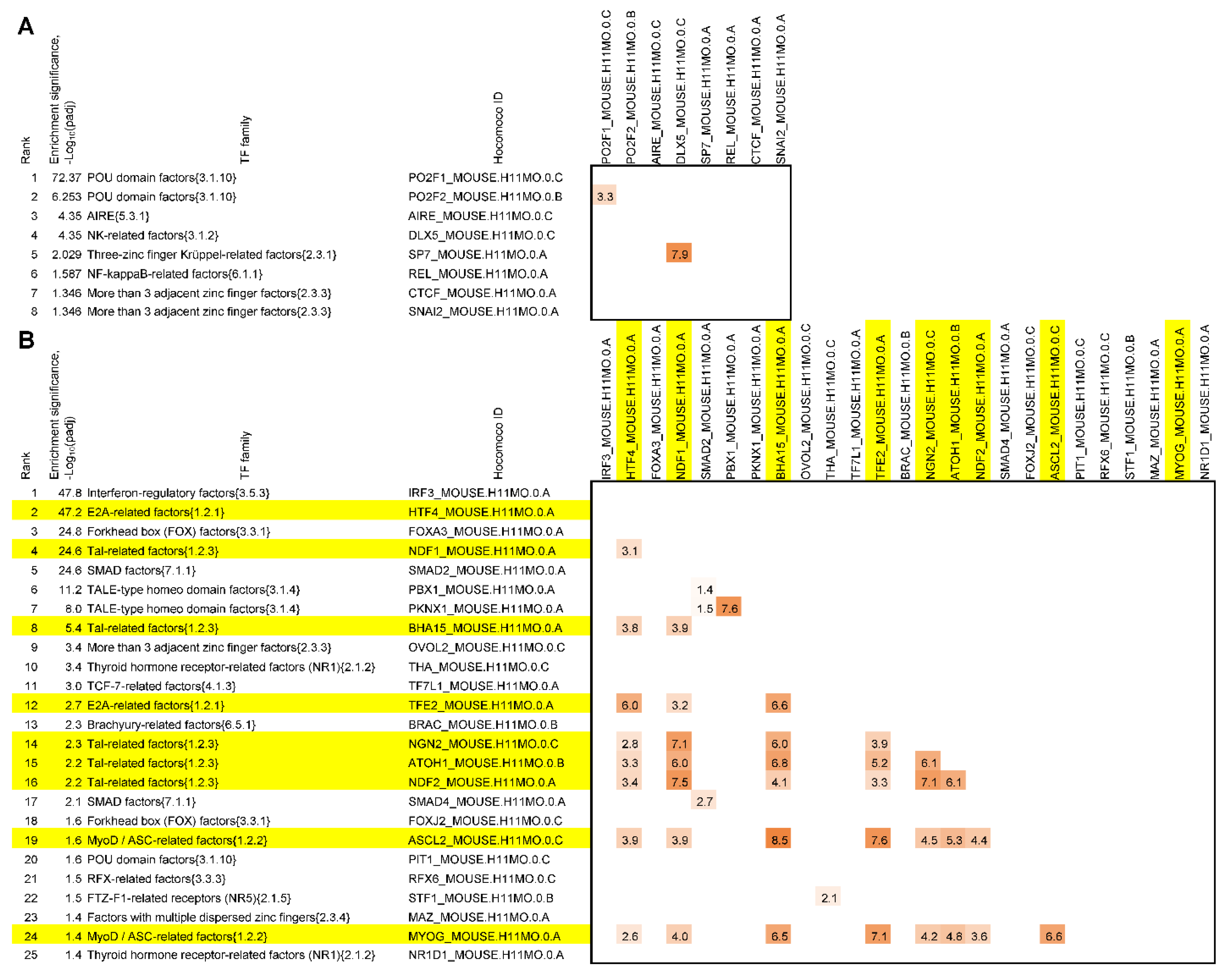
2.3. Analysis of Enrichment with Binding Sites for TFs
3. Discussion
3.1. Genes Encoding Transcription Factors Ascl3, Fos, and Fosb and Gene of Transcription Regulator Arc
3.2. The Effect of the SNP in the TATA Box of the Ascl3 Gene
3.3. ASCL3 as an Important Regulator of Changes in Behavior through Domestication of Gray Rats
4. Materials and Methods
4.1. Experimental Animals
4.2. Total RNA Extraction, Library Construction, and RNA-Seq Data Analysis
4.3. qPCR
4.4. Motif Enrichment Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASCL | achaete-scute complex like |
| bHLH | basic helix–loop–helix |
| DEG | differentially expressed gene |
| MT | tegmental region of the midbrain |
| PAG | periaqueductal gray |
| qPCR | quantitative PCR |
| SNP | single-nucleotide polymorphism |
| TF | transcription factor |
| TBP | TATA-binding protein |
References
- Rubin, C.J.; Zody, M.; Eriksson, J.; Meadows, J.R.S.; Sherwood, E.; Webster, M.T.; Jiang, L.; Ingman, M.; Sharpe, T.; Ka, S.; et al. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature 2010, 464, 587–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axelsson, E.; Ratnakumar, A.; Arendt, M.-L.; Maqbool, K.; Webster, M.T.; Perloski, M.; Liberg, O.; Arnemo, J.M.; Hedhammar, A.; Lindblad-Toh, K. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 2013, 495, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.; Rubin, C.-J.; Di Palma, F.; Albert, F.W.; Alföldi, J.; Barrio, A.M.; Pielberg, G.; Rafati, N.; Sayyab, S.; Turner-Maier, J.; et al. Rabbit genome analysis reveals a polygenic basis for phenotypic change during domestication. Science 2014, 345, 1074–1079. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, C.S.; Hekman, J.P.; Johnson, J.L.; Lyu, Z.; Ortega, M.T.; Joshi, T.; Mao, J.; Vladimirova, A.V.; Gulevich, R.G.; Kharlamova, A.V.; et al. Hypothalamic transcriptome of tame and aggressive silver foxes (Vulpes vulpes) identifies gene expression differences shared across brain regions. Genes Brain Behav. 2020, 19, e12614. [Google Scholar] [CrossRef] [PubMed]
- Chadaeva, I.; Ponomarenko, P.; Kozhemyakina, R.; Suslov, V.; Bogomolov, A.; Klimova, N.; Shikhevich, S.; Savinkova, L.; Oshchepkov, D.; Kolchanov, N.A.; et al. Domestication explains two-thirds of differential-gene-expression variance between domestic and wild animals, the remaining one-third reflects intraspecific and interspecific variation. Animals 2021, 11, 2667. [Google Scholar] [CrossRef]
- Oshchepkov, D.; Chadaeva, I.; Kozhemyakina, R.; Zolotareva, K.; Khandaev, B.; Sharypova, E.; Ponomarenko, P.; Bogomolov, A.; Klimova, N.V.; Shikhevich, S.; et al. Stress reactivity, susceptibility to hypertension, and differential expression of genes in hypertensive compared to normotensive patients. Int. J. Mol. Sci. 2022, 23, 2835. [Google Scholar] [CrossRef]
- Wilkins, A.S.; Wrangham, R.W.; Fitch, W.T. The “domestication syndrome” in mammals: A unified explanation based on neural crest cell behavior and genetics. Genetics 2014, 197, 795–808. [Google Scholar] [CrossRef] [Green Version]
- Kruska, D. The effect of domestication of brain size and composition in the mink (Mustela vison). J. Zool. 1996, 239, 645–661. [Google Scholar] [CrossRef]
- Jensen, P. Domestication—From behaviour to genes and back again. Appl. Anim. Behav. Sci. 2006, 97, 3–15. [Google Scholar] [CrossRef]
- Setchell, B.P. Domestication and reproduction. Anim. Reprod. Sci. 1992, 28, 195–202. [Google Scholar] [CrossRef]
- Belyaev, D.K.; Plyusnina, I.Z.; Trut, L.N. Domestication in the silver fox (Vulpes fulvus desm): Changes in physiological boundaries of the sensitive period of primary socialization. Appl. Anim. Behav. Sci. 1984, 13, 359–370. [Google Scholar] [CrossRef]
- Trut, L.; Oskina, I.; Kharlamova, A. Animal evolution during domestication: The domesticated fox as a model. Bioessays 2009, 31, 349–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belyaev, D.K.; Borodin, P.M. The influence of stress on variation and its role in evolution. Biol. Zent. 1982, 101, 705–714. [Google Scholar]
- Plyusnina, I.; Oskina, I. Behavioral and adrenocortical responses to open-field test in rats selected for reduced aggressiveness toward humans. Physiol. Behav. 1997, 61, 381–385. [Google Scholar] [CrossRef]
- Hrabovszky, E.; Halász, J.; Meelis, W.; Kruk, M.R.; Liposits, Z.; Haller, J. Neurochemical characterization of hypothalamic neurons involved in attack behavior: Glutamatergic dominance and co-expression of thyrotropin-releasing hormone in a subset of glutamatergic neurons. Neuroscience 2005, 133, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Lee, A.C.H. The role of the hippocampus in approach-avoidance conflict decision-making: Evidence from rodent and human studies. Behav. Brain Res. 2016, 313, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Wingender, E.; Schoeps, T.; Dönitz, J. TFClass: An expandable hierarchical classification of human transcription factors. Nucleic Acids Res. 2013, 41, D165–D170. [Google Scholar] [CrossRef] [PubMed]
- Torres-Machorro, A.L. Homodimeric and heterodimeric interactions among vertebrate basic helix-loop-helix transcription factors. Int. J. Mol. Sci. 2021, 22, 12855. [Google Scholar] [CrossRef]
- Sanes, D.H. Development of the Nervous System, 3rd ed.; Sanes, D., Reh, T., Harris, W., Eds.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar] [CrossRef]
- Guillemot, F.; Lo, L.-C.; Johnson, J.E.; Auerbach, A.; Anderson, D.J.; Joyner, A.L. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 1993, 75, 463–476. [Google Scholar] [CrossRef]
- Gyoja, F. Basic helix-loop-helix transcription factors in evolution: Roles in development of mesoderm and neural tissues. Genesis 2017, 55, e23051. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Moghimyfiroozabad, S.; Moghimyfiroozabad, A.; Tierney, T.S.; Alavian, K.N. The factors for the early and late development of midbrain dopaminergic neurons segregate into two distinct evolutionary clusters. Brain Disord. 2021, 1, 100002. [Google Scholar] [CrossRef]
- Shaikh, M.B.; Brutus, M.; Siegel, H.E.; Siegel, A. Differential control of aggression by the midbrain. Exp. Neurol. 1984, 83, 436–442. [Google Scholar] [CrossRef]
- Coimbra, B.; Soares-Cunha, C.; Vasconcelos, N.; Domingues, A.V.; Borges, S.; Sousa, N.; Rodrigues, A.J. Role of laterodorsal tegmentum projections to nucleus accumbens in reward-related behaviors. Nat. Commun. 2019, 10, 4138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudryavtseva, N.N.; Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Vishnivetskaya, G.B.; Babenko, V.N.; Orlov, Y.L. Serotonergic genes in the development of anxiety/depression-like state and pathology of aggressive behavior in male mice: RNA-seq data. Mol. Biol. 2017, 51, 288–300. [Google Scholar] [CrossRef]
- Mahadevia, D.; Saha, R.; Manganaro, A.; Chuhma, N.; Ziolkowski-Blake, A.; Morgan, A.A.; Dumitriu, D.; Rayport, S.; Ansorge, M.S. Dopamine promotes aggression in mice via ventral tegmental area to lateral septum projections. Nat. Commun. 2021, 12, 6796. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Kulakovskiy, I.V.; Vorontsov, I.E.; Yevshin, I.S.; Sharipov, R.N.; Fedorova, A.D.; Rumynskiy, E.I.; Medvedeva, Y.A.; Magana-Mora, A.; Bajic, V.B.; Papatsenko, D.A.; et al. HOCOMOCO: Towards a complete collection of transcription factor binding models for human and mouse via large-scale ChIP-Seq analysis. Nucleic Acids Res. 2018, 46, D252–D259. [Google Scholar] [CrossRef] [PubMed]
- Levitsky, V.; Zemlyanskaya, E.; Oshchepkov, D.; Podkolodnaya, O.; Ignatieva, E.; Grosse, I.; Mironova, V.; Merkulova, T. A single ChIP-seq dataset is sufficient for comprehensive analysis of motifs co-occurrence with MCOT package. Nucleic Acids Res. 2019, 47, e139. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, G.; Vorontsov, I.; Penzar, D.; Groux, R.; Fornes, O.; Nikolaeva, D.D.; Ballester, B.; Grau, J.; Grosse, I.; Makeev, V.; et al. Insights gained from a comprehensive all-against-all transcription factor binding motif benchmarking study. Genome Biol. 2020, 21, 114. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ohbo, K.; Takakura, A.; Takebayashi, H.; Okada, T.; Abe, K.; Nabeshima, Y. Sgn1, a basic helix-loop-helix transcription factor delineates the salivary gland duct cell lineage in mice. Dev. Biol. 2001, 240, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.; Masgutova, G.; Collin, A.; Toch, M.; Hidalgo-Figueroa, M.; Jacob, B.; Corcoran, L.M.; Francius, C.; Clotman, F. Onecut factors and Pou2f2 regulate the distribution of V2 interneurons in the mouse developing spinal cord. Front. Cell. Neurosci. 2019, 13, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragunow, M.; Faull, R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J. Neurosci. Methods 1989, 29, 261–265. [Google Scholar] [CrossRef]
- Kovács, K.J. Measurement of immediate-early gene activation-c-fos and beyond. J. Neuroendocrinol. 2008, 20, 665–672. [Google Scholar] [CrossRef]
- Hamann, C.S.; Bankmann, J.; Mora Maza, H.; Kornhuber, J.; Zoicas, I.; Schmitt-Böhrer, A. Social fear affects limbic system neuronal activity and gene expression. Int. J. Mol. Sci. 2022, 23, 8228. [Google Scholar] [CrossRef]
- Leung, H.-W.; Foo, G.; VanDongen, A. Arc regulates transcription of genes for plasticity; excitability and alzheimer’s disease. Biomedicines 2022, 10, 1946. [Google Scholar] [CrossRef] [PubMed]
- Guzowski, J.F.; Timlin, J.A.; Roysam, B.; McNaughton, B.L.; Worley, P.F.; Barnes, C.A. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr. Opin. Neurobiol. 2005, 15, 599–606. [Google Scholar] [CrossRef]
- Boutros, S.W.; Kessler, K.; Unni, V.K.; Raber, J. Infusion of etoposide in the CA1 disrupts hippocampal immediate early gene expression and hippocampus-dependent learning. Sci. Rep. 2022, 12, 12834. [Google Scholar] [CrossRef]
- Lamprecht, R.; Dudai, Y. Transient expression of c-Fos in rat amygdala during training is required for encoding conditioned taste aversion memory. Learn. Mem. 1996, 3, 31–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honkaniemi, J.; Kononen, J.; Kainu, T.; Pyykönen, I.; Pelto-Huikko, M. Induction of multiple immediate early genes in rat hypothalamic paraventricular nucleus after stress. Brain Res. Mol. Brain Res. 1994, 25, 234–241. [Google Scholar] [CrossRef]
- Ons, S.; Martí, O.; Armario, A. Stress-induced activation of the immediate early gene Arc (activity-regulated cytoskeleton-associated protein) is restricted to telencephalic areas in the rat brain: Relationship to c-fos mRNA. J. Neurochem. 2004, 89, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Grueter, B.A.; Robison, A.J.; Neve, R.L.; Nestler, E.J.; Malenka, R.C. ∆FosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc. Natl. Acad. Sci. USA 2013, 110, 1923–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korb, E.; Wilkinson, C.L.; Delgado, R.N.; Lovero, K.L.; Finkbeiner, S. Arc in the nucleus regulates PML-dependent GluA1 transcription and homeostatic plasticity. Nat. Neurosci. 2013, 16, 874–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberi, L.; Liu, S.; Wang, Y.; Badie, R.; Smith-Hicks, C.; Wu, J.; Pierfelice, T.J.; Abazyan, B.; Mattson, M.P.; Kuhl, D.; et al. Activity-induced Notch signaling in neurons requires Arc/Arg3.1 and is essential for synaptic plasticity in hippocampal networks. Neuron 2011, 69, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Messaoudi, E.; Kanhema, T.; Soulé, J.; Tiron, A.; Dagyte, G.; da Silva, B.; Bramham, C.R. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J. Neurosci. 2007, 27, 10445–10455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peebles, C.L.; Yoo, J.; Thwin, M.T.; Palop, J.J.; Noebels, J.L.; Finkbeiner, S. Arc regulates spine morphology and maintains network stability in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 18173–18178. [Google Scholar] [CrossRef] [Green Version]
- Epstein, I.; Finkbeiner, S. The Arc of cognition: Signaling cascades regulating Arc and implications for cognitive function and disease. Semin. Cell Dev. Biol. 2018, 77, 63–72. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, J.; Angel, P.; Schorpp-Kistner, M. AP-1 subunits: Quarrel and harmony among siblings. J. Cell Sci. 2004, 117 Pt 25, 5965–5973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharypova, E.B.; Drachkova, I.A.; Chadaeva, I.V.; Ponomarenko, M.P.; Savinkova, M.P. An experimental study of the effects of SNPs in the TATA boxes of the GRIN1; ASCL3 and NOS1 genes on interactions with the TATA-binding protein. Vavilovskii Zhurnal Genet. Sel. 2022, 26, 227–233. [Google Scholar] [CrossRef]
- Day, I.N. dbSNP in the detail and copy number complexities. Hum. Mutat. 2010, 31, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, M.; Rasskazov, D.; Arkova, O.; Ponomarenko, P.; Suslov, V.; Savinkova, L.; Kolchanov, N. How to use SNP_TATA_Comparator to find a significant change in gene expression caused by the regulatory SNP of this gene’s promoter via a change in affinity of the TATA-binding protein for this promoter. Biomed. Res. Int. 2015, 2015, 359835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponomarenko, M.; Kleshchev, M.; Ponomarenko, P.; Chadaeva, I.; Sharypova, E.; Rasskazov, D.; Kolmykov, S.; Drachkova, I.; Vasiliev, G.; Gutorova, N.; et al. Disruptive natural selection by male reproductive potential prevents underexpression of protein-coding genes on the human Y chromosome as a self-domestication syndrome. BMC Genet. 2020, 21 (Suppl. S1), 89. [Google Scholar] [CrossRef] [PubMed]
- Haeussler, M.; Raney, B.; Hinrichs, A.; Clawson, H.; Zweig, A.; Karolchik, D.; Casper, J.; Speir, M.; Haussler, D.; Kent, W. Navigating protected genomics data with UCSC Genome Browser in a box. Bioinformatics 2015, 31, 764–766. [Google Scholar] [CrossRef] [Green Version]
- Ponomarenko, P.; Savinkova, L.; Drachkova, I.; Lysova, M.; Arshinova, T.; Ponomarenko, M.; Kolchanov, N. A step-by-step model of TBP/TATA box binding allows predicting human hereditary diseases by single nucleotide polymorphism. Dokl. Biochem. Biophys. 2008, 419, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo, R.; Whittington, J.; Parkhurst, L.; Parkhurst, L. The TBP core domain in solution variably bends TATA sequences via a three-step binding mechanism. Biochemistry 2009, 48, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.A.; Pugh, B.F. Evidence for functional binding and stable sliding of the TATA binding protein on nonspecific DNA. J. Biol. Chem. 1995, 270, 13850–13859. [Google Scholar] [CrossRef] [Green Version]
- Berg, O.G.; von Hippel, P.H. Selection of DNA binding sites by regulatory proteins: Statistical-mechanical theory and application to operators and promoters. J. Mol. Biol. 1987, 193, 723–750. [Google Scholar] [CrossRef]
- Flatters, D.; Lavery, R. Sequence-dependent dynamics of TATA-Box binding sites. Biophys. J. 1998, 75, 372–381. [Google Scholar] [CrossRef] [Green Version]
- Hahn, S.; Buratowski, S.; Sharp, P.; Guarente, L. Yeast TATA-binding protein TFIID binds to TATA elements with both consensus and nonconsensus DNA sequences. Proc. Natl. Acad. Sci. USA 1989, 86, 5718–5722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucher, P. Weight matrix descriptions of four eukaryotic RNA polymerase II promoter elements derived from 502 unrelated promoter sequences. J. Mol. Biol. 1990, 212, 563–578. [Google Scholar] [CrossRef]
- Karas, H.; Knuppel, R.; Schulz, W.; Sklenar, H.; Wingender, E. Combining structural analysis of DNA with search routines for the detection of transcription regulatory elements. Comput. Applic. Biosci. 1996, 12, 441–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponomarenko, M.P.; Ponomarenko, J.V.; Frolov, A.S.; Podkolodny, N.L.; Savinkova, L.K.; Kolchanov, N.A.; Overton, G.C. Identification of sequence-dependent features correlating to activity of DNA sites interacting with proteins. Bioinformatics 1999, 15, 687–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waardenberg, A.; Basset, S.; Bouveret, R.; Harvey, R. CompGO: An R package for comparing and visualizing Gene Ontology enrichment differences between DNA binding experiments. BMC Bioinform. 2015, 16, 275. [Google Scholar] [CrossRef] [Green Version]
- Pugh, B. Purification of the human TATA-binding protein, TBP. Methods Mol. Biol. 1995, 37, 359–367. [Google Scholar] [CrossRef]
- Savinkova, L.; Drachkova, I.; Arshinova, T.; Ponomarenko, P.; Ponomarenko, M.; Kolchanov, N. An experimental verification of the predicted effects of promoter TATA-box polymorphisms associated with human diseases on interactions between the TATA boxes and TATA-binding protein. PLoS ONE 2013, 8, e54626. [Google Scholar] [CrossRef] [Green Version]
- Drachkova, I.; Savinkova, L.; Arshinova, T.; Ponomarenko, M.; Peltek, S.; Kolchanov, N. The mechanism by which TATA-box polymorphisms associated with human hereditary diseases influence interactions with the TATA-binding protein. Hum. Mutat. 2014, 35, 601–608. [Google Scholar] [CrossRef]
- Herbeck, Y.E.; Zakharov, I.K.; Trapezov, O.V.; Shumny, V.K. Evolution compressed in time. Philos. Sci. 2013, 1, 115–139. [Google Scholar]
- Liu, Z.; Wang, X.; Jiang, K.; Ji, X.; Zhang, Y.A.; Chen, Z. TNFα-induced up-regulation of Ascl2 affects the differentiation and proliferation of neural stem cells. Aging Dis. 2019, 10, 1207–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.E.; Birren, S.J.; Anderson, D.J. Two rat homologues of Drosophila achaete-scute specifically expressed in neuronal precursors. Nature 1990, 346, 858–861. [Google Scholar] [CrossRef]
- Pacary, E.; Heng, J.L.; Azzarelli, R.; Riou, P.; Castro, D.; Lebel-Potter, M.; Parras, C.; Bell, D.M.; Ridley, A.J.; Parsons, M.; et al. Proneural transcription factors regulate different steps of cortical neuron migration through Rnd-mediated inhibition of RhoA signaling. Neuron 2011, 69, 1069–1084. [Google Scholar] [CrossRef] [Green Version]
- Jessberger, S.; Gage, F.H. Fate plasticity of adult hippocampal progenitors: Biological relevance and therapeutic use. Trends Pharmacol. Sci. 2009, 30, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Miao, Q.; Yuan, J.; Han, S.; Zhang, P.; Li, S.; Rao, Z.; Zhao, W.; Ye, Q.; Geng, J.; et al. Ascl1 converts dorsal midbrain astrocytes into functional neurons in vivo. J. Neurosci. 2015, 35, 9336–9355. [Google Scholar] [CrossRef] [Green Version]
- Henrique, D.; Tyler, D.; Kintner, C.; Heath, J.K.; Lewis, J.H.; Ish-Horowicz, D.; Storey, K.G. Cash4, a novel achaete-scute homolog induced by Hensen’s node during generation of the posterior nervous system. Genes Dev. 1997, 11, 603–615. [Google Scholar] [CrossRef] [Green Version]
- Kempermann, G. The pessimist’s and optimist’s views of adult neurogenesis. Cell 2011, 145, 1009–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farzanehfar, P.; Lu, S.S.; Dey, A.; Musiienko, D.; Baagil, H.; Horne, M.K.; Aumann, T.D. Evidence of functional duplicity of Nestin expression in the adult mouse midbrain. Stem Cell Res. 2017, 19, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Farzanehfar, P.; Horne, M.K.; Aumann, T.D. Can valproic acid regulate neurogenesis from Nestin+ cells in the adult midbrain? Neurochem. Res. 2017, 42, 2127–2134. [Google Scholar] [CrossRef]
- Fauser, M.; Pan-Montojo, F.; Richter, C.; Kahle, P.J.; Schwarz, S.C.; Schwarz, J.; Storch, A.; Hermann, A. Chronic-progressive dopaminergic deficiency does not induce midbrain neurogenesis. Cells 2021, 10, 775. [Google Scholar] [CrossRef]
- Prasolova, L.A.; Gerbek, Y.E.; Gulevich, R.G.; Shikhevich, S.G.; Konoshenko, M.Y.; Kozhemyakina, R.V.; Oskina, I.N.; Plyusnina, I.Z. The effects of prolonged selection for behavior on the stress response and activity of the reproductive system of male grey mice (Rattus norvegicus) Russ. J. Genet. 2014, 50, 959–966. [Google Scholar] [CrossRef]
- Penz, O.K.; Fuzik, J.; Kurek, A.B.; Romanov, R.; Larson, J.; Park, T.J.; Harkany, T.; Keimpema, E. Protracted brain development in a rodent model of extreme longevity. Sci. Rep. 2015, 5, 11592. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Slomianka, L.; Farmer, A.J.; Kharlamova, A.V.; Gulevich, R.G.; Herbeck, Y.E.; Trut, L.N.; Wolfer, D.P.; Amrein, I. Selection for tameness, a key behavioral trait of domestication, increases adult hippocampal neurogenesis in foxes. Hippocampus 2015, 25, 963–975. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Plyusnina, I.Z. Play fighting in rats, selected for the elimination and the enhancement of aggressiveness towards human. Zhurnal Vyss. Nervn. Deiatelnosti Im. I P Pavlov. 2017, 67, 93–105. [Google Scholar]
- Trut, L.N.; Plyusnina, I.Z.; Os’kina, I.N. An experiment on fox domestication and debatable issues of evolution of the dog. Genetika 2004, 40, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, C.A.; Macdonald, D.W.; O’Brien, S.J. From wild animals to domestic pets; an evolutionary view of domestication. PNAS 2009, 106 (Suppl. S1), 9971–9978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, E.O. Behavioral development in animals undergoing domestication. Appl. Anim. Behav. Sci. 1999, 65, 245–271. [Google Scholar] [CrossRef]
- Naumenko, E.; Popova, N.; Nikulina, E.; Dygalo, N.; Shishkina, G.; Borodin, P.; Markel, A. Behavior, adrenocortical activity; and brain monoamines in Norway rats selected for reduced aggressiveness towards man. Pharmacol. Biochem. Behav. 1989, 33, 85–91. [Google Scholar] [CrossRef]
- Plyusnina, I.Z.; Solov’eva, M.Y.; Oskina, I.N. Effect of domestication on aggression in gray Norway rats. Behav. Genet. 2011, 41, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C.R. The Rat Brain in Stereotaxic Coordinates, 7th ed.; Academic Press: London, UK, 2013; p. 472. [Google Scholar]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Gholami, K.; Loh, S.Y.; Salleh, N.; Lam, S.K.; Hoe, S.Z. Selection of suitable endogenous reference genes for qPCR in kidney and hypothalamus of rats under testosterone influence. PLoS ONE 2017, 12, e0176368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penning, L.C.; Vrieling, H.E.; Brinkhof, B.; Riemers, F.M.; Rothuizen, J.; Rutteman, G.R.; Hazewinkel, H.A. A validation of 10 feline reference genes for gene expression measurements in snap-frozen tissues. Vet. Immunol. Immunopathol. 2007, 120, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Hartung, J. A note on combining dependent tests of significance. Biom. J. 1999, 41, 849–855. [Google Scholar] [CrossRef]


| Group | Count |
|---|---|
| Total number of sequence reads (NCBI SRA ID: PRJNA668014) | 182,197,974 |
| Reads mapped to reference rat genome RGSC Rnor_6.0, UCSC Rn6, July 2014 (%) | 158,310,590 (86.9%) |
| Expressed genes identified | 14,039 |
| Statistically significant DEGs (|log2 fold| > 1, PADJ < 0.1, Fisher’s Z-test with Benjamini–Hochberg correction) | 42 |
| #No. | Rat Gene, Name | Symbol | log2 fold | PADJ |
|---|---|---|---|---|
| 1 | defensin beta 17 | Defb17 | 7.31 | 1.86 × 10−3 |
| 2 | leukocyte immunoglobulin-like receptor, subfamily B member 3-like | Lilrb3l | 5.71 | 9.15 × 10−2 |
| 3 | lipoxygenase homology PLAT domains 1 | Loxhd1 | 4.02 | 7.91 × 10−2 |
| 4 | sucrase-isomaltase | Si | 3.30 | 8.66 × 10−2 |
| 5 | achaete-scute family bHLH transcription factor 3 | Ascl3 * | 2.82 | 3.16 × 10−4 |
| 6 | protocadherin gamma subfamily A, 1 | Pcdhga1 | 2.80 | 2.21 × 10−2 |
| 7 | LOC100910802 | 2.25 | 6.64 × 10−2 | |
| 8 | Fc gamma receptor IIb | Fcgr2b | 2.01 | 2.46 × 10−2 |
| 9 | cysteine-rich secretory protein LCCL domain containing 1 | Crispld1 | 1.49 | 7.40 × 10−2 |
| 10 | collagen triple helix repeat containing 1 | Cthrc1 | 1.44 | 6.13 × 10−2 |
| 11 | solute carrier family 7 member 11 | Slc7a11 | 1.37 | 9.84 × 10−2 |
| 12 | nicotinamide nucleotide adenylyltransferase 1 | Nmnat1 | 1.31 | 1.76 × 10−11 |
| 13 | 5-hydroxytryptamine receptor 3A | Htr3a | 1.30 | 4.33 × 10−2 |
| 14 | macrophage expressed 1 | Mpeg1 | 1.28 | 8.30 × 10−4 |
| 15 | schlafen family member 13 | Slfn13 | 1.26 | 6.30 × 10−2 |
| 16 | syncoilin, intermediate filament protein | Sync | 1.19 | 5.31 × 10−2 |
| 17 | olfactomedin-like 1 | Olfml1 | 1.14 | 6.73 × 10−2 |
| 18 | phosphotriesterase related | Pter | 1.13 | 7.16 × 10−2 |
| 19 | collagen type XV alpha 1 chain | Col15a1 | 1.12 | 5.45 × 10−2 |
| 20 | MORN repeat containing 1 | Morn1 | 1.10 | 1.43 × 10−4 |
| 21 | somatostatin receptor 2 | Sstr2 | −1.01 | 2.46 × 10−2 |
| 22 | retinol saturase | Retsat | −1.03 | 1.33 × 10−2 |
| 23 | glycerol-3-phosphate dehydrogenase 1 | Gpd1 | −1.05 | 2.46 × 10−2 |
| 24 | aquaporin 9 | Aqp9 | −1.22 | 9.89 × 10−2 |
| 25 | serine peptidase inhibitor, Kunitz type 1 | Spint1 | −1.29 | 1.43 × 10−4 |
| 26 | activity-regulated cytoskeleton-associated protein | Arc | −1.35 | 6.13 × 10−2 |
| 27 | t-complex-associated testis expressed 1 | Tcte1 | −1.41 | 6.30 × 10−2 |
| 28 | tetratricopeptide repeat domain 22 | Ttc22 | −1.44 | 5.08 × 10−2 |
| 29 | keratin2 | Krt2 | −1.54 | 8.63 × 10−5 |
| 30 | Fos proto-oncogene, AP-1 transcription factor subunit | Fos* | −1.63 | 6.87 × 10−2 |
| 31 | synaptophysin-like 2 | Sypl2 | −1.65 | 8.72 × 10−2 |
| 32 | Ly6/Plaur domain containing 3 | Lypd3 | −1.86 | 5.08 × 10−2 |
| 33 | 5-hydroxytryptamine (serotonin) receptor 5B | Htr5b | −1.94 | 9.17 × 10−2 |
| 34 | FosB proto-oncogene, AP-1 transcription factor subunit | Fosb* | −1.95 | 2.46 × 10−2 |
| 35 | heat shock protein family A (Hsp70) member 1A | Hspa1a | −2.10 | 4.25 × 10−2 |
| 36 | heat shock protein family A (Hsp70) member 1B | Hspa1b | −2.19 | 3.42 × 10−8 |
| 37 | vasoactive intestinal peptide | Vip | −2.35 | 8.93 × 10−3 |
| 38 | RGD1565611 | −2.94 | 6.69 × 10−2 | |
| 39 | tachykinin precursor 3 | Tac3 | −3.64 | 2.46 × 10−2 |
| 40 | protein tyrosine phosphatase, non-receptor type 20 | Ptpn20 | −4.49 | 9.17 × 10−2 |
| 41 | hypocretin neuropeptide precursor | Hcrt | −5.15 | 6.56 × 10−2 |
| 42 | hemoglobin, beta adult major chain | Hbb-b1 | −7.78 | 1.09 × 10−5 |
| No. | Gene | Forward, 5′→3′ | Reverse, 5′→3′ |
|---|---|---|---|
| DEGs identified in the hippocampus of tame versus aggressive adult male rats [This Work] | |||
| 1 | Ascl3 | CCTCTGCTGCCCTTTTCCAG | ACTTGACTCGCTGCCTCTCT |
| 2 | Defb17 | TGGTAGCTTGGACTTGAGGAAAGAA | TGCAGCAGTGTGTTCCAGGTC |
| Reference Genes | |||
| 3 | Ppia | TTCCAGGATTCATGTGCCAG | CTTGCCATCCAGCCACTC |
| 4 | Rpl30 | CATCTTGGCGTCTGATCTTG | TCAGAGTCTGTTTGTACCCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshchepkov, D.; Chadaeva, I.; Kozhemyakina, R.; Shikhevich, S.; Sharypova, E.; Savinkova, L.; Klimova, N.V.; Tsukanov, A.; Levitsky, V.G.; Markel, A.L. Transcription Factors as Important Regulators of Changes in Behavior through Domestication of Gray Rats: Quantitative Data from RNA Sequencing. Int. J. Mol. Sci. 2022, 23, 12269. https://doi.org/10.3390/ijms232012269
Oshchepkov D, Chadaeva I, Kozhemyakina R, Shikhevich S, Sharypova E, Savinkova L, Klimova NV, Tsukanov A, Levitsky VG, Markel AL. Transcription Factors as Important Regulators of Changes in Behavior through Domestication of Gray Rats: Quantitative Data from RNA Sequencing. International Journal of Molecular Sciences. 2022; 23(20):12269. https://doi.org/10.3390/ijms232012269
Chicago/Turabian StyleOshchepkov, Dmitry, Irina Chadaeva, Rimma Kozhemyakina, Svetlana Shikhevich, Ekaterina Sharypova, Ludmila Savinkova, Natalya V. Klimova, Anton Tsukanov, Victor G. Levitsky, and Arcady L. Markel. 2022. "Transcription Factors as Important Regulators of Changes in Behavior through Domestication of Gray Rats: Quantitative Data from RNA Sequencing" International Journal of Molecular Sciences 23, no. 20: 12269. https://doi.org/10.3390/ijms232012269






