Photoluminescent Histidine-Stabilized Gold Nanoclusters as Efficient Sensors for Fast and Easy Visual Detection of Fe Ions in Water Using Paper-Based Portable Platform
Abstract
:1. Introduction
2. Results and Discussion
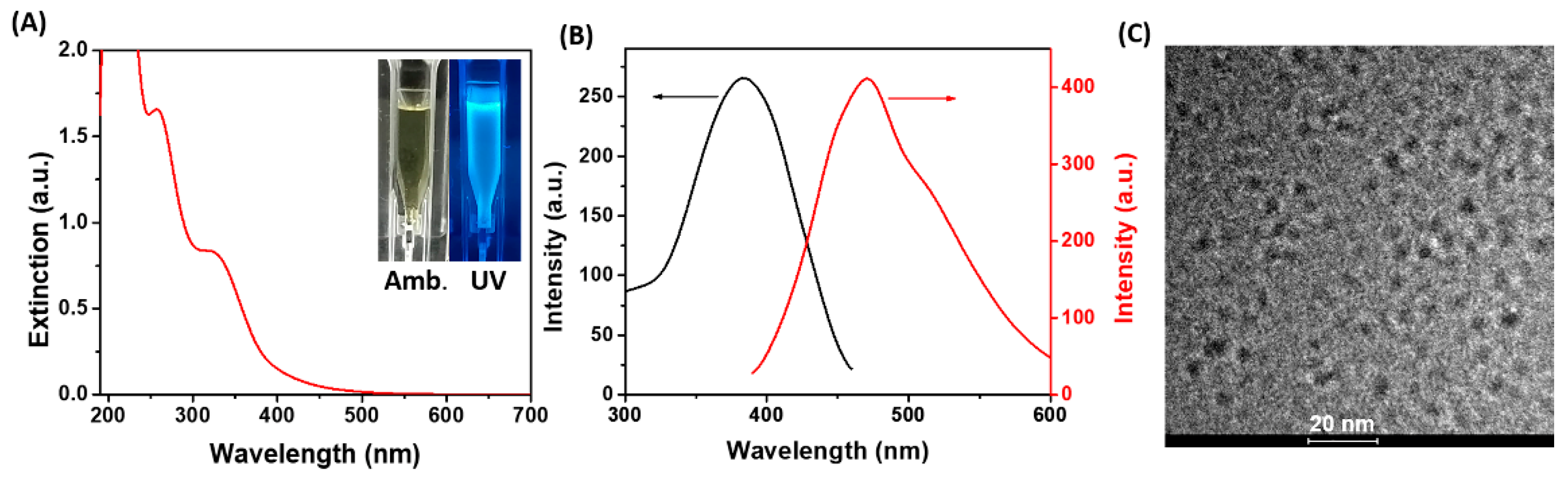
2.1. Characterization of His-AuNCs
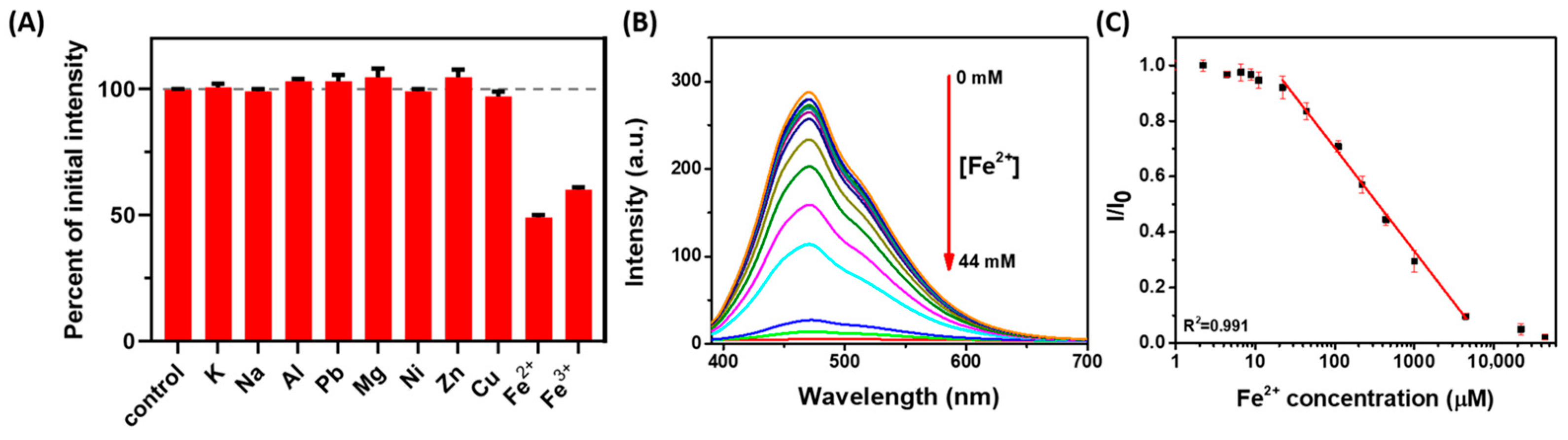
2.2. Performance of His-AuNCs-Based Colloidal Sensor
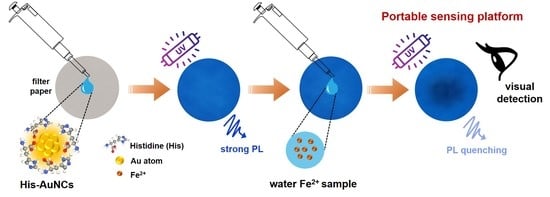
2.3. Fe2+ Sensing with His-AuNCs-Paper-Based Platform
2.4. Fe Sensing from Real Water Samples with His-AuNCs-Paper-Based Platform
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Colloidal His-AuNCs
3.3. Selectivity Assay
3.4. Sensitivity Assay
3.5. Fabrication and Performance of His-AuNCs-Paper-Based Sensor
3.6. His-AuNCs-Paper-Based Fe Sensing in Real Water Samples
3.7. Instrumentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.-J.; Qiao, D.; Zhao, J.; Weng, G.-J.; Zhu, J.; Zhao, J.-W. Ratiometric Fluorescence Detection of Hg2+ and Fe3+ Based on BSA-Protected Au/Ag Nanoclusters and His-Stabilized Au Nanoclusters. Methods Appl. Fluoresc. 2019, 7, 045001. [Google Scholar] [CrossRef]
- Dai, R.; Deng, W.; Hu, P.; You, C.; Yang, L.; Jiang, X.; Xiong, X.; Huang, K. One-Pot Synthesis of Bovine Serum Albumin Protected Gold/Silver Bimetallic Nanoclusters for Ratiometric and Visual Detection of Mercury. Microchem. J. 2018, 139, 1–8. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, J.; Weng, G.; Li, J.; Zhu, J.; Zhao, J. Modification-Free Colorimetric and Visual Detection of Hg2+ Based on the Etching from Core-Shell Structural Au-Ag Nanorods to Nanorices. Sens. Actuators B Chem. 2018, 267, 181–190. [Google Scholar] [CrossRef]
- Li, W.; Wen, X.; Zhao, H.; Yan, W.; Trant, J.F.; Li, Y. Acid-Triggered Self-Assembled Egg White Protein-Coated Gold Nanoclusters for Selective Fluorescent Detection of Fe3+, NO2−, and Cysteine. ACS Appl. Nano Mater. 2020, 3, 11838–11849. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality; Health Criteria and Other Supporting Information: Addendum; World Health Organization: Geneva, Switzerland, 1998; Volume 2.
- Zheng, M.; Tan, H.; Xie, Z.; Zhang, L.; Jing, X.; Sun, Z. Fast Response and High Sensitivity Europium Metal Organic Framework Fluorescent Probe with Chelating Terpyridine Sites for Fe3+. ACS Appl. Mater. Interfaces 2013, 5, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-Y.; Jiang, S.-J.; Sahayam, A.C. Combined Use of HPLC–ICP-MS and Microwave-Assisted Extraction for the Determination of Cobalt Compounds in Nutritive Supplements. Food Chem. 2014, 147, 215–219. [Google Scholar] [CrossRef]
- Salnikova, E.V.; Burtseva, T.I.; Skalnaya, M.G.; Skalny, A.V.; Tinkov, A.A. Copper and Zinc Levels in Soil, Water, Wheat, and Hair of Inhabitants of Three Areas of the Orenburg Region, Russia. Environ. Res. 2018, 166, 158–166. [Google Scholar] [CrossRef]
- van den Berg, C.M. Chemical Speciation of Iron in Seawater by Cathodic Stripping Voltammetry with Dihydroxynaphthalene. Anal. Chem. 2006, 78, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yang, B.; Yang, Y.; Wang, L. SERS-Based Mercury Ion Detections: Principles, Strategies and Recent Advances. Sci. China Chem. 2016, 59, 16–29. [Google Scholar] [CrossRef]
- Arnold, G.L.; Weyer, S.; Anbar, A.D. Fe Isotope Variations in Natural Materials Measured Using High Mass Resolution Multiple Collector ICPMS. Anal. Chem. 2004, 76, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Dong, Y.; Feng, L.; Wang, S.; Zhang, S.; Zhang, X. Horseradish Peroxidase Functionalized Fluorescent Gold Nanoclusters for Hydrogen Peroxide Sensing. Anal. Chem. 2011, 83, 1193–1196. [Google Scholar] [CrossRef]
- Hada, A.-M.; Craciun, A.-M.; Astilean, S. Intrinsic Photoluminescence of Solid-State Gold Nanoclusters: Towards Fluorescence Lifetime Imaging of Tissue-like Phantoms under Two-Photon near-Infrared Excitation. Front. Chem. 2021, 9, 761711. [Google Scholar] [CrossRef]
- Liu, J.-M.; Chen, J.-T.; Yan, X.-P. Near Infrared Fluorescent Trypsin Stabilized Gold Nanoclusters as Surface Plasmon Enhanced Energy Transfer Biosensor and in Vivo Cancer Imaging Bioprobe. Anal. Chem. 2013, 85, 3238–3245. [Google Scholar] [CrossRef]
- Sun, C.; Yang, H.; Yuan, Y.; Tian, X.; Wang, L.; Guo, Y.; Xu, L.; Lei, J.; Gao, N.; Anderson, G.J. Controlling Assembly of Paired Gold Clusters within Apoferritin Nanoreactor for in Vivo Kidney Targeting and Biomedical Imaging. J. Am. Chem. Soc. 2011, 133, 8617–8624. [Google Scholar] [CrossRef]
- Hada, A.-M.; Craciun, A.-M.; Focsan, M.; Borlan, R.; Soritau, O.; Todea, M.; Astilean, S. Folic Acid Functionalized Gold Nanoclusters for Enabling Targeted Fluorescence Imaging of Human Ovarian Cancer Cells. Talanta 2021, 225, 121960. [Google Scholar] [CrossRef]
- Yin, H.-Q.; Bi, F.-L.; Gan, F. Rapid Synthesis of Cyclic RGD Conjugated Gold Nanoclusters for Targeting and Fluorescence Imaging of Melanoma A375 Cells. Bioconjug. Chem. 2015, 26, 243–249. [Google Scholar] [CrossRef]
- Bi, F.; Yin, H.; Zheng, S.; Zhu, Q.; Yang, H.; Kang, M.; Gan, F.; Chen, X. One-Step Synthesis of Peptide Conjugated Gold Nanoclusters for the High Expression of FGFR2 Tumor Targeting and Imaging. RSC Adv. 2016, 6, 4627–4633. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, D.; Zhen, Y.; Guo, R. Amino Acid-Mediated ‘Turn-off/Turn-on’Nanozyme Activity of Gold Nanoclusters for Sensitive and Selective Detection of Copper Ions and Histidine. Biosens. Bioelectron. 2017, 92, 140–146. [Google Scholar] [CrossRef]
- Liu, G.; Shao, Y.; Ma, K.; Cui, Q.; Wu, F.; Xu, S. Synthesis of DNA-Templated Fluorescent Gold Nanoclusters. Gold Bull. 2012, 45, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Venkatesh, V.; Shukla, A.; Sivakumar, S.; Verma, S. Purine-Stabilized Green Fluorescent Gold Nanoclusters for Cell Nuclei Imaging Applications. ACS Appl. Mater. Interfaces 2014, 6, 2185–2191. [Google Scholar] [CrossRef]
- Lee, D.; Donkers, R.L.; Wang, G.; Harper, A.S.; Murray, R.W. Electrochemistry and Optical Absorbance and Luminescence of Molecule-like Au38 Nanoparticles. J. Am. Chem. Soc. 2004, 126, 6193–6199. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R. On the Ligand’s Role in the Fluorescence of Gold Nanoclusters. Nano Lett. 2010, 10, 2568–2573. [Google Scholar] [CrossRef]
- Yu, Q.; Gao, P.; Zhang, K.Y.; Tong, X.; Yang, H.; Liu, S.; Du, J.; Zhao, Q.; Huang, W. Luminescent Gold Nanocluster-Based Sensing Platform for Accurate H2S Detection in Vitro and in Vivo with Improved Anti-Interference. Light Sci. Appl. 2017, 6, e17107. [Google Scholar] [CrossRef]
- Xu, M.-M.; Jia, T.-T.; Li, B.; Ma, W.; Chen, X.; Zhao, X.; Zang, S.-Q. Tuning the Properties of Atomically Precise Gold Nanoclusters for Biolabeling and Drug Delivery. Chem. Commun. 2020, 56, 8766–8769. [Google Scholar] [CrossRef]
- Li, G.; Jin, R. Atomically Precise Gold Nanoclusters as New Model Catalysts. Acc. Chem. Res. 2013, 46, 1749–1758. [Google Scholar] [CrossRef]
- Chen, D.; Li, B.; Cai, S.; Wang, P.; Peng, S.; Sheng, Y.; He, Y.; Gu, Y.; Chen, H. Dual Targeting Luminescent Gold Nanoclusters for Tumor Imaging and Deep Tissue Therapy. Biomaterials 2016, 100, 1–16. [Google Scholar] [CrossRef]
- Desai, M.L.; Basu, H.; Saha, S.; Singhal, R.K.; Kailasa, S.K. Fluorescence Enhancement of Bovine Serum Albumin Gold Nanoclusters from La3+ Ion: Detection of Four Divalent Metal Ions (Hg2+, Cu2+, Pb2+ and Cd2+). J. Mol. Liq. 2021, 336, 116239. [Google Scholar] [CrossRef]
- Cheng, Y.; Kang, W.; Guo, Y.; Du, C.; Xie, Y.; Chen, Y.; Yao, W.; Qian, H. Visual Detection of Cu2+ Based on Fluorescence Quenching of Green-Synthesized Gold Nanoclusters Using Soy Protein as Template. Food Agric. Immunol. 2017, 28, 848–858. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Kong, D.; Jin, R.; Li, H.; Yan, X.; Liu, F.; Sun, P.; Gao, Y.; Lu, G. On-Site Monitoring of Thiram via Aggregation-Induced Emission Enhancement of Gold Nanoclusters Based on Electronic-Eye Platform. Sens. Actuators B Chem. 2019, 296, 126641. [Google Scholar] [CrossRef]
- Mu, X.; Qi, L.; Dong, P.; Qiao, J.; Hou, J.; Nie, Z.; Ma, H. Facile One-Pot Synthesis of L-Proline-Stabilized Fluorescent Gold Nanoclusters and Its Application as Sensing Probes for Serum Iron. Biosens. Bioelectron. 2013, 49, 249–255. [Google Scholar] [CrossRef]
- Su, Y.; Qi, L.; Mu, X.; Wang, M. A Fluorescent Probe for Sensing Ferric Ions in Bean Sprouts Based on L-Histidine-Stabilized Gold Nanoclusters. Anal. Methods 2015, 7, 684–689. [Google Scholar] [CrossRef]
- Hada, A.-M.; Zetes, M.; Focsan, M.; Nagy-Simon, T.; Craciun, A.-M. Novel Paper-Based Sensing Platform Using Photoluminescent Gold Nanoclusters for Easy, Sensitive and Selective Naked-Eye Detection of Cu2+. J. Mol. Struct. 2021, 1244, 130990. [Google Scholar] [CrossRef]
- Liana, D.D.; Raguse, B.; Gooding, J.J.; Chow, E. Recent Advances in Paper-Based Sensors. Sensors 2012, 12, 11505–11526. [Google Scholar] [CrossRef] [Green Version]
- Susu, L.; Campu, A.; Craciun, A.M.; Vulpoi, A.; Astilean, S.; Focsan, M. Designing Efficient Low-Cost Paper-Based Sensing Plasmonic Nanoplatforms. Sensors 2018, 18, 3035. [Google Scholar] [CrossRef] [Green Version]
- Pal, N.K.; Kryschi, C. A Facile UV-Light Mediated Synthesis of l-Histidine Stabilized Silver Nanocluster for Efficient Photodegradation of Methylene Blue. J. Mol. Catal. A Chem. 2015, 404, 27–35. [Google Scholar] [CrossRef]
- Yang, T.-Q.; Peng, B.; Shan, B.-Q.; Zong, Y.-X.; Jiang, J.-G.; Wu, P.; Zhang, K. Origin of the Photoluminescence of Metal Nanoclusters: From Metal-Centered Emission to Ligand-Centered Emission. Nanomaterials 2020, 10, 261. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Zeng, C.; Li, Q.; Higaki, T.; Jin, R. Gold Nanoclusters: Bridging Gold Complexes and Plasmonic Nanoparticles in Photophysical Properties. Nanomaterials 2019, 9, 933. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Di, J.; Li, L.; Tu, Y.; Yan, J. Copper ion detection with improved sensitivity through catalytic quenching of gold nanocluster fluorescence. Talanta 2018, 187, 231–236. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, S.; Zhang, L.; Huang, H.; Zeng, Y.; Liu, F. Multiplex Sensor for Detection of Different Metal Ions Based on on–off of Fluorescent Gold Nanoclusters. Anal. Chim. Acta 2014, 852, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, B.; Desimoni, E. About Estimating the Limit of Detection by the Signal to Noise Approach. Pharm. Anal. Acta 2015, 6, 2. [Google Scholar] [CrossRef]


| Type of Water | Added (μM) | Found (μM) | Recovered (%) |
|---|---|---|---|
| River | 0 | ND 1 | - |
| 35 | 36.9 ± 1.6 | 105.4 ± 4.5 | |
| Spring | 0 | ND | - |
| 35 | 36.2 ± 1.4 | 103.4 ± 4.0 | |
| Tap | 0 | ND | - |
| 35 | 35.7 ± 2.0 | 102.0 ± 5.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hada, A.-M.; Zetes, M.; Focsan, M.; Astilean, S.; Craciun, A.-M. Photoluminescent Histidine-Stabilized Gold Nanoclusters as Efficient Sensors for Fast and Easy Visual Detection of Fe Ions in Water Using Paper-Based Portable Platform. Int. J. Mol. Sci. 2022, 23, 12410. https://doi.org/10.3390/ijms232012410
Hada A-M, Zetes M, Focsan M, Astilean S, Craciun A-M. Photoluminescent Histidine-Stabilized Gold Nanoclusters as Efficient Sensors for Fast and Easy Visual Detection of Fe Ions in Water Using Paper-Based Portable Platform. International Journal of Molecular Sciences. 2022; 23(20):12410. https://doi.org/10.3390/ijms232012410
Chicago/Turabian StyleHada, Alexandru-Milentie, Markus Zetes, Monica Focsan, Simion Astilean, and Ana-Maria Craciun. 2022. "Photoluminescent Histidine-Stabilized Gold Nanoclusters as Efficient Sensors for Fast and Easy Visual Detection of Fe Ions in Water Using Paper-Based Portable Platform" International Journal of Molecular Sciences 23, no. 20: 12410. https://doi.org/10.3390/ijms232012410