Natural and Synthetic Xanthone Derivatives Counteract Oxidative Stress via Nrf2 Modulation in Inflamed Human Macrophages
Abstract
:1. Introduction
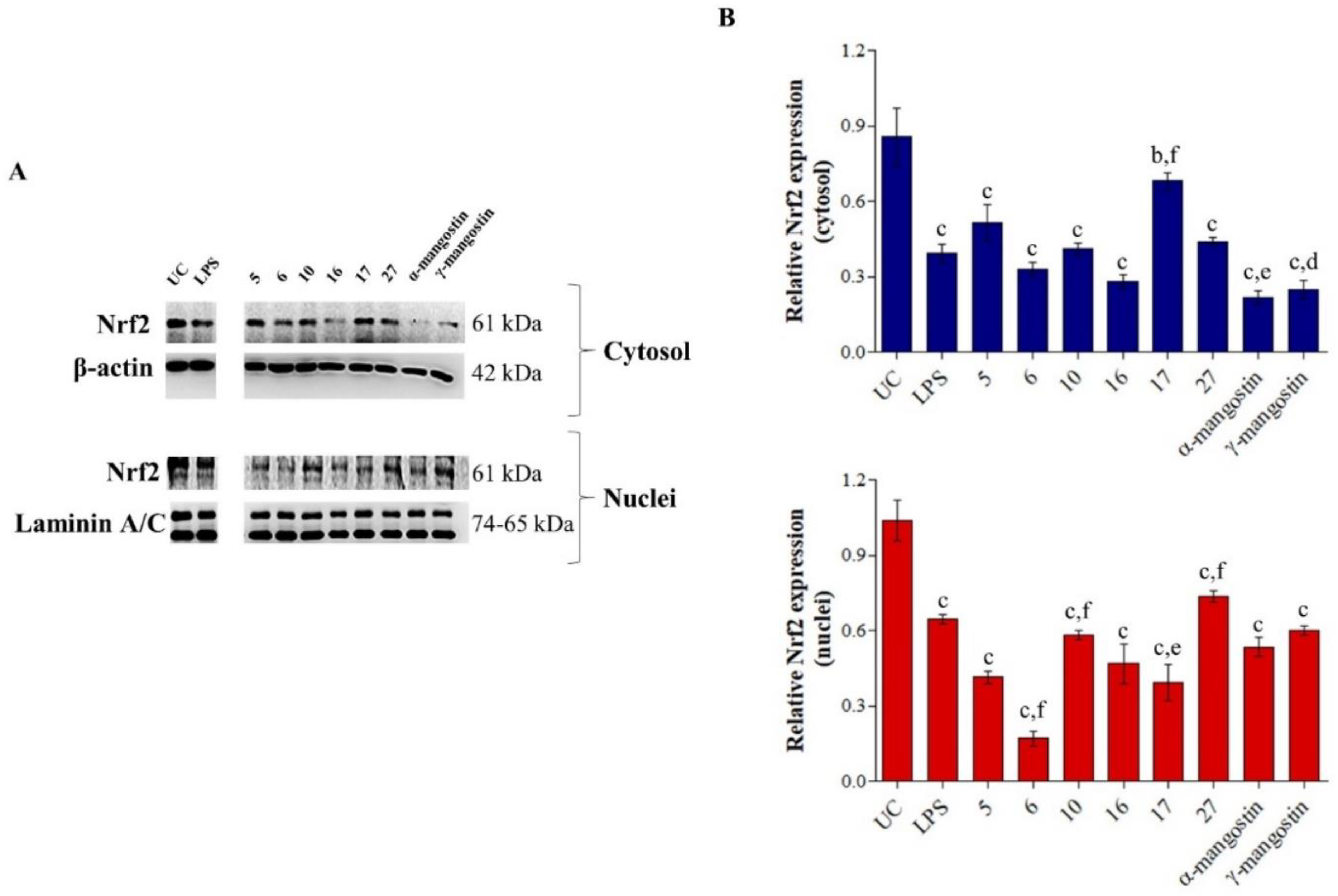
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis
3.2. Synthesis of 1-(((4-fluorobenzyl)amino)methyl)-3,4-dimethoxy-9H-xanthen-9-one hydrochloride (38)
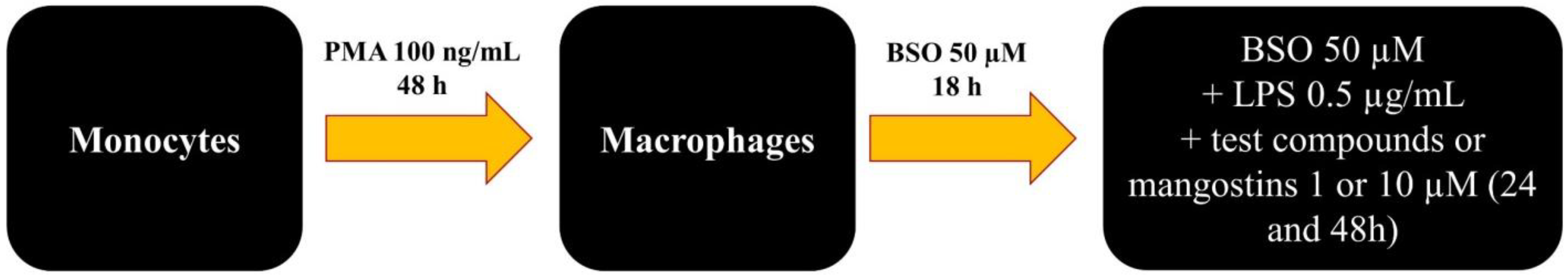
3.3. Cell Culture and Differentiation
3.4. Establishment of the Inflamed Environment and Cell Treatment
3.5. Cell Metabolic Activity (MTT Assay)
3.6. Cytotoxicity Assay (LDH Assay)
3.7. Protein Extraction
3.8. Immunoblotting (Western Blot)
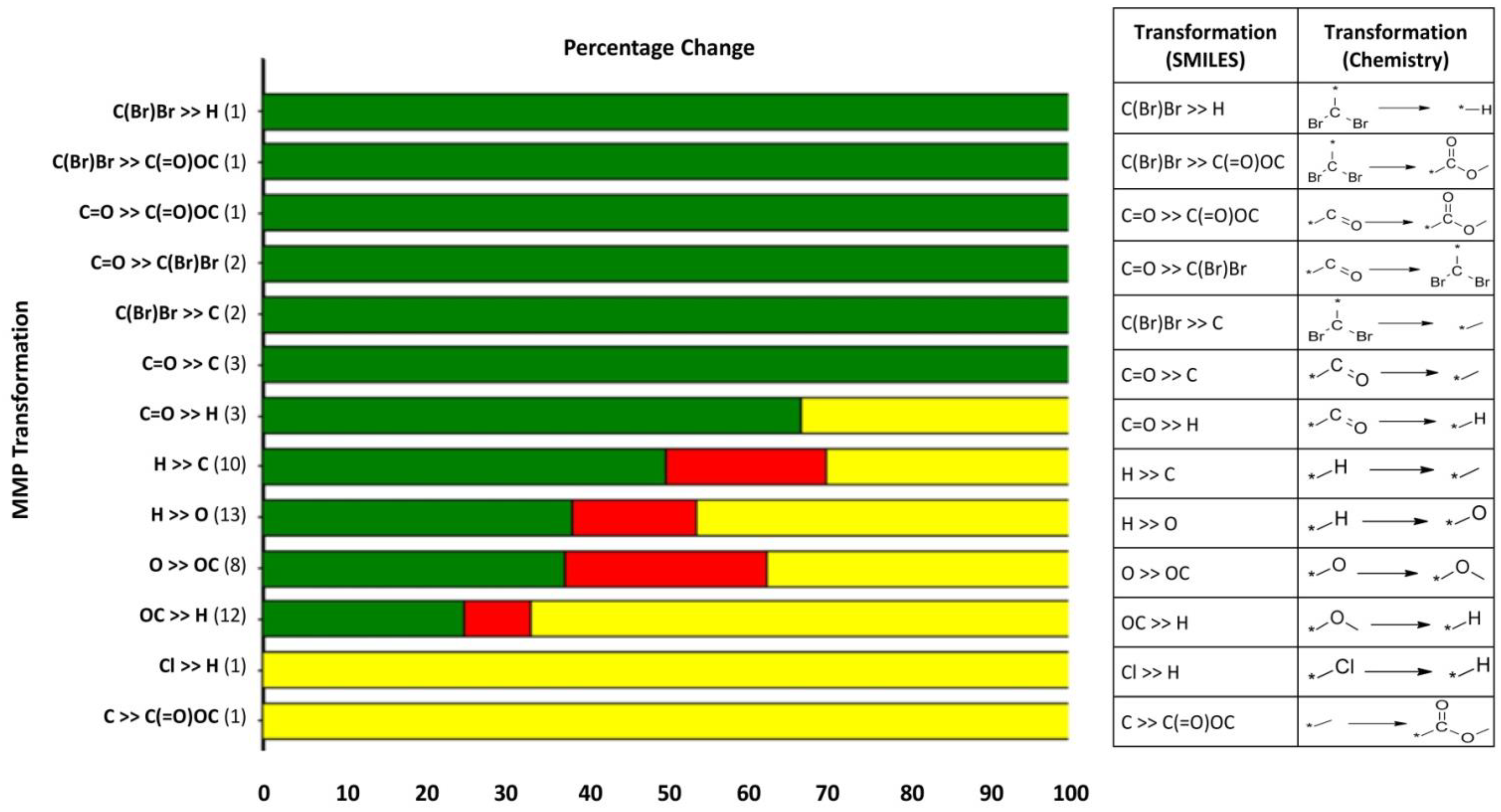
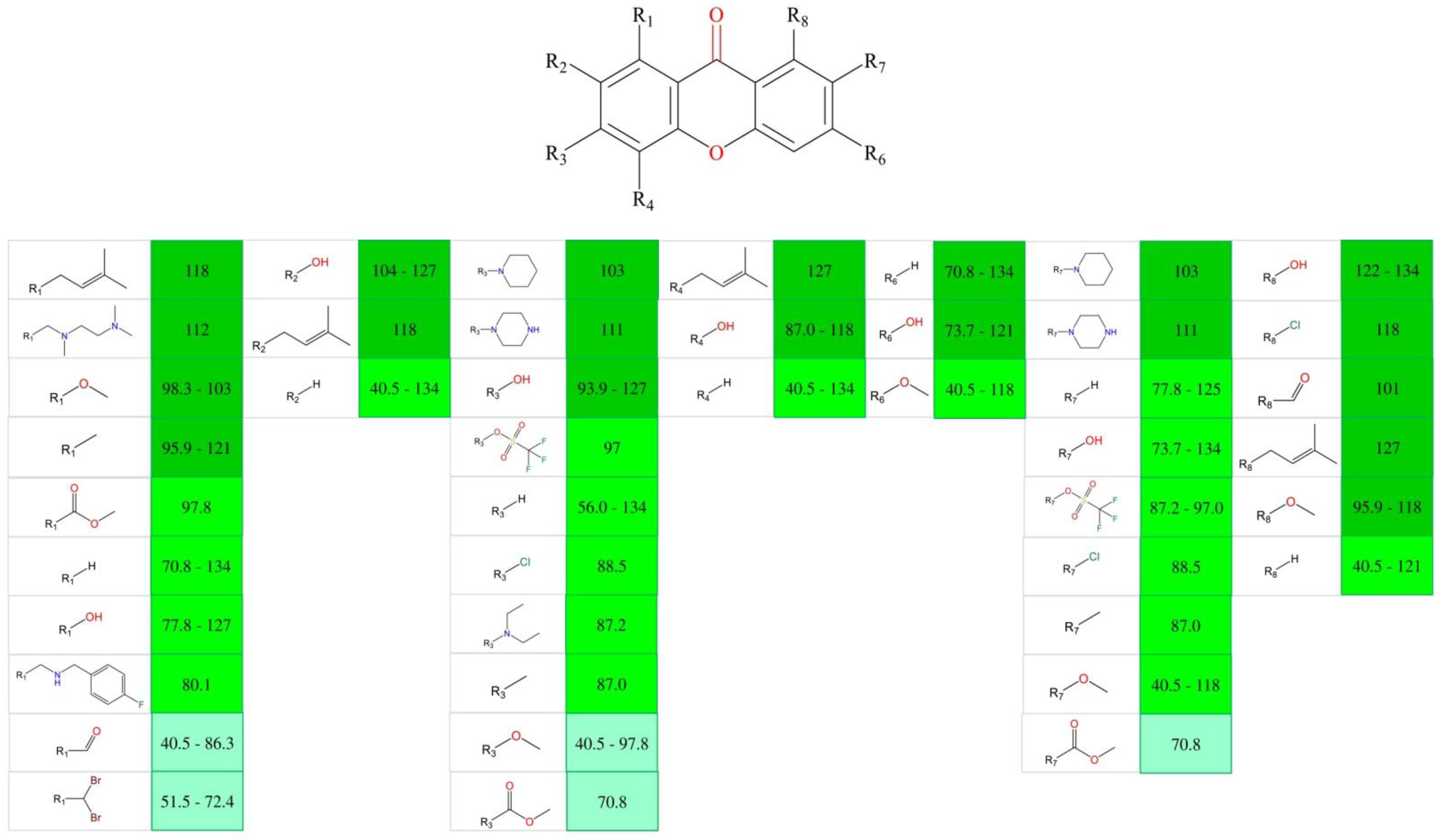
3.9. Structure–Activity Analysis Using Matched Molecular Pairs (MMPs)
3.10. Quantitative Structure–Activity Relationship (QSAR)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Q.; Wang, Y.; Wu, H.; Yuan, M.; Zheng, C.; Xu, H. Xanthone Glucosides: Isolation, Bioactivity and Synthesis. Molecules 2021, 26, 5575. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.M.M.; Palmeira, A.; Fernandes, C.; Resende, D.I.S.P.; Sousa, E.; Cidade, H.; Tiritan, M.E.; Correia-da-Silva, M.; Cravo, S. From Natural Products to New Synthetic Small Molecules: A Journey through the World of Xanthones. Molecules 2021, 26, 431. [Google Scholar] [CrossRef] [PubMed]
- Gunter, N.V.; Teh, S.S.; Lim, Y.M.; Mah, S.H. Natural Xanthones and Skin Inflammatory Diseases: Multitargeting Mechanisms of Action and Potential Application. Front. Pharmacol. 2020, 11, 594202. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.P.; Shukla, A.; Choudhury, S.; Singh, R.; Anand, M.; Prabhu, S.N. IL1β/ TNFα/COX-2/VEGF axis responsible for effective healing potential of C-glucoside xanthone (mangiferin) based ointment in immunocompromised rats. Cytokine 2022, 158, 156012. [Google Scholar] [CrossRef]
- Wang, M.-H.; Zhang, K.-J.; Gu, Q.-L.; Bi, X.-L.; Wang, J.-X. Pharmacology of mangostins and their derivatives: A comprehensive review. Chin. J. Nat. Med. 2017, 15, 81–93. [Google Scholar] [CrossRef]
- Ibrahim, M.Y.; Hashim, N.M.; Mariod, A.A.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, I.A. α-Mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arab. J. Chem. 2016, 9, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Li, Y.; Wang, W.; Deng, L. Bioactivity and pharmacological properties of α-mangostin from the mangosteen fruit: A review. Expert. Opin. Ther. Pat. 2018, 28, 415–427. [Google Scholar] [CrossRef]
- Meylina, L.; Muchtaridi, M.; Joni, I.M.; Mohammed, A.F.A.; Wathoni, N. Nanoformulations of α-Mangostin for Cancer Drug Delivery System. Pharmaceutics 2021, 13, 1993. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Kalpravidh, R.W.; Chuang, C.-C.; Overman, A.; Martinez, K.; Kennedy, A.; McIntosh, M. Xanthones from mangosteen inhibit inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. J. Nutr. 2010, 140, 842–847. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Schweikl, H.; Gallorini, M.; Pöschl, G.; Urmann, V.; Petzel, C.; Bolay, C.; Hiller, K.-A.; Cataldi, A.; Buchalla, W. Functions of transcription factors NF-κB and Nrf2 in the inhibition of LPS-stimulated cytokine release by the resin monomer HEMA. Dent. Mater. 2018, 34, 1661–1678. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Su, T.; Qiu, X.; Mao, P.; Xu, Y.; Hu, Z.; Zhang, Y.; Zheng, X.; Xie, P.; Liu, Q. Protective effect of alpha-mangostin against oxidative stress induced-retinal cell death. Sci. Rep. 2016, 6, 21018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, T.; Li, H.; Zhao, Y.; Cai, E.; Zhu, H.; Li, P.; Liu, J. Hepatoprotective effect of α-mangostin against lipopolysaccharide/d-galactosamine-induced acute liver failure in mice. Biomed. Pharmacother. 2018, 106, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.G.R.; Silva, A.M.S.; Cavaleiro, J.A.S.; Silva, F.M.; Borges, M.F.M.; Pinto, M.M.M. 1H and 13C NMR spectroscopy of mono-, di-, tri- and tetrasubstituted xanthones. Magn. Reson. Chem. 1998, 36, 305–309. [Google Scholar] [CrossRef]
- Pedro, M.; Cerqueira, F.; Sousa, M.E.; Nascimento, M.S.J.; Pinto, M. Xanthones as inhibitors of growth of human cancer cell lines and Their effects on the proliferation of human lymphocytes In Vitro. Bioorg. Med. Chem. 2002, 10, 3725–3730. [Google Scholar] [CrossRef]
- Pinto, E.; Afonso, C.; Duarte, S.; Vale-Silva, L.; Costa, E.; Sousa, M.E.; Pinto, M. Antifungal Activity of Xanthones: Evaluation of their Effect on Ergosterol Biosynthesis by High-performance Liquid Chromatography. Chem. Biol. Drug Des. 2011, 77, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Leão, M.; Pereira, C.; Bisio, A.; Ciribilli, Y.; Paiva, A.M.; Machado, N.; Palmeira, A.; Fernandes, M.X.; Sousa, E.; Pinto, M.; et al. Discovery of a new small-molecule inhibitor of p53–MDM2 interaction using a yeast-based approach. Biochem. Pharmacol. 2013, 85, 1234–1245. [Google Scholar] [CrossRef]
- Bessa, L.J.; Palmeira, A.; Gomes, A.S.; Vasconcelos, V.; Sousa, E.; Pinto, M.; da Costa, P.M. Synergistic Effects Between Thioxanthones and Oxacillin Against Methicillin-Resistant Staphylococcus aureus. Microb. Drug Resist. 2015, 21, 404–415. [Google Scholar] [CrossRef]
- Cidade, H.; Rocha, V.; Palmeira, A.; Marques, C.; Tiritan, M.E.; Ferreira, H.; Lobo, J.S.; Almeida, I.F.; Sousa, M.E.; Pinto, M. In silico and in vitro antioxidant and cytotoxicity evaluation of oxygenated xanthone derivatives. Arab. J. Chem. 2020, 13, 17–26. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Pereira-Terra, P.; Inácio, Â.S.; da Costa, P.M.; Pinto, E.; Sousa, E.; Pinto, M.M.M. Lichen Xanthones as Models for New Antifungal Agents. Molecules 2018, 23, 2617. [Google Scholar] [CrossRef]
- Urbatzka, R.; Freitas, S.; Palmeira, A.; Almeida, T.; Moreira, J.; Azevedo, C.; Afonso, C.; Correia-Da-Silva, M.; Sousa, M.E.; Pinto, M.; et al. Lipid reducing activity and toxicity profiles of a library of polyphenol derivatives. Eur. J. Med. Chem. 2018, 151, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Correia-Da-Silva, M.; Nunes, C.; Campos, J.; Sousa, E.; Silva, P.M.; Bousbaa, H.; Rodrigues, F.; Ferreira, D.; Costa, P.C.; et al. Discovery of a New Xanthone against Glioma: Synthesis and Development of (Pro)liposome Formulations. Molecules 2019, 24, 409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemos, A.; Gomes, A.S.; Loureiro, J.B.; Brandão, P.; Palmeira, A.; Pinto, M.M.M.; Saraiva, L.; Sousa, M.E. Synthesis, Biological Evaluation, and In Silico Studies of Novel Aminated Xanthones as Potential p53-Activating Agents. Molecules 2019, 24, 1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, E.; Silva, V.; Lemos, A.; Palmeira, A.; Puthongking, P.; de Sousa, M.E.; Rocha-Pereira, C.; Ghanem, C.I.; Carmo, H.; Remião, F.; et al. Newly Synthesized Oxygenated Xanthones as Potential P-Glycoprotein Activators: In Vitro, Ex Vivo, and In Silico Studies. Molecules 2019, 24, 707. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.; Cerqueira, F.; Nazareth, N.; Medeiros, R.; Sarmento, A.; Sousa, E.; Pinto, M. 1,2-Dihydroxyxanthone: Effect on A375-C5 Melanoma Cell Growth Associated with Interference with THP-1 Human Macrophage Activity. Pharmaceuticals 2019, 12, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resende, D.I.S.P.; Almeida, M.C.; Maciel, B.; Carmo, H.; Lobo, J.S.; Pozzo, C.D.; Cravo, S.M.; Rosa, G.P.; Kane-Pagès, A.; Barreto, M.D.C.; et al. Efficacy, Stability, and Safety Evaluation of New Polyphenolic Xanthones Towards Identification of Bioactive Compounds to Fight Skin Photoaging. Molecules 2020, 25, 2782. [Google Scholar] [CrossRef] [PubMed]
- Resende, D.I.S.P.; Pereira-Terra, P.; Moreira, J.; Freitas-Silva, J.; Lemos, A.; Gales, L.; Pinto, E.; de Sousa, M.E.; Da Costa, P.M.; Pinto, M.M.M. Synthesis of a Small Library of Nature-Inspired Xanthones and Study of Their Antimicrobial Activity. Molecules 2020, 25, 2405. [Google Scholar] [CrossRef]
- Silva, V.; Gil Martins, E.; Rocha-Pereira, C.; Lemos, A.; Palmeira, A.; Puthongking, P.; Sousa, E.; Bastos, M.D.L.; Remião, F.; Silva, R. Oxygenated xanthones as P-glycoprotein modulators at the intestinal barrier: In vitro and docking studies. Med. Chem. Res. 2020, 29, 1041–1057. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Almeida, J.R.; Pereira, S.; Campos, A.; Lemos, A.; Plowman, J.E.; Thomas, A.; Clerens, S.; Vasconcelos, V.; Pinto, M.; et al. From Natural Xanthones to Synthetic C-1 Aminated 3,4-Dioxygenated Xanthones as Optimized Antifouling Agents. Mar. Drugs 2021, 19, 638. [Google Scholar] [CrossRef]
- Rosa, G.; Palmeira, A.; Resende, D.; Almeida, I.; Kane-Pagès, A.; Barreto, M.; Sousa, E.; Pinto, M. Xanthones for melanogenesis inhibition: Molecular docking and QSAR studies to understand their anti-tyrosinase activity. Bioorg. Med. Chem. 2020, 29, 115873. [Google Scholar] [CrossRef]
- Soidinsalo, O.; Wähälä, K. Aromatic Chlorination with Thionyl Chloride. Applications in the Synthesis of Chlorinated Isoflavones. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 2761–2767. [Google Scholar] [CrossRef]
- Schweikl, H.; Birke, M.; Gallorini, M.; Petzel, C.; Bolay, C.; Waha, C.; Hiller, K.-A.; Buchalla, W. HEMA-induced oxidative stress inhibits NF-κB nuclear translocation and TNF release from LTA- and LPS-stimulated immunocompetent cells. Dent. Mater. 2021, 37, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Tyrchan, C.; Evertsson, E. Matched Molecular Pair Analysis in Short: Algorithms, Applications and Limitations. Comput. Struct. Biotechnol. J. 2016, 15, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Griffen, E.; Leach, A.G.; Robb, G.R.; Warner, D.J. Matched Molecular Pairs as a Medicinal Chemistry Tool. J. Med. Chem. 2011, 54, 7739–7750. [Google Scholar] [CrossRef]
- Ding, X.; Cui, C.; Wang, D.; Zhao, J.; Zheng, M.; Luo, X.; Jiang, H.; Chen, K. Bioactivity Prediction Based on Matched Molecular Pair and Matched Molecular Series Methods. Curr. Pharm. Des. 2020, 26, 4195–4205. [Google Scholar] [CrossRef]
- Arodz, T.; Galvez, J. Computational Methods in Developing Quantitative Structure-Activity Relationships (QSAR): A Review. Comb. Chem. High Throughput Screen. 2006, 9, 213–228. [Google Scholar] [CrossRef]
- Ćwik, J.; Koronacki, J. A Heuristic Method of Model Choice for Nonlinear Regression. In Rough Sets and Current Trends in Computing, Proceedings of the First International Conference, RSCTC’98, Warsaw, Poland, 22–26 June 1998; Polkowski, L., Skowron, A., Eds.; Springer: Berlin/Heidelberg, Germany; pp. 68–74.
- Dunn, W.J.; Hopfinger, A.J. 3D QSAR of Flexible Molecules using Tensor Representation; Kluwer Academic Publishers: New York, NY, USA, 2002. [Google Scholar]
- Kubinyi, H. QSAR: Hansch Analysis and Related Approaches; VCH: Weinheim, Germany; New York, NY, USA, 1993. [Google Scholar]
- Veerasamy, R.; Rajak, H.; Jain, A.; Sivadasan, S.; Varghese, C.P.; Agrawal, R.K. Validation of QSAR Models—Strategies and Importance. Int. J. Drug Discov. 2011, 2, 511–519. [Google Scholar]
- Alexander, D.L.J.; Tropsha, A.; Winkler, D.A. Beware of R2: Simple, Unambiguous Assessment of the Prediction Accuracy of QSAR and QSPR Models. J. Chem. Inform. Mod. 2015, 55, 1316–1322. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Long, W. Current Mathematical Methods Used in QSAR/QSPR Studies. Int. J. Mol. Sci. 2009, 10, 1978–1998. [Google Scholar] [CrossRef]
- Gramatica, P. On the Development and Validation of QSAR Models. In Computational Toxicology: Volume II; Reisfeld, B., Mayeno, A.N., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 499–526. [Google Scholar]
- Golbraikh, A.; Shen, M.; Xiao, Z.; Xiao, Y.-D.; Lee, K.-H.; Tropsha, A. Rational selection of training and test sets for the development of validated QSAR models. J. Comput. Aided Mol. Des. 2003, 17, 241–253. [Google Scholar] [CrossRef]
- Devillers, J.; Balaban, A.T. Topological Indices and Related Descriptors in QSAR and QSPR; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Katritzky, A.R.; Gordeeva, E.V. Traditional topological indices vs electronic, geometrical, and combined molecular descriptors in QSAR/QSPR research. J. Chem. Inf. Comput. Sci. 1993, 33, 835–857. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Tamm, T.; Wang, Y.; Sild, S.; Karelson, M. QSPR Treatment of Solvent Scales. J. Chem. Inf. Comput. Sci. 1999, 39, 684–691. [Google Scholar] [CrossRef]
- Todeschini, R.; Consonni, V. Methods and Principles in Medicinal Chemistry—Molecular Descriptors for Chemoinformatics; VCH: Weinheim, Germany, 2009. [Google Scholar]
- Carson, J.R.; Codd, E.; Razler, C.M.; Works, A.; McDonnell, M.; McNally, J.J. Tricyclic-Bridged Piperidinylidene Derivatives as 8-Opioid Modulators. U.S. Patent No. US7589103B2, 15 September 2009. [Google Scholar]
- Wu, L.; Burgess, K. Synthesis and Spectroscopic Properties of Rosamines with Cyclic Amine Substituents. J. Org. Chem. 2008, 73, 8711–8718. [Google Scholar] [CrossRef] [PubMed]
- Berrino, E.; Carradori, S.; Angeli, A.; Carta, F.; Supuran, C.; Guglielmi, P.; Coletti, C.; Paciotti, R.; Schweikl, H.; Maestrelli, F.; et al. Dual Carbonic Anhydrase IX/XII Inhibitors and Carbon Monoxide Releasing Molecules Modulate LPS-Mediated Inflammation in Mouse Macrophages. Antioxidants 2021, 10, 56. [Google Scholar] [CrossRef]
- Gallorini, M.; Zara, S.; Ricci, A.; Mangano, F.G.; Cataldi, A.; Mangano, C. The Open Cell Form of 3D-Printed Titanium Improves Osteconductive Properties and Adhesion Behavior of Dental Pulp Stem Cells. Materials 2021, 14, 5308. [Google Scholar] [CrossRef]
- Gallorini, M.; Petzel, C.; Bolay, C.; Hiller, K.-A.; Cataldi, A.; Buchalla, W.; Krifka, S.; Schweikl, H. Activation of the Nrf2-regulated antioxidant cell response inhibits HEMA-induced oxidative stress and supports cell viability. Biomaterials 2015, 56, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Katritsky, A.; Karelson, M.; Lobanov, V.; Dennington, R.; Keith, T. CODESSA 2.7. 10; Semichem, Inc.: Shawnee, KS, USA, 2004. [Google Scholar]



| Compound | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 |
|---|---|---|---|---|---|---|---|---|
| 1 | H | H | H | H | H | H | H | H |
| 2 | OH | H | H | H | H | H | H | H |
| 3 | H | OH | H | H | H | H | H | H |
| 4 | H | H | OH | H | H | H | H | H |
| 5 | H | H | H | OH | H | H | H | H |
| 6 | OCH3 | H | H | H | H | H | H | H |
| 7 | H | OCH3 | H | H | H | H | H | H |
| 8 | H | H | H | OCH3 | H | H | H | H |
| 9 | OH | OH | H | H | H | H | H | H |
| 10 | H | OH | OH | H | H | H | H | H |
| 11 | H | H | OH | OH | H | H | H | H |
| 12 | OH | H | H | H | H | H | OH | H |
| 13 | H | H | OH | H | H | OH | H | H |
| 14 | OCH3 | OCH3 | H | H | H | H | H | H |
| 15 | H | OCH3 | OCH3 | H | H | H | H | H |
| 16 | H | H | OCH3 | OCH3 | H | H | H | H |
| 17 | H | H | OCH3 | OH | H | H | H | H |
| 18 | OH | OCH3 | OH | H | H | H | H | H |
| 19 | CHO | H | OH | OH | H | H | H | H |
| 20 | CHO | H | OCH3 | OCH3 | H | H | H | H |
| 21 | CHO | H | OCH3 | OCH3 | H | OCH3 | H | H |
| 22 | CHO | H | OCH3 | OH | H | H | H | H |
| 23 | COOCH3 | H | OCH3 | OCH3 | H | OCH3 | H | H |
| 24 | H | CHO | OH | OCH3 | H | H | H | H |
| 25 | CH3 | H | OH | OH | H | H | H | H |
| 26 | CH3 | H | OCH3 | OCH3 | H | H | H | H |
| 27 | CH3 | Cl | OCH3 | OCH3 | H | H | H | H |
| 28 | CH3 | H | OCH3 | OCH3 | H | OCH3 | H | H |
| 29 | CHBr2 | H | OCH3 | OCH3 | H | H | H | H |
| 30 | CHBr2 | H | OCH3 | OCH3 | H | OCH3 | H | H |
| 31 | OH | H | CH3 | H | H | CH3 | H | OH |
| 32 | H | H | Cl | H | H | Cl | H | H |
| 33 | H | H | CO2CH3 | H | H | CO2CH3 | H | H |
| 34 | H | H | OTf | H | H | OTf | H | H |
| 35 | H | H | 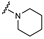 | H | H | 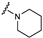 | H | H |
| 36 | H | H | N(CH3)2 | H | H | N(CH3)2 | H | H |
| 37 | H | H | 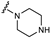 | H | H |  | H | H |
| 38 |  | H | OCH3 | OCH3 | H | H | H | H |
| 49 | 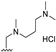 | H | OCH3 | OCH3 | H | H | H | H |
| 40 |  | H | OCH3 | OCH3 | H | H | H | H |
| α-mangostin | OH | 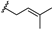 | OH | H | H | OH | OCH3 |  |
| γ-mangostin | OH | 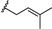 | OH | H | H | OH | OH | 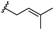 |
| Compound (1 µM) | Viability 24 h (% LPS) | Viability 48 h (% LPS) |
|---|---|---|
| 1 | 81.3 ± 5.1 d,g | 118.7 ± 23.1 |
| 2 | 77.9 ± 2.6 a,e,h | 98.6 ± 25.5 |
| 3 | 116.5 ± 28.9 | 137.1 ± 16.5 |
| 4 | 123.4 ± 4.1 | 130.8 ± 6.7 a |
| 5 | 151.7 ± 5.2 a | 144.6 ± 7.0 b |
| 6 | 168.7 ± 5.2 c | 147.4 ± 3.1 b |
| 7 | 110.8 ± 0.7 | 105.9 ± 15.0 |
| 8 | 97.6 ± 17.0 | 100.6 ± 18.8 |
| 9 | 135.2 ± 39.6 b | 147.2 ± 6.4 |
| 10 | 131.8 ± 3.3 a | 127.1 ± 5.3 a |
| 11 | 119.3 ± 7.6 | 104.7 ± 17.8 g |
| 12 | 92.6 ± 5.0 f,i | 107.3 ± 15.4 |
| 13 | 114.7 ± 0.6 | 101.4 ± 12.8 d,g |
| 14 | 122.3 ± 1.8 | 102.0 ± 11.6 g |
| 15 | 109.5 ± 1.9 h | 108.9 ± 3.0 |
| 16 | 111.3 ± 10.0 g | 130.9 ± 1.9 |
| 17 | 143.4 ± 14.5 a | 123.5 ± 5.3 |
| 18 | 104.8 ± 12.0 d,h | 109.7 ± 5.9 |
| 19 | 105.4 ± 4.9 d,h | 111.8 ± 2.5 |
| 20 | 134.5 ± 2.8 a | 101.3 ± 2.4 d,g |
| 21 | 90.8 ± 0.5 f,i | 93.3 ± 6.6 e,i |
| 22 | 93.4 ± 0.4 f,i | 107.6 ± 11.1 |
| 23 | 112.7 ± 3.6 g | 91.4 ± 6.0 e,i |
| 24 | 102.0 ± 11.4 e,i | 83.7 ± 17.1 f,i |
| 25 | 110.7 ± 8.4 h | 99.9 ± 15.0 d,h |
| 26 | 100.8 ± 7.0 f,h | 99.9 ± 7.2 |
| 27 | 101.6 ± 16.9 e,h | 124.8 ± 8.4 a |
| 28 | 112.5 ± 2.0 g | 108.8 ± 8.1 |
| 29 | 89.4 ± 1.5 f,i | 98.4 ± 2.2 d,h |
| 30 | 104.7 ± 1.5 e,i | 108.4 ± 7.3 |
| 31 | 104.0 ± 2.7 e,i | 105.5 ± 10.6 g |
| 32 | 96.6 ± 8.8 f,i | 94.8 ± 3.4 e,i |
| 33 | 98.0 ± 2.9 f,i | 82.8 ± 9.0 f,i |
| 34 | 87.0 ± 5.6 f,i | 85.5 ± 3.6 f,i |
| 35 | 111.8 ± 3.5 f,i | 115.3 ± 2.2 a,h |
| 36 | 117.1 ± 3.8 a,f,i | 100.9 ± 2.7 e,h |
| 37 | 112.9 ± 0.3 f,i | 103.3 ± 0.1 |
| 38 | 90.0 ± 4.2 b,f,i | 84.5 ± 11.5 f,i |
| 39 | 96.7 ± 0.5 f,i | 83.5 ± 7.0 f,i |
| 40 | 82.9 ± 1.4 f,i | 85.3 ± 2.0 a,f,i |
| α-mangostin | 135.4 ± 0.2 b | 120.3 ± 1.4 a |
| γ-mangostin | 140.2 ± 1.4 c | 123.2 ± 3.5 a |
| Compound (10 µM) | Viability 24 h (% LPS) | Viability 48 h (% LPS) |
|---|---|---|
| 1 | 108.0 ± 0.3 m | 100.7 ± 7.5 |
| 2 | 81.2 ± 0.4 | 77.7 ± 15.8 g |
| 3 | 112.4 ± 19.8 | 122.3 ± 1.3 |
| 4 | 117.5 ± 6.8 | 99.0 ± 3.5 m |
| 5 | 144.1 ± 19.3 a | 96.3 ± 2.7 n |
| 6 | 123.8 ± 5.5 n | 102.9 ± 5.8 n |
| 7 | 133.2 ± 11.3 a | 95.8 ± 1.7 |
| 8 | 92.8 ± 10.5 | 97.0 ± 1.3 |
| 9 | 127.2 ± 5.7 | 124.7 ± 1.8 |
| 10 | 134.3 ± 9.3 a | 134.2 ± 4.6 a |
| 11 | 115.7 ± 2.9 | 91.9 ± 8.0 |
| 12 | 100.1 ± 3.9 i | 103.7 ± 16.1 |
| 13 | 111.9 ± 1.1 | 93.8 ± 3.9 |
| 14 | 111.2 ± 2.4 | 98.3 ± 0.1 |
| 15 | 98.3 ± 10.1 | 101.1 ± 0.2 |
| 16 | 116.6 ± 10.6 | 103.3 ± 3.7 |
| 17 | 111.2 ± 5.9 | 92.3 ± 15.0 |
| 18 | 106.1 ± 14.2 | 115.0 ± 5.3 |
| 19 | 116.4 ± 1.9 | 73.7 ± 4.2 m,e,h |
| 20 | 86.0 ± 2.3 n,d,h | 56.0 ± 6.4 a,m,f,i |
| 21 | 51.8 ± 9.9 a,m,f,i | 40.4 ± 11.3 b,m,f,i |
| 22 | 100.1 ± 0.1 | 86.2 ± 3.7 g |
| 23 | 111.8 ± 6.1 | 97.7 ± 16.1 |
| 24 | 101.9 ± 0.9 | 100.4 ± 1.3 |
| 25 | 101.6 ± 2.0 | 120.5 ± 7.1 |
| 26 | 114.0 ± 7.9 | 115.0 ± 8.2 |
| 27 | 102.0 ± 1.3 | 118.0 ± 3.0 a |
| 28 | 100.8 ± 2.7 | 95.9 ± 11.3 |
| 29 | 77.5 ± 3.3 a,f,i | 72.3 ± 1.3 c,r,e,i |
| 30 | 53.0 ± 6.6 b,r,f,i | 51.5 ± 1.3 c,r,f,i |
| 31 | 91.1 ± 1.1 h | 87.0 ± 8.2 g |
| 32 | 92.4 ± 3.7 g | 88.5 ± 2.5 |
| 33 | 88.3 ± 10.7 g | 70.7 ± 7.1 a,e,i |
| 34 | 94.6 ± 7.1 g | 96.9 ± 8.6 |
| 35 | 109.0 ± 0.8 h | 103.3 ± 0.7 h |
| 36 | 91.7 ± 2.1 n,f,i | 87.1 ± 7.6 f,i |
| 37 | 92.8 ± 0.1 m,f,i | 110.6 ± 3.4 h |
| 38 | 82.2 ± 0.7 a,f,i | 80.0 ± 7.1 f,i |
| 39 | 91.8 ± 2.2 a,f,i | 112.2 ± 1.2 a,h |
| 40 | 76.86 ± 1.7 m,f,i | 91.0 ± 1.4 n,f,i |
| α-mangostin | 115.1 ± 0.1 | 118.2 ± 1.3 a |
| γ-mangostin | 120.1 ± 0.6 a,m | 126.9 ± 0.7 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallorini, M.; Carradori, S.; Resende, D.I.S.P.; Saso, L.; Ricci, A.; Palmeira, A.; Cataldi, A.; Pinto, M.; Sousa, E. Natural and Synthetic Xanthone Derivatives Counteract Oxidative Stress via Nrf2 Modulation in Inflamed Human Macrophages. Int. J. Mol. Sci. 2022, 23, 13319. https://doi.org/10.3390/ijms232113319
Gallorini M, Carradori S, Resende DISP, Saso L, Ricci A, Palmeira A, Cataldi A, Pinto M, Sousa E. Natural and Synthetic Xanthone Derivatives Counteract Oxidative Stress via Nrf2 Modulation in Inflamed Human Macrophages. International Journal of Molecular Sciences. 2022; 23(21):13319. https://doi.org/10.3390/ijms232113319
Chicago/Turabian StyleGallorini, Marialucia, Simone Carradori, Diana I. S. P. Resende, Luciano Saso, Alessia Ricci, Andreia Palmeira, Amelia Cataldi, Madalena Pinto, and Emília Sousa. 2022. "Natural and Synthetic Xanthone Derivatives Counteract Oxidative Stress via Nrf2 Modulation in Inflamed Human Macrophages" International Journal of Molecular Sciences 23, no. 21: 13319. https://doi.org/10.3390/ijms232113319