Luminescence, Paramagnetic, and Electrochemical Properties of Copper Oxides-Decorated TiO2/Graphene Oxide Nanocomposites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photoluminescence (PL) Data
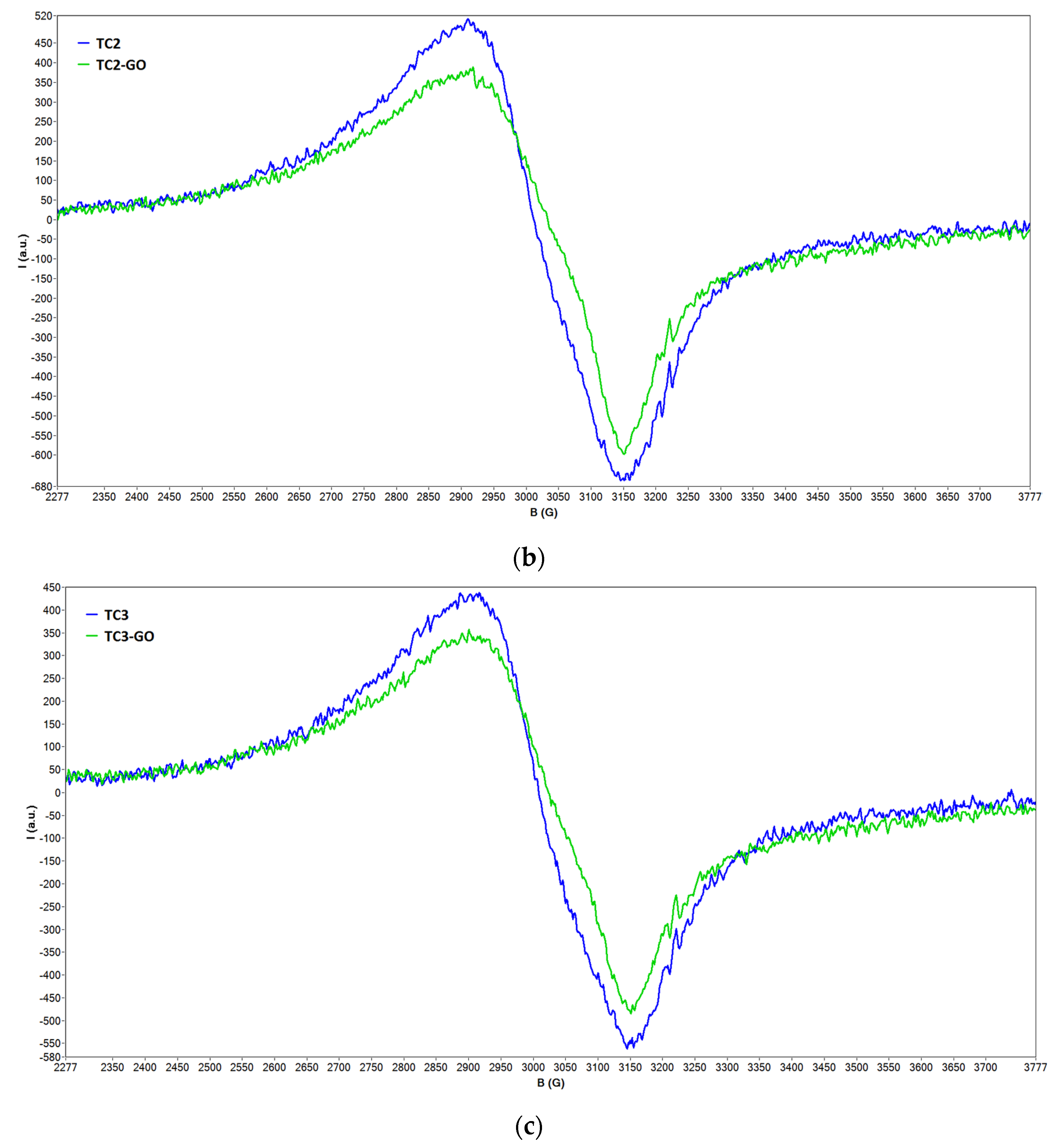
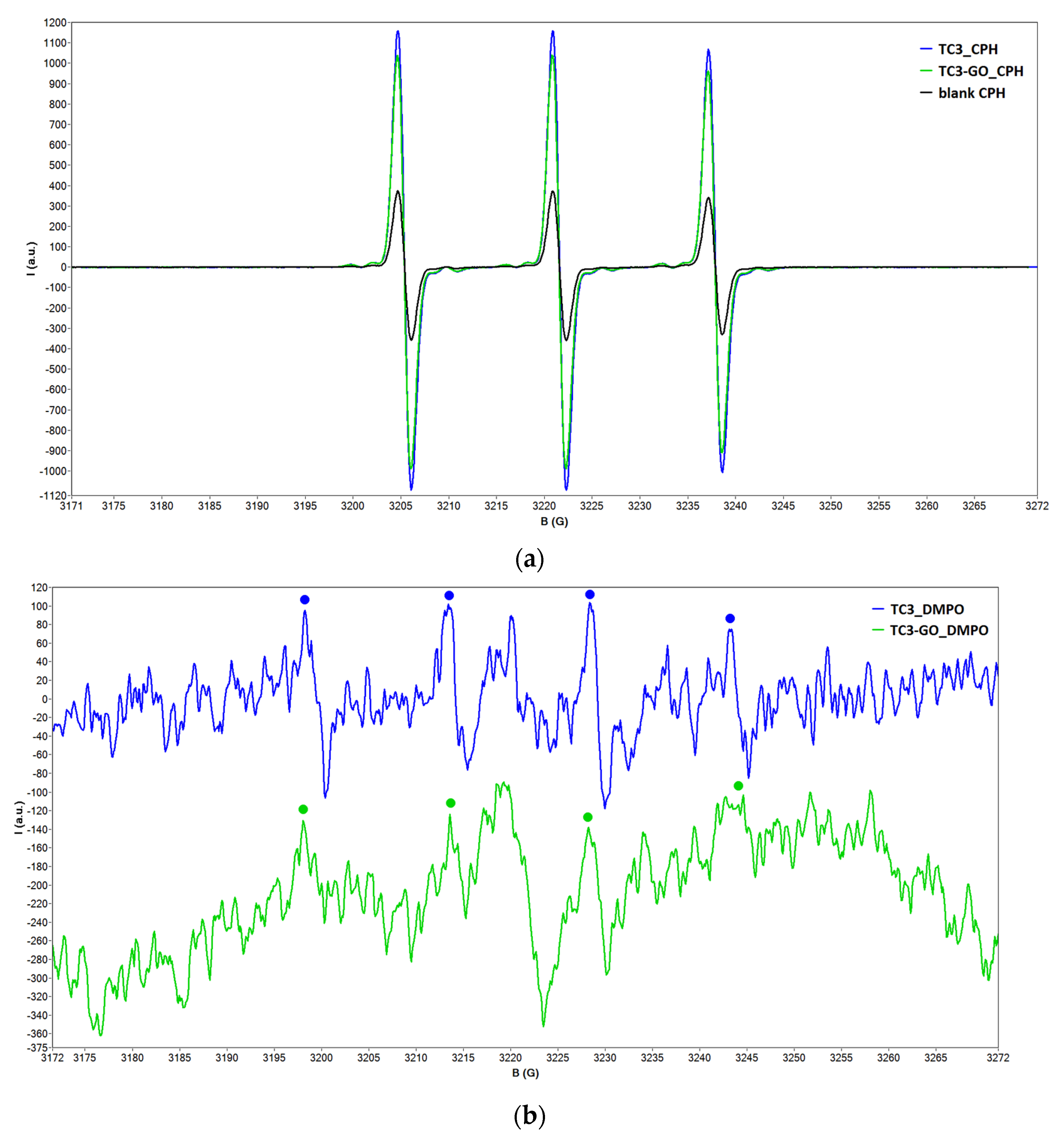
2.2. EPR Spectroscopy Data
2.2.1. EPR Spectra of Solid Samples
2.2.2. Spin-Trapping Measurements
2.3. Electrochemical Characterization
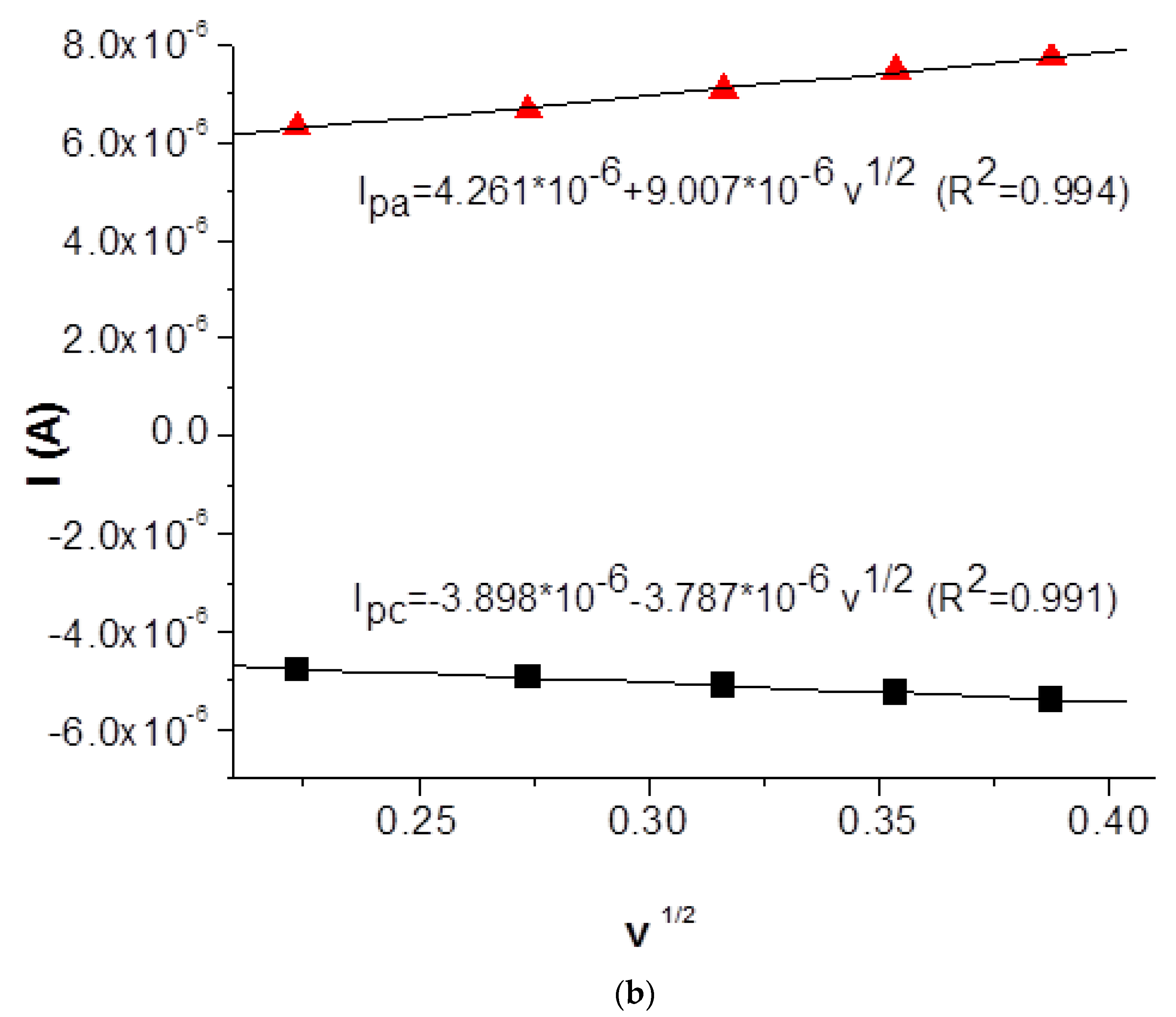
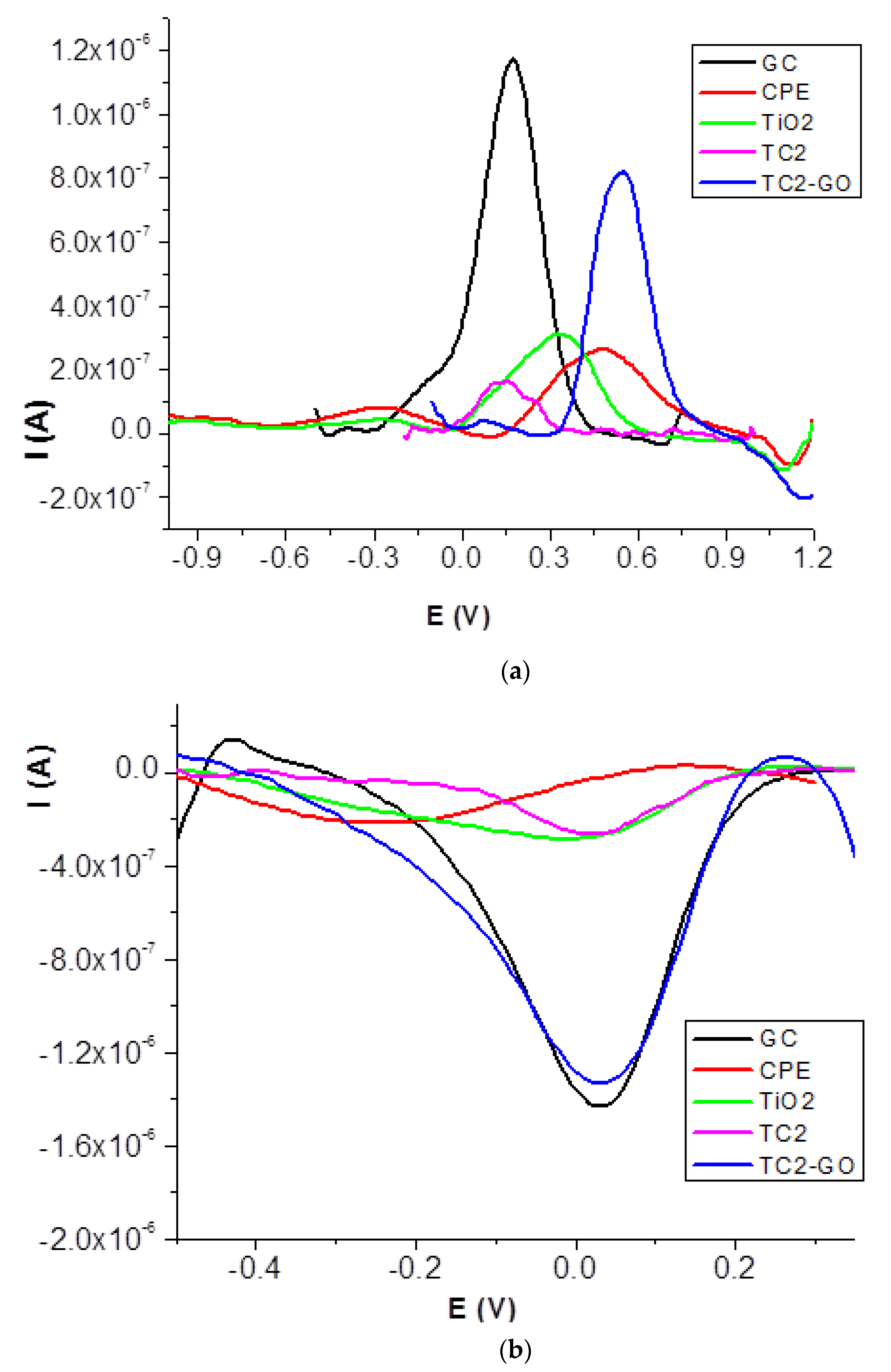
2.3.1. Cyclic Voltammetry Study
2.3.2. Differential Pulse Voltammetry Results
3. Materials and Methods
3.1. Nanomaterials Preparation
3.2. Photoluminescence and EPR Spectroscopy Characterization
3.3. Preparation of the Carbon Paste Electrodes
3.4. Testing the Modified Carbon Paste Electrodes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brezová, V.; Billik, P.; Vrecková, Z.; Plesch, G. Photoinduced formation of reactive oxygen species in suspensions of titania mechanochemically synthesized from TiCl4. J. Mol. Catal. A Chem. 2010, 327, 101–109. [Google Scholar] [CrossRef]
- Do Kim, K.; Lee, T.J.; Kim, H.T. Optimal conditions for synthesis of TiO2 nanoparticles in semi-batch reactor. Colloids Surf. A 2003, 224, 1–9. [Google Scholar] [CrossRef]
- Kim, I.; Kim, G.; Choi, Y.; Lee, W.; Smith, C., Jr.; Kim, Y. Method for Synthesizing Nano-Sized Titanium Dioxide Particles. US Patent WO 2006/044495 A1, 27 April 2006. [Google Scholar]
- Yang, J.; Mei, S.; Ferreira, J. Hydrothermal synthesis of TiO2 nanopowders from tetraalkylammonium hydroxide peptized sols. Mater. Sci. Eng. C 2001, 15, 183–185. [Google Scholar] [CrossRef]
- Zhang, C.J.; Anasori, B.; Seral-Ascaso, A.; Park, S.H.; McEvoy, N.; Shmeliov, A.; Dues-berg, G.S.; Coleman, J.N.; Gogotsi, Y.; Nicolosi, V. Transparent, flexible, and conductive 2D titanium carbide (MXene) films with high volumetric capacitance. Adv. Mater. 2017, 29, 1702678. [Google Scholar] [CrossRef] [PubMed]
- Ajmala, A.; Majeed, I.; Malik, R.N.; Iqbal, M.; Arif Nadeem, M.; Hussain, I.; Yousaf, S.; Zeshan, M.G.; Zafar, M.I.; Amtiaz Nadeem, M. Photocatalytic degradation of textile dyes on Cu2O-CuO/TiO2 anatase powders. J. Environ. Chem. Eng. 2016, 4, 2138–2146. [Google Scholar] [CrossRef]
- Padmanabhan, N.T.; Thomas, N.; Louis, J.; Mathew, D.T.; Ganguly, P.; John, H.; Pillai, S.C. Graphene coupled TiO2 photocatalysts for environmental applications: A review. Chemosphere 2021, 271, 129506. [Google Scholar] [CrossRef]
- Darabi, H.; Adelifard, M.; Rajabi, Y. Characterization of nonlinear optical refractive index for graphene oxide–silicon oxide nanohybrid composite. J. Nonlinear Opt. Phys. Mater. 2019, 28, 1950005. [Google Scholar] [CrossRef]
- Safa, M.; Rajabi, Y.; Ardyanian, M. Influence of preparation method on the structural, linear, and nonlinear optical properties of TiN nanoparticles. J. Mate-Rials Sci. Mater. Electron. 2021, 32, 19455–19477. [Google Scholar] [CrossRef]
- Dadkhah, S.; Rajabi, Y.; Zare, E.N. Thermal Lensing Effect in Laser Nanofluids Based on Poly (aniline-co-ortho phenylenediamine)@TiO2 Interaction. J. Electron. Mater. 2021, 50, 4896–4907. [Google Scholar] [CrossRef]
- Altin, I. CuO-TiO2/graphene ternary nanocomposite for highly efficient visible-light-driven photocatalytic degradation of bisphenol A. J. Mol. Struct. 2022, 1252, 132199. [Google Scholar] [CrossRef]
- Mirza-Aghayan, M.; Saeedi, M.; Boukherroub, R. An efficient CuO/rGO/TiO2 photocatalyst for the synthesis of benzopyranopyrimidine compounds under visible light irradiation. New J. Chem. 2022, 46, 3817–3830. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, K.-J.; Niu, D.-J.; Yang, C.-P.; Jing, Q.-S. TiO2-graphene nanocomposite for electrochemical sensing of adenine and guanine. Electrochim. Acta 2011, 56, 4685–4690. [Google Scholar] [CrossRef]
- Liu, A.; Wei, M.D.; Honma, I.; Zhou, H. Biosensing properties of titanate nanotube films: Selective detection of dopamine in the presence of ascorbate and uric acid. Adv. Funct. Mater. 2006, 16, 371–376. [Google Scholar] [CrossRef]
- Tavakkoli, N.; Soltani, N.; Salavati, H.; Talakoub, M. New carbon paste electrode modified with graphene/TiO2/V2O5 for electrochemical measurement of chlorpromazine hydrochloride. J. Taiwan Inst. Chem. Eng. 2018, 83, 50–58. [Google Scholar] [CrossRef]
- Choudhury, B.; Dey, M.; Choudhury, A. Shallow and deep trap emission and luminescence quenching of TiO2 nanoparticles on Cu doping. Appl. Nanosci. 2014, 4, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Kernazhitsky, L.; Shymanovska, V.; Gavrilko, T.; Naumov, V.; Fedorenko, L.; Kshnyakin, V.; Baran, J. Room temperature photoluminescence of anatase and rutile TiO2 powders. J. Lumin. 2014, 146, 199–204. [Google Scholar] [CrossRef]
- Nair, R.V.; Gayathri, P.K.; Gummaluri, V.S.; Nambissan, P.M.G.; Vijayan, C. Large bandgap narrowing in rutile TiO2 aimed towards visible light applications and its correlation with vacancy-type defects history and transformation. J. Phys. D Appl. Phys. 2018, 51, 045107. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E. On the origin of enhanced photocatalytic activity of copper-modified titania in the oxidative reaction systems. Catalysts 2017, 7, 317. [Google Scholar] [CrossRef] [Green Version]
- Yadav, H.M.; Kim, J.S. Solvothermal synthesis of anatase TiO2-graphene oxide nanocomposites and their photocatalytic performance. J. Alloys Compd. 2016, 688, 123–129. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Xue, N.; Wang, P.; Pei, M.; Guo, W. Ultra-highly efficient removal of methylene blue based on graphene oxide/TiO2/bentonite sponge. Materials 2020, 13, 824. [Google Scholar] [CrossRef] [PubMed]
- Hagen, W.R. EPR spectroscopy as a probe of metal centres in biological systems. Dalton Trans. 2006, 37, 4415–4434. [Google Scholar] [CrossRef] [PubMed]
- Dvoranová, D.; Barbieriková, Z.; Brezová, V. Radical intermediates in photoinduced reactions on TiO2 (An EPR spin trapping study). Molecules 2014, 19, 17279–17304. [Google Scholar] [CrossRef] [Green Version]
- Carter, E.; Carley, A.F.; Murphy, D.M. Evidence for O2-radical stabilization at surface oxygen vacancies on polycrystalline TiO2. J. Phys. Chem. C 2007, 111, 10630–10638. [Google Scholar] [CrossRef]
- Hellack, B.; Nickel, C.; Schins, R.P.F. Oxidative potential of silver nanoparticles measured by electron paramagnetic resonance spectroscopy. J. Nanopart. Res. 2017, 19, 404. [Google Scholar] [CrossRef]
- Pou, S.; Ramos, C.L.; Gladwell, T.; Renks, E.; Centra, M.; Young, D.; Cohen, M.S.; Rosen, G.M. A Kinetic Approach to the Selection of a Sensitive Spin Trapping System for the Detection of Hydroxyl Radical. Anal. Biochem. 1994, 217, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R. Spin trapping: ESR parameters of spin adducts. Free Radic. Biol. Med. 1987, 3, 259–303. [Google Scholar] [CrossRef]
- Lin, W.J.; Liao, C.S.; Jhang, J.H.; Tsai, Y.C. Graphene modified basal and edge plane pyrolytic graphite electrodes for electrocatalytic oxidation of hydrogen peroxide and β-nicotinamide adenine dinucleotide. Electrochem. Commun. 2009, 11, 2153. [Google Scholar] [CrossRef]
- Cosma, D.; Urda, A.; Radu, T.; Rosu, M.C.; Mihet, M.; Socaci, C. Evaluation of the Photocatalytic Properties of Copper Oxides/Graphene/TiO2 Nanoparticles Composites. Molecules 2022, 27, 5803. [Google Scholar] [CrossRef]
- Pogacean, F.; Socaci, C.; Pruneanu, S.; Biris, A.R.; Coros, M.; Magerusan, L.; Katona, G.; Turcu, R.; Borodi, G. Graphene based nanomaterials as chemical sensors for hydrogen peroxide–A comparison study of their intrinsic peroxidase catalytic behavior. Sens. Actuators B Chem. 2015, 213, 474–483. [Google Scholar] [CrossRef]
- Dikalov, S.S.; Grigor’ev, I.A.; Voinov, M.; Bassenge, E. Detection of superoxide radicals and peroxynitrite by 1-hydroxy-4-phosphonooxy-2,2,6,6-tetramethylpiperidine: Quantification of extracellular superoxide radicals formation. Biochem. Biophys. Res. Commun. 1998, 248, 211–215. [Google Scholar] [CrossRef]
- Duling, D.R. PEST Winsim, version 0.96; National Institute of Environmental Health Sciences: Triangle Park, NC, USA, 1996. [Google Scholar]
- Duling, D.R. Simulation of multiple isotropic spin-trap EPR spectra. J. Magn. Reson. B 1994, 104, 105–110. [Google Scholar] [CrossRef] [PubMed]




| Sample | ν (GHz) | B (mT) | g |
|---|---|---|---|
| TC1 | 9.046638 | 300.419 | 2.1516 |
| TC1-GO | 9.047555 | 299.914 310.639 | 2.1554 2.0810 |
| TC2 | 9.046588 | 301.141 | 2.1464 |
| TC2-GO | 9.047630 | 298.793 310.079 | 2.1635 2.0848 |
| TC3 | 9.047059 | 300.865 | 2.1485 |
| TC3-GO | 9.046954 | 298.233 309.038 | 2.1674 2.0916 |
| Ec (V) | Ic (A) | Ea (V) | Ia (A) | ΔE (V) | |
|---|---|---|---|---|---|
| CPE | −0.568 | −2.69 × 10−6 | 0.706 | 6.22 × 10−6 | 1.274 |
| TiO2 | −0.424 | −4.69 × 10−6 | 0.567 | 7.28 × 10−6 | 0.991 |
| TC2 | −0.203 | −5.00 × 10−6 | 0.466 | 7.08 × 10−6 | 0.669 |
| TC2-GO | −0.557 | −1.07 × 10−6 | 0.768 | 6.04 × 10−6 | 1.325 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bala, D.; Matei, I.; Ionita, G.; Cosma, D.-V.; Rosu, M.-C.; Stanca, M.; Gaidau, C.; Baleanu, M.; Virgolici, M.; Stanculescu, I. Luminescence, Paramagnetic, and Electrochemical Properties of Copper Oxides-Decorated TiO2/Graphene Oxide Nanocomposites. Int. J. Mol. Sci. 2022, 23, 14703. https://doi.org/10.3390/ijms232314703
Bala D, Matei I, Ionita G, Cosma D-V, Rosu M-C, Stanca M, Gaidau C, Baleanu M, Virgolici M, Stanculescu I. Luminescence, Paramagnetic, and Electrochemical Properties of Copper Oxides-Decorated TiO2/Graphene Oxide Nanocomposites. International Journal of Molecular Sciences. 2022; 23(23):14703. https://doi.org/10.3390/ijms232314703
Chicago/Turabian StyleBala, Daniela, Iulia Matei, Gabriela Ionita, Dragos-Viorel Cosma, Marcela-Corina Rosu, Maria Stanca, Carmen Gaidau, Maria Baleanu, Marian Virgolici, and Ioana Stanculescu. 2022. "Luminescence, Paramagnetic, and Electrochemical Properties of Copper Oxides-Decorated TiO2/Graphene Oxide Nanocomposites" International Journal of Molecular Sciences 23, no. 23: 14703. https://doi.org/10.3390/ijms232314703





