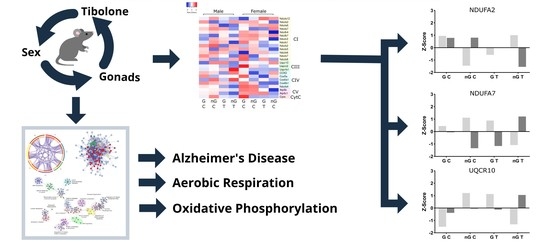
Respirasome Proteins Are Regulated by Sex-Hormone Interactions in the Brain
Abstract
:1. Introduction
2. Results
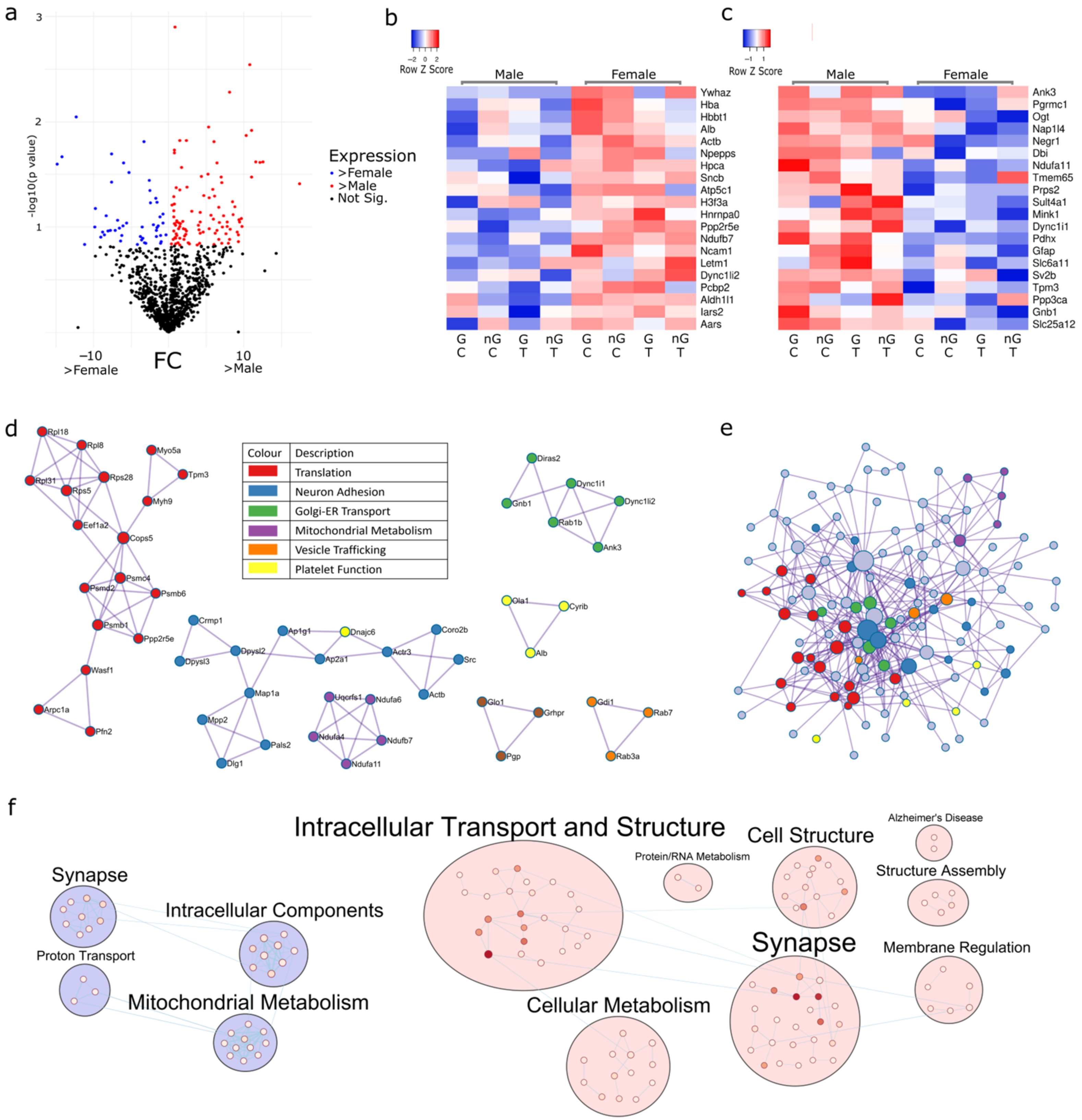
2.1. Sex Regulates Metabolism, Transcription and Organelle Localisation
2.2. Gonadectomy Impacts Cellular Functions, including Endocytosis, Translation and Carbon Metabolism
2.3. Multiple Pathways in Mitochondrial Metabolism Can Be Targeted with Tibolone
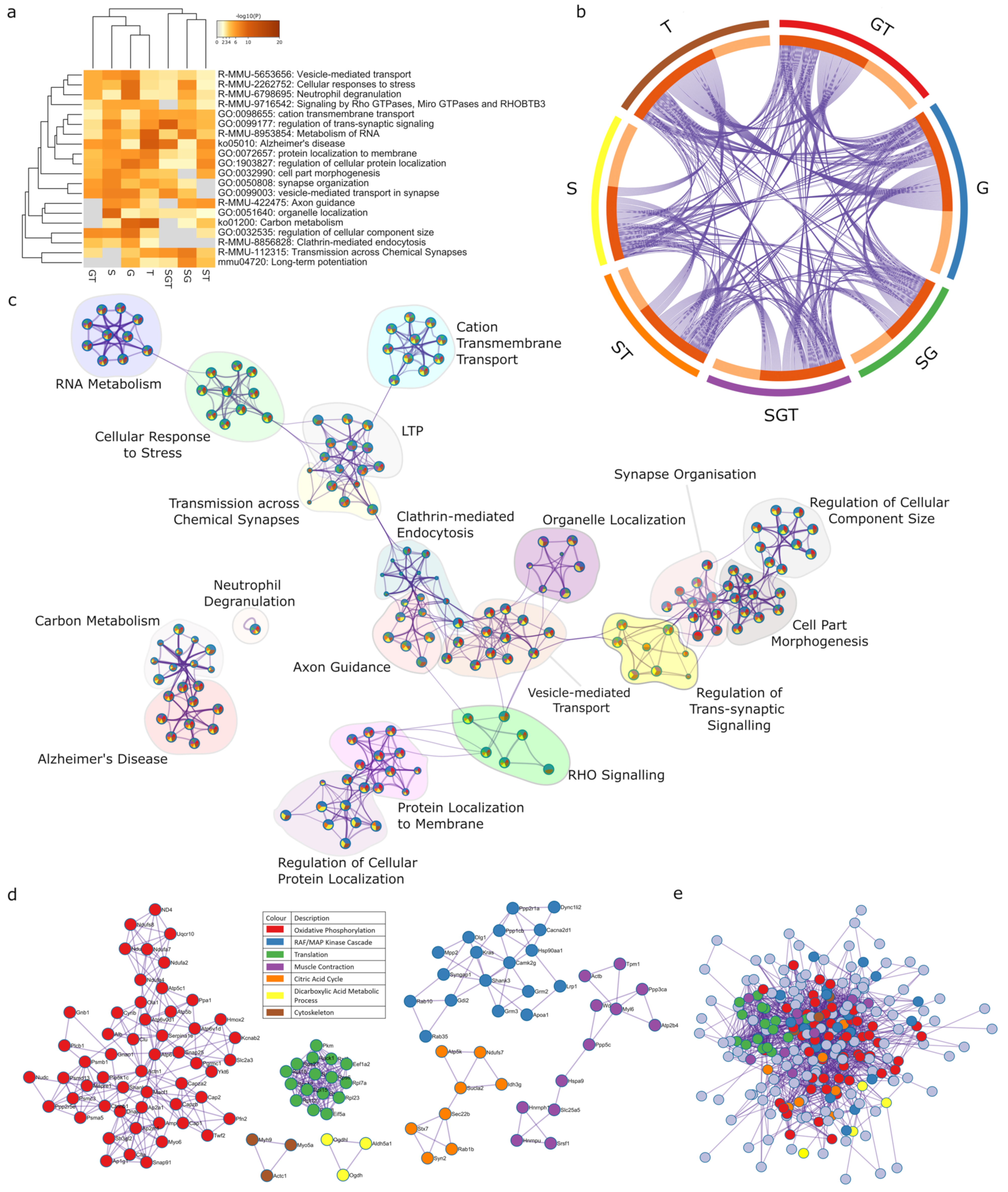
2.4. Two-Way Interactions of Sex-Associated Factors
2.4.1. Sex and Gonadectomy Interact to Regulate Nonsense-Mediated Decay and Metabolism
2.4.2. Sex–Tibolone Interactions Impact Complex 1 of the Electron Transport Chain
2.4.3. Gonadectomy and Tibolone Interact to Regulate Translation and Post-Translation-Associated Pathways
2.5. Three-Way Interaction of Sex, Gonads and Tibolone Implicate Connection to Respiration and Alzheimer’s Disease
2.6. Meta-Analysis for Enriched Functions amongst Proteins Impacted by Sex-Specific Factors Reveals Connection to Alzheimer’s Disease
2.7. NDUFA2, NDUFA7 and UQCR10 Are Regulated by Sex–Gonad–Tibolone Interactions with Relevance to Alzheimer’s Disease
3. Discussion
3.1. Functional Enrichment Analysis Reveals Altered Pathways Associated with Alzheimer’s Disease and the Electron Transport Chain
3.2. Complex 1 Proteins Are Regulated by Sex-Hormone Interactions
3.3. Complex 3 Protein UQCR10 Is Regulated by Sex-Hormone Interactions
3.4. Future Studies and Limitations
4. Materials and Methods
4.1. Model Preparation
4.2. Proteomic Dataset Production
4.2.1. Protein Processing for Proteomics
4.2.2. Analysis via Liquid Chromatography Coupled to TRIPLE-TOF Mass Spectrometry
4.2.3. Data Preparation
4.3. Bioinformatic Analysis
4.3.1. Functional Enrichment Mapping
4.3.2. Protein–Protein Interactions (PPI)
4.3.3. Pathway and Process Enrichment Analysis
4.4. Graphing
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Institutes of Health. NIH guidelines on the inclusion of women and minorities as subjects in clinical research. Fed. Regist. 1994, 59, 1408–1413. [Google Scholar]
- Tang, M.-X.; Jacobs, D.; Stern, Y.; Marder, K.; Schofield, P.; Gurland, B.; Andrews, H.; Mayeux, R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet 1996, 348, 429–432. [Google Scholar] [CrossRef]
- Christensen, A.; Pike, C.J. Menopause, obesity and inflammation: Interactive risk factors for Alzheimer’s disease. Front. Aging Neurosci. 2015, 7, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moisan, F.; Kab, S.; Mohamed, F.; Canonico, M.; Le Guern, M.; Quintin, C.; Carcaillon, L.; Nicolau, J.; Duport, N.; Singh-Manoux, A. Parkinson disease male-to-female ratios increase with age: French nationwide study and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 952–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreto, G.E.; McGovern, A.J.; Garcia-Segura, L.M. Role of Neuroglobin in the Neuroprotective Actions of Estradiol and Estrogenic Compounds. Cells 2021, 10, 1907. [Google Scholar] [CrossRef] [PubMed]
- Del Río, J.P.; Molina, S.; Hidalgo-Lanussa, O.; Garcia-Segura, L.M.; Barreto, G.E. Tibolone as Hormonal Therapy and Neuroprotective Agent. Trends Endocrinol. Metab. 2020, 31, 742–759. [Google Scholar] [CrossRef]
- Arevalo, M.-A.; Azcoitia, I.; Garcia-Segura, L.M. The neuroprotective actions of oestradiol and oestrogen receptors. Nat. Rev. Neurosci. 2015, 16, 17. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Moulin, M.; Piquereau, J.; Lemaire, C.; Mericskay, M.; Veksler, V.; Garnier, A. Mitochondria: A central target for sex differences in pathologies. Clin. Sci. 2017, 131, 803–822. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.-J.; Lin, C.-H.; Lane, H.-Y. From Menopause to Neurodegeneration—Molecular Basis and Potential Therapy. Int. J. Mol. Sci. 2021, 22, 8654. [Google Scholar] [CrossRef]
- Wang, Y.; Mishra, A.; Brinton, R.D. Transitions in metabolic and immune systems from pre-menopause to post-menopause: Implications for age-associated neurodegenerative diseases. F1000Research 2020, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- López-Grueso, R.; Gambini, J.; Abdelaziz, K.M.; Monleón, D.; Díaz, A.; El Alami, M.; Bonet-Costa, V.; Borrás, C.; Viña, J. Early, But Not Late Onset Estrogen Replacement Therapy Prevents Oxidative Stress and Metabolic Alterations Caused by Ovariectomy. Antioxid. Redox Signal. 2013, 20, 236–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Jiménez, C.; González, J.; Vesga, D.; Aristizabal, A.; Barreto, G.E. Tibolone Ameliorates the Lipotoxic Effect of Palmitic Acid in Normal Human Astrocytes. Neurotox. Res. 2020, 38, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.Á.; Garcia-Segura, L.M.; Cabezas, R.; Torrente, D.; Capani, F.; Gonzalez, J.; Barreto, G.E. Tibolone protects T98G cells from glucose deprivation. J. Steroid Biochem. Mol. Biol. 2014, 144, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Avila-Rodriguez, M.; Garcia-Segura, L.M.; Hidalgo-Lanussa, O.; Baez, E.; Gonzalez, J.; Barreto, G.E. Tibolone protects astrocytic cells from glucose deprivation through a mechanism involving estrogen receptor beta and the upregulation of neuroglobin expression. Mol. Cell. Endocrinol. 2016, 433, 35–46. [Google Scholar] [CrossRef]
- González-Giraldo, Y.; Garcia-Segura, L.M.; Echeverria, V.; Barreto, G.E. Tibolone preserves mitochondrial functionality and cell morphology in astrocytic cells treated with palmitic acid. Mol. Neurobiol. 2018, 55, 4453–4462. [Google Scholar] [CrossRef]
- Hidalgo-Lanussa, O.; Ávila-Rodriguez, M.; Baez-Jurado, E.; Zamudio, J.; Echeverria, V.; Garcia-Segura, L.M.; Barreto, G.E. Tibolone reduces oxidative damage and inflammation in microglia stimulated with palmitic acid through mechanisms involving estrogen receptor beta. Mol. Neurobiol. 2018, 55, 5462–5477. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Arnold, A.P.; Ball, G.F.; Blaustein, J.D.; De Vries, G.J. Sex differences in the brain: The not so inconvenient truth. J. Neurosci. 2012, 32, 2241–2247. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Sifuentes, Y.; Maney, D.L. Reporting and Misreporting of Sex Differences in the Biological Sciences. eLife 2021, 10, e70817. [Google Scholar] [CrossRef]
- Ullah, M.F.; Ahmad, A.; Bhat, S.H.; Abu-Duhier, F.M.; Barreto, G.E.; Ashraf, G.M. Impact of sex differences and gender specificity on behavioral characteristics and pathophysiology of neurodegenerative disorders. Neurosci. Biobehav. Rev. 2019, 102, 95–105. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Kushnareva, Y.; Murphy, A.N.; Andreyev, A. Complex I-mediated reactive oxygen species generation: Modulation by cytochrome c and NAD (P)+ oxidation–reduction state. Biochem. J. 2002, 368, 545–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stroud, D.A.; Surgenor, E.E.; Formosa, L.E.; Reljic, B.; Frazier, A.E.; Dibley, M.G.; Osellame, L.D.; Stait, T.; Beilharz, T.H.; Thorburn, D.R. Accessory subunits are integral for assembly and function of human mitochondrial complex I. Nature 2016, 538, 123–126. [Google Scholar] [CrossRef] [Green Version]
- Hoefs, S.J.; Dieteren, C.E.; Distelmaier, F.; Janssen, R.J.; Epplen, A.; Swarts, H.G.; Forkink, M.; Rodenburg, R.J.; Nijtmans, L.G.; Willems, P.H. NDUFA2 complex I mutation leads to Leigh disease. Am. J. Hum. Genet. 2008, 82, 1306–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrier, S.; Gauquelin, L.; Tétreault, M.; Tran, L.T.; Webb, N.; Srour, M.; Mitchell, J.J.; Brunel-Guitton, C.; Majewski, J.; Long, V.; et al. Recessive mutations in NDUFA2 cause mitochondrial leukoencephalopathy. Clin. Genet. 2018, 93, 396–400. [Google Scholar] [CrossRef]
- Alagia, M.; Cappuccio, G.; Torella, A.; D’Amico, A.; Mazio, F.; Romano, A.; Fecarotta, S.; Casari, G.; Nigro, V.; Brunetti-Pierri, N. Cavitating and tigroid-like leukoencephalopathy in a case of NDUFA2-related disorder. JIMD Rep. 2020, 52, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Li, H.; Pan, S. Discovery and Validation of Key Biomarkers Based on Immune Infiltrates in Alzheimer’s Disease. Front. Genet. 2021, 12, 886. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, H.; Xue, L.; He, W.; Shu, W.; Wu, H.; Wang, Z. High throughput proteomic and metabolic profiling identified target correction of metabolic abnormalities as a novel therapeutic approach in head and neck paraganglioma. Transl. Oncol. 2021, 14, 101146. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Y.; Chen, R.; Gong, Y.; Zhang, M.; Guan, R.; Rotstein, O.D.; Liu, X.; Wen, X.Y. ndufa7 plays a critical role in cardiac hypertrophy. J. Cell. Mol. Med. 2020, 24, 13151–13162. [Google Scholar] [CrossRef]
- Wu, J.; Dai, F.; Li, C.; Zou, Y. Gender differences in cardiac hypertrophy. J. Cardiovasc. Transl. Res. 2020, 13, 73–84. [Google Scholar] [CrossRef]
- Xiao, M.-H.; Lin, Y.-F.; Xie, P.-P.; Chen, H.-X.; Deng, J.-W.; Zhang, W.; Zhao, N.; Xie, C.; Meng, Y.; Liu, X.; et al. Downregulation of a mitochondrial micropeptide, MPM, promotes hepatoma metastasis by enhancing mitochondrial complex I activity. Mol. Ther. 2021, 30, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Mitsunaga, S.; Hosomichi, K.; Okudaira, Y.; Nakaoka, H.; Suzuki, Y.; Kuwana, M.; Sato, S.; Kaneko, Y.; Homma, Y.; Oka, A. Aggregation of rare/low-frequency variants of the mitochondria respiratory chain-related proteins in rheumatoid arthritis patients. J. Hum. Genet. 2015, 60, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Pikwer, M.; Nilsson, J.-Å.; Bergström, U.; Jacobsson, L.T.; Turesson, C. Early menopause and severity of rheumatoid arthritis in women older than 45 years. Arthritis Res. Ther. 2012, 14, R190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unni, S.; Thiyagarajan, S.; Srinivas Bharath, M.M.; Padmanabhan, B. Tryptophan Oxidation in the UQCRC1 Subunit of Mitochondrial Complex III (Ubiquinol-Cytochrome C Reductase) in a Mouse Model of Myodegeneration Causes Large Structural Changes in the Complex: A Molecular Dynamics Simulation Study. Sci. Rep. 2019, 9, 10694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Páleníková, P.; Harbour, M.E.; Prodi, F.; Minczuk, M.; Zeviani, M.; Ghelli, A.; Fernández-Vizarra, E. Duplexing complexome profiling with SILAC to study human respiratory chain assembly defects. Biochim. Biophys. Acta (BBA)—Bioenerg. 2021, 1862, 148395. [Google Scholar] [CrossRef]
- Burska, D.; Stiburek, L.; Krizova, J.; Vanisova, M.; Martinek, V.; Sladkova, J.; Zamecnik, J.; Honzik, T.; Zeman, J.; Hansikova, H.; et al. Homozygous missense mutation in UQCRC2 associated with severe encephalomyopathy, mitochondrial complex III assembly defect and activation of mitochondrial protein quality control. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2021, 1867, 166147. [Google Scholar] [CrossRef]
- Manners, H.N.; Roy, S.; Kalita, J.K. Intrinsic-overlapping co-expression module detection with application to Alzheimer’s Disease. Comput. Biol. Chem. 2018, 77, 373–389. [Google Scholar] [CrossRef]
- Zhong, R.; Zhang, Y.; Chen, D.; Cao, S.; Han, B.; Zhong, H. Single-cell RNA sequencing reveals cellular and molecular immune profile in a Pembrolizumab-responsive PD-L1-negative lung cancer patient. Cancer Immunol. Immunother. 2021, 70, 2261–2274. [Google Scholar] [CrossRef]
- Coyoy-Salgado, A.; Segura-Uribe, J.J.; Manuel Gallardo, J.; Estrada-Cruz, N.A.; Camacho-Arroyo, I.; Guerra-Araiza, C. Tibolone regulates systemic metabolism and the expression of sex hormone receptors in the central nervous system of ovariectomised rats fed with high-fat and high-fructose diet. Brain Res. 2020, 1748, 147096. [Google Scholar] [CrossRef]
- Chabrun, F.; Dieu, X.; May-Panloup, P.; Chupin, S.; Bourreau, J.; Henrion, D.; Letournel, F.; Procaccio, V.; Bonneau, D.; Lenaers, G.; et al. Metabolomic Sexual Dimorphism of the Mouse Brain is Predominantly Abolished by Gonadectomy with a Higher Impact on Females. J. Proteome Res. 2021, 20, 2772–2779. [Google Scholar] [CrossRef]
- Demarest, T.G.; Varma, V.R.; Estrada, D.; Babbar, M.; Basu, S.; Mahajan, U.V.; Moaddel, R.; Croteau, D.L.; Thambisetty, M.; Mattson, M.P. Biological sex and DNA repair deficiency drive Alzheimer’s disease via systemic metabolic remodeling and brain mitochondrial dysfunction. Acta Neuropathol. 2020, 140, 25–47. [Google Scholar] [CrossRef] [PubMed]
- Abyadeh, M.; Gupta, V.; Chitranshi, N.; Gupta, V.; Wu, Y.; Saks, D.; Wander Wall, R.; Fitzhenry, M.J.; Basavarajappa, D.; You, Y.; et al. Mitochondrial dysfunction in Alzheimer’s disease—A proteomics perspective. Expert Rev. Proteom. 2021, 18, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Palau, X.; Lee, B.S.T.; Adav, S.S.; Qian, J.; Serra, A.; Park, J.E.; Lai, M.K.P.; Chen, C.P.; Kalaria, R.N.; Sze, S.K. Gender differences in white matter pathology and mitochondrial dysfunction in Alzheimer’s disease with cerebrovascular disease. Mol. Brain 2016, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacCannell, A.D.V.; Futers, T.S.; Whitehead, A.; Moran, A.; Witte, K.K.; Roberts, L.D. Sexual dimorphism in adipose tissue mitochondrial function and metabolic flexibility in obesity. Int. J. Obes. 2021, 45, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Velarde, M.C. Pleiotropic actions of estrogen: A mitochondrial matter. Physiol. Genom. 2013, 45, 106–109. [Google Scholar] [CrossRef] [Green Version]
- Cagnacci, A.; Venier, M. The Controversial History of Hormone Replacement Therapy. Medicina 2019, 55, 602. [Google Scholar] [CrossRef] [Green Version]
- Collaborative Group on Hormonal Factors in Breast, C. Type and timing of menopausal hormone therapy and breast cancer risk: Individual participant meta-analysis of the worldwide epidemiological evidence. Lancet 2019, 394, 1159–1168. [Google Scholar] [CrossRef]
- Martinkova, J.; Quevenco, F.-C.; Karcher, H.; Ferrari, A.; Sandset, E.C.; Szoeke, C.; Hort, J.; Schmidt, R.; Chadha, A.S.; Ferretti, M.T. Proportion of Women and Reporting of Outcomes by Sex in Clinical Trials for Alzheimer Disease: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2124124. [Google Scholar] [CrossRef]
- Schwartz, J.B.; Weintraub, S. Treatment for Alzheimer Disease—Sex and Gender Effects Need to Be Explicitly Analyzed and Reported in Clinical Trials. JAMA Netw. Open 2021, 4, e2124386. [Google Scholar] [CrossRef]
- Rich-Edwards, J.W.; Kaiser, U.B.; Chen, G.L.; Manson, J.E.; Goldstein, J.M. Sex and Gender Differences Research Design for Basic, Clinical, and Population Studies: Essentials for Investigators. Endocr. Rev. 2018, 39, 424–439. [Google Scholar] [CrossRef] [Green Version]
- Wichmann, C.; Meier, F.; Virreira Winter, S.; Brunner, A.-D.; Cox, J.; Mann, M. MaxQuant.Live Enables Global Targeting of More than 25,000 Peptides. Mol. Cell. Proteom. 2019, 18, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Reimand, J.; Isserlin, R.; Voisin, V.; Kucera, M.; Tannus-Lopes, C.; Rostamianfar, A.; Wadi, L.; Meyer, M.; Wong, J.; Xu, C.; et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019, 14, 482–517. [Google Scholar] [CrossRef]
- Oesper, L.; Merico, D.; Isserlin, R.; Bader, G.D. WordCloud: A Cytoscape plugin to create a visual semantic summary of networks. Source Code Biol. Med. 2011, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.H.; Apeltsin, L.; Newman, A.M.; Baumbach, J.; Wittkop, T.; Su, G.; Bader, G.D.; Ferrin, T.E. clusterMaker: A multi-algorithm clustering plugin for Cytoscape. BMC Bioinform. 2011, 12, 436. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGovern, A.J.; Arevalo, M.A.; Ciordia, S.; Garcia-Segura, L.M.; Barreto, G.E. Respirasome Proteins Are Regulated by Sex-Hormone Interactions in the Brain. Int. J. Mol. Sci. 2022, 23, 14754. https://doi.org/10.3390/ijms232314754
McGovern AJ, Arevalo MA, Ciordia S, Garcia-Segura LM, Barreto GE. Respirasome Proteins Are Regulated by Sex-Hormone Interactions in the Brain. International Journal of Molecular Sciences. 2022; 23(23):14754. https://doi.org/10.3390/ijms232314754
Chicago/Turabian StyleMcGovern, Andrew J., Maria Angeles Arevalo, Sergio Ciordia, Luis Miguel Garcia-Segura, and George E. Barreto. 2022. "Respirasome Proteins Are Regulated by Sex-Hormone Interactions in the Brain" International Journal of Molecular Sciences 23, no. 23: 14754. https://doi.org/10.3390/ijms232314754